Abstract
The mechanisms by which the human T-cell leukemia virus type I (HTLV-I) Tax oncoprotein deregulates cellular signaling for oncogenesis have been extensively studied, but how Tax itself is regulated remains largely unknown. Here we report that Tax was negatively regulated by PDLIM2, which promoted Tax K48-linked polyubiquitination. In addition, PDLIM2 recruited Tax from its functional sites into the nuclear matrix where the polyubiquitinated Tax was degraded by the proteasome. Consistently, PDLIM2 suppressed Tax-mediated signaling activation, cell transformation, and oncogenesis both in vitro and in animal. Notably, PDLIM2 expression was down-regulated in HTLV-I–transformed T cells, and PDLIM2 reconstitution reversed the tumorigenicity of the malignant cells. These studies indicate that the counterbalance between HTLV-I/Tax and PDLIM2 may determine the outcome of HTLV-I infection. These studies also suggest a potential therapeutic strategy for cancers and other diseases associated with HTLV-I infection and/or PDLIM2 deregulation.
Introduction
The human T-cell leukemia virus type I (HTLV-I) is the etiologic agent of adult T-cell leukemia (ATL), an aggressive T-cell malignancy, and HTLV-I–associated myelopathy/tropical spastic paraparesis (HAM/TSP), a neuroinflammatory disease.1,2 HTLV-I infection also associates with the formation of several autoimmune diseases, increases the risk of primary malignant neoplasms, and promotes the development of AIDS when coinfecting with the human immunodeficiency virus (HIV). The pathogenesis, particularly the leukemogenesis, of HTLV-I is mediated mainly by its encoded regulatory protein Tax.
Tax not only transforms rodent fibroblasts but also immortalizes human primary T cells in vitro.3-6 Compared with cells transformed by many cellular oncogenes, Tax-transformed cells have an apparently higher resistance to the induction of apoptosis.7 In addition, Tax-transformed lymphoid cells and fibroblasts induce tumors when introduced into nude mice.3,4,8 More importantly, the HTLV-I genome without Tax loses its original transforming ability,9 whereas Tax transgenic mice develop various tumors, depending on the type of the promoters used to drive Tax expression.10-12 Of note, Tax-immortalized lymphocytes in vitro and Tax-mediated T-cell lymphoma in animals resemble closely the phenotype of HTLV-I–transformed T cells and HTLV-I–induced ATL, respectively.13-15
The Tax oncoprotein exerts its oncogenic role largely through deregulation of cellular transcription factors that are critical for cell growth and division, such as CREB/ATF and NF-κB.1,2 The mechanisms by which Tax deregulates these important transcription factors have been well studied. For example, Tax activates transcription activity of CREB/ATF proteins by directly interacting with their basic region-leucine zipper (bZIP) DNA-binding domains in the nucleus thereby enhancing DNA binding.2 On the other hand, Tax intervenes at multiple levels to activate NF-κB. In the cytoplasm, Tax binds to and recruits the IκB (inhibitor of NF-κB) kinase (IKK) complex, via its regulator IKKγ (also known as NEMO), into specific compartments for IKK activation, resulting in degradation of IκB and subsequent nuclear translocation of NF-κB factors including RelA (also known as p65).16 In the nucleus, Tax recruits p65 as well as other cellular transcriptional components into interchromatin granules to form discrete transcriptional hot spots termed “Tax nuclear bodies” for full NF-κB transcriptional activation.17,18 Interestingly, the critical cytoplasmic and nuclear steps of NF-κB activation require 2 distinct posttranslational modifications of Tax proteins, ubiquitination and sumoylation.19,20
Protein ubiquitination involves the sequential action of the ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin ligases (E3). This reaction starts with the formation of a thiolester linkage between E1 and ubiquitin, followed by transfer of ubiquitin to an E2. Finally, E3 recruits a specific protein substrate to the E2-ubiquitin, where the ubiquitin is conjugated, via its C-terminal glycine, to a specific lysine in the protein substrate. Ubiquitin itself can be further ubiquitinated to form a polyubiquitin chain, which is mainly through lysine 48 (K48) or lysine 63 (K63) of ubiquitin.21-23 Strikingly, K48-linked and K63-linked polyubiquitinations lead to different fates of the substrates: the former targets substrates for proteaosomal degradation, whereas the latter usually acts as a scaffold to alter substrate functions through proteasome-independent mechanisms.24
In this study, we show that Tax could be ubiquitinated in both the cytoplasm and the nucleus. Whereas the cytoplasmic ubiquitination of Tax was via either K63 linkage or K48 linkage, the nuclear ubiquitination was mainly K48 linked. The K48-linked ubiquitination of Tax involved PDLIM2, a newly identified ubiquitin E3 ligase. PDLIM2 bound to and recruited Tax from both the cytoplasm and the Tax nuclear bodies into the nuclear matrix where the ubiquitinated Tax was degraded by the preteasome. Whereas PDLIM2 coexpression was able to suppress Tax-mediated signaling activation and oncogenesis, its genetic knockout further enhanced Tax's functions. Notably, the PDLIM2 was down-regulated in various HTLV-I–transformed T cells. Reconstitution of PDLIM2 into these malignant cells blocked their tumorigenicity. These findings demonstrate a novel mechanism of Tax regulation that could be targeted for clinical therapy.
Methods
Expression vectors and reagents
Expression vectors encoding Tax, PDLIM2, and its LIM domain deletion mutant have been described before.25,26 Ubiquitin and its Ub-K48 and Ub-K63 mutants were gifts of Z. Chen. The Tax and PDLIM2 cDNAs were also subcloned into retroviral vectors pCLXSN and/or pTRIP by routine cloning strategies as described.27 The HA monoclonal antibody (12CA5) and HRP-conjugated HA monoclonal antibody (3F10) were from Roche Molecular Biochemicals (Indianapolis, IN). The ubiquitin, Sp1, lamin B, Hsp90, and PML antibodies as well as the FITC-conjugated antigoat secondary antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). The SC-35 antibody was from Sigma (St Louis, MO). The Texas red–conjugated antirabbit secondary antibody was from Amersham Pharmacia Biotech (Piscataway, NJ). Texas red–conjugated antimouse secondary antibody, FITC-conjugated antirabbit secondary antibody, and Hoechst 33258 were from Molecular Probes (Eugene, OR). The 20S proteasome antibodies, proteasome inhibitor MG132, and protein synthesis inhibitor cycloheximide (CHX) were from Biomol (Plymouth Meeting, PA). The Tax and PDLIM2 antibodies were described previously.26,28
Cell culture and transfection
HEK293 cells, Hela cells, Rat-1 fibroblasts, and mouse embryonic fibroblasts (MEFs) were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum and 2 mM l-glutamine. Human T-lymphocyte Jurkat, and HTLV-I–transformed T cell lines C8166, HuT102, MT-4, and SLB were maintained in suspension in RPMI 1640 medium supplemented with 10% fetal bovine serum and 2 mM l-glutamine.29 293 and Jurkat cells were transfected with DEAE-Dextran, Hela, and MEF cells with Lipofectamine 2000 (Invitrogen, Frederick, MD), and HTLV-I–transformed T cells with Transfast reagent (Promega, Madison, WI).30,31
Retroviral transduction and generation of stable transfectant
Rat-1 cells and HTLV-I–transformed T cells were infected with virus expressing Tax or PDLIM2, respectively. The Rat-1 cells stably expressing Tax were also reinfected with virus expressing PDLIM2 for simultaneously expressing both Tax and PDLIM2. The viruses expressing GFP were used as a control. The stable transfectants were obtained by selection with G418 and/or blasticidin selections as described previously.32
Subcellular fractionation, immunoblotting, and immunoprecipitation assays
Cytoplasmic, soluble, and insoluble nuclear extracts were prepared using the hypotonic buffer (20 mM HEPES, pH 8.0, 10 mM KCl, 1 mM MgCl2, 0.1% [vol/vol] Triton X-100, and 20% [vol/vol] glycerol), hypertonic buffer (20 mM HEPES, pH 8.0, 1 mM EDTA, 20% [vol/vol] glycerol, 0.1% [vol/vol] Triton X-100, and 400 mM NaCl), and insoluble buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 1% [wt/vol] SDS, 1% [vol/vol] NP-40, and 10 mM iodoacetamide), respectively.25 The purity of the obtained fractions was confirmed by checking Hsp90 (cytoplasm), Sp1 (soluble nuclear fraction), or lamin B (insoluble nuclear fraction). For the nuclear matrix, the cells were lysed sequentially to remove the cytoplasm, nucleoplasm, and chromatin using the hypotonic buffer, hypertonic buffer, and digestion buffer (200 U/mL DNase I, 100 mM NaCl, 300 mM sucrose, 10 mM PIPES, pH 6.8, 3 mM MgCl2, 0.5% Triton X-100, 1 mM PMSF, 1 μg/mL leupeptin, and 1 μg/mL pepstatin A), respectively. After 3 washes with the high salt buffer (2 M NaCl, 300 mM sucrose, 10 mM PIPES, pH 6.8, 3 mM MgCl2, 0.5% Triton X-100, 1 mM PMSF, 1 μg/mL leupeptin, and 1 μg/mL pepstatin A), the left pellet was the nuclear matrix fraction.33 Total nuclear extracts were prepared by simply lysing pellets in insoluble buffer after the cytoplasm was extracted. Whole-cell extracts were prepared by lysing cells in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.25% [wt/vol] Na-deoxycholate, 1% [vol/vol] NP-40, 1 mM DTT). All the lysis buffers were supplemented with 1 mM PMSF and a protease inhibitor cocktail (Roche Molecular Biochemicals). The cells extracts were used for immunoprecipitation (IP) and/or immunoblotting (IB) assays as described before.34
In vivo ubiquitin conjugation assay
Cytoplasmic and nuclear extracts were prepared from HTLV-I–transformed T cells or 293 cells transfected with Tax together with HA-tagged ubiquitin or its mutants in the presence or absence of PDLIM2, immediately followed by IP using anti-Tax. The ubiquitin-conjugated Tax pulled down by IP was detected by IB using antiubiquitin or anti–HA-HRP.35
Protein stability assay
Cells were treated with 10 μM CHX, followed by chase of the indicated time period in the presence or absence of MG132, and IB to detect the indicated proteins.36
Real-time PCR analysis
Total RNA was prepared with TRIZOL reagent and cDNA was generated with SuperScript II reverse transcriptase (Invitrogen), followed by real-time polymerase chain reaction (PCR) assays as described before.34 Primer pairs were as follows: human PDLIM2, forward 5′-GCCCATCATGGTGACTAAGG, reverse 5′-ATGGCCACGATTATGTCTCC; human β-actin, forward 5′-ATCAAGATCATTGCTCCTCCT, reverse 5′-GAGAGCGAGGCCAGGATGGA.
Luciferase gene reporter assays
Jurkat, 293, and MEF cells were transfected with luciferase reporter and Tax in the presence of increasing amounts of PDLIM2. For MT-4 cells, the luciferase reporter together with increasing amounts of PDLIM2 was transfected. At 40 hours after transfection, luciferase activity was measured as we described before.37
Immunofluorescence and confocal microscopic analysis
Hela cells expressing GFP-Tax and/or PDLIM2, and HTLV-I–transformed T cells stably expressing PDLIM2 or an empty vector were directly fixed, permeabilized, and subsequently incubated with the indicated primary antibodies, followed by incubation with the indicated secondary antibodies. The subcellular localization of stained proteins was detected using a Nikon Eclipse E800 (Tokyo, Japan; 100 × 1.40 Navil objective) fluorescence microscope (for Hela cells) or the Olympus Fluoview 1000 confocal microscope (Melville, NY) (100 × 11.4 NA oil objective) (for HTLV-I–transformed T cells). The cells were also counterstained with Hoechst 33258 for nuclear staining.32
Colony formation assays
Soft agar assays were performed as previously described.32 Briefly, 6-well plates were coated with an initial underlay of 1% SeaPlaque low-melting agarose (Cambrex Bio Science Rockland, Rockland, ME) in culture medium. Cell suspension in culture medium containing 0.6% SeaPlaque low-melting agarose was then added to the coated plates. Colony growth was scored and pictured using the Olympus CKX41 microscope (Tokyo, Japan) (10 10.25 NA oil objective) after 21 days of cell incubation at the normal condition. All the colony formation assays presented in this study were repeated in at least 3 independent experiments.
In vivo tumorigenicity assays
All experiments involving mice were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC). Five-week-old female severe combined immunodeficient (SCID) mice (C.B-17/IcrCrl-scidBR; from Charles River Laboratories, Wilmington, MA) were challenged subcutaneously in hind-back with Rat-1 or MEF stable cell lines, or subcutaneously in the postauricular region with C8166 or MT-4 stably expressing PDLIM2 or an empty vector. The recipient mice were monitored, and killed and dissected for tumor evaluation at the indicated postinjection days.
Results
Cytoplasmic and nuclear Tax shows different ubiquitin modifications and stabilities
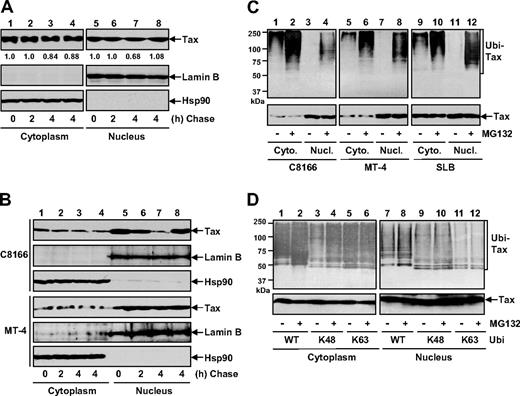
To study how Tax is regulated in the different subcellular locations, we initially compared the stabilities of cytoplasmic and nuclear Tax in human embryonic kidney 293 cells and various HTLV-I–transformed T cells by performing CHX-chase assays. As shown in Figure 1A and B, the cytoplasmic Tax in all these cells decreased marginally following a chase of up to 4 hours (lanes 1-3). However, the nuclear Tax decreased much more dramatically, although to different extents in different cell types (Figure 1A,B lanes 5-7). Moreover, the nuclear degradation was completely blocked when the proteasome was blocked (Figure 1A,B lane 8). It is worthy to note that the different dynamic of Tax turnover in those cells inversely correlated with the expression levels of PDLIM2 (Figure 6), which shuttles Tax into the nucleus for degradation (Figures 2,3). Nevertheless, these results indicated that different from its cytoplasmic portion, which is relatively stable, Tax in the nucleus is unstable and undergoes proteasomal degradation under both overexpression and pathophysiological conditions.
Tax shows different ubiquitin modifications and stabilities in the cytoplasm and in the nucleus. (A) 293 cells transiently transfected with Tax were CHX chased at the indicated time (in hours), followed by cytoplasmic and nuclear extractions. For the 4-hour time point, the cells were also chased in the presence of 10 μM MG132 (lanes 4 and 8). The expression levels of Tax, lamin B, and Hsp90 in these 2 fractions were detected by IB. The Tax levels were also quantitated by densitometry. (B) C8166 and MT-4 were used for Tax stability assays as described in panel A. (C) C8166, MT-4, and SLB cells were left untreated or treated with MG132 for 3 hours, followed by cytoplasmic and nuclear extractions. The ubiquitinated Tax proteins were then analyzed by IP using Tax antibody and IB using ubiquitin antibody. The levels of Tax in the extractions were analyzed by direct IB. (D) 293 cells transfected with Tax plus the indicated HA-tagged ubiquitin mutants were left untreated or treated with MG132 for 3 hours, followed by the ubiquitination assays as described in panel C.
Tax shows different ubiquitin modifications and stabilities in the cytoplasm and in the nucleus. (A) 293 cells transiently transfected with Tax were CHX chased at the indicated time (in hours), followed by cytoplasmic and nuclear extractions. For the 4-hour time point, the cells were also chased in the presence of 10 μM MG132 (lanes 4 and 8). The expression levels of Tax, lamin B, and Hsp90 in these 2 fractions were detected by IB. The Tax levels were also quantitated by densitometry. (B) C8166 and MT-4 were used for Tax stability assays as described in panel A. (C) C8166, MT-4, and SLB cells were left untreated or treated with MG132 for 3 hours, followed by cytoplasmic and nuclear extractions. The ubiquitinated Tax proteins were then analyzed by IP using Tax antibody and IB using ubiquitin antibody. The levels of Tax in the extractions were analyzed by direct IB. (D) 293 cells transfected with Tax plus the indicated HA-tagged ubiquitin mutants were left untreated or treated with MG132 for 3 hours, followed by the ubiquitination assays as described in panel C.
Since ubiquitination is the major mechanism leading to proteasomal degradation, we examined the Tax ubiquitination in both cytoplasm and nucleus. In agreement with recent studies,38-41 we found that cytoplasmic Tax in various HTLV-I–transformed T cells was polyubiquitinated (Figure 1C lanes 1, 5, and 9). Interestingly, this ubiquitination could be further enhanced, although only modestly, by proteasome inhibition (Figure 1C lanes 2, 6, and 10). On the contrary, Tax ubiquitination in the nucleus was recovered only when the proteasome was blocked (Figure 1C lanes 3, 4, 7, 8, 11, 12). It thus seemed that both cytoplasmic and nuclear Tax can be polyubiquitinated, but in different modes, which may account for their different stabilities and different sensitivities to the proteasome.
Given the distinct roles of the K48- and K63-linked polyubiquitination in protein stability, we used wild-type ubiquitin (Ub-WT) and its 2 mutants harboring lysine to arginine substitutions on all the lysine residues of ubiquitin except lysine 48 (Ub-K48) or lysine 63 (Ub-K63) for the Tax in vivo ubiquitination assays. As shown in Figure 1D, abundant polyubiquitinated Tax proteins in the cytoplasm were readily detected when Ub-WT or Ub-K63 was coexpressed (lanes 1 and 5). However, proteasome inhibition could enhance only Ub-WT– but not Ub-K63–conjugated polyubiquitination (Figure 1D lanes 2 and 6), suggesting that the enhanced polyubiquitination of Tax was K48 linked. Indeed, a lower level of polyubiquitinated Tax in the cytoplasm could be detected and markedly increased by proteasome inhibition when Ub-K48 was coexpressed (Figure 1D lanes 3 and 4). In the nucleus, polyubiquitinated Tax was recovered when Ub-WT or Ub-K48, but not Ub-K63, was coexpressed (Figure 1D lanes 7, 9, and 11). Consistently, the proteasome inhibition could further increase the level of the nuclear polyubiquitinated Tax in Ub-WT or Ub-K48, but not Ub-K63, coexpressing cells (Figure 1D lanes 8, 10, and 12). Together, these studies indicated that whereas Tax polyubiquitination in the cytoplasm is via either K48 or K63 linkage, its nuclear polyubiquitination is K48 linked and constitutively targeted for proteasomal degradation.
PDLIM2 induces Tax K48-linked polyubiquitination and proteasomal degradation
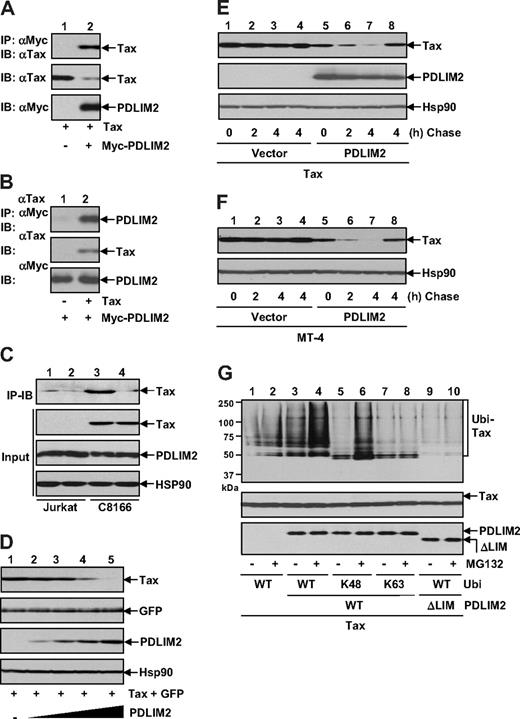
To identify the cellular factor involved in the Tax ubiquitination and degradation, we tested the possible role of PDLIM2. PDLIM2 is a novel ubiquitin E3 ligase whose activity depends on its LIM domain, a motif similar to RING finger domain.25,28 We first examined whether PDLIM2 physically associates with Tax. When coexpressed together in 293 cells, PDLIM2 and Tax formed a stable complex, as evidenced by our reciprocal immunoprecipitation assays (Figure 2A,B). In fact, endogenous PDLIM2 and Tax also form a complex in HTLV-I–transformed T cells (Figure 2C).
PDLIM2 promotes K48-linked ubiquitination and proteasomal degradation of Tax. (A) 293 cells were transfected with Tax in the presence or absence of Myc-PDLIM2, followed by IP using Myc antibody and IB using Tax antibody. The expression levels of Tax and Myc-PDLIM2 were analyzed by direct IB. (B) 293 cells transfected with Myc-PDLIM2 alone or together with Tax were subjected to IP using Tax antibody and IB using Myc antibody. The expression levels of Tax and Myc-PDLIM2 were also analyzed. (C) Jurkat and C8166 cells were subjected to IP using PDLIM2 antibody (lanes 1 and 3) or control IgG (lanes 2 and 4) and IB using Tax antibody. The input was monitored by direct IB using Tax, PDLIM2, or Hsp90 antibody. (D) 293 cells were transfected with Tax and GFP in the presence of increasing amounts of PDLIM2, followed by IB to detect expression levels of Tax, GFP, PDLIM2, and Hsp90. (E) 293 cells transfected with Tax in the presence or absence of PDLIM2 were CHX chased at the indicated time (in hours). In lanes 4 and 8, the cells were chased in the presence of 10 μM MG132. The protein levels of Tax, Myc-PDLIM2, and Hsp90 were analyzed by direct IB. (F) MT-4 cells stably expressing PDLIM2 or an empty vector were CHX chased as described in panel D. (G) 293 cells transfected with the indicated constructs were left untreated or treated with MG132 for 3 hours, followed by IP-IB to detect the ubiquitinated Tax in the nucleus (top). To better compare Tax ubiquitination, the similar amounts of Tax proteins were used in each lane for IP (middle). The expression levels of PDLIM2 and its LIM deletion mutants were analyzed by IB (bottom).
PDLIM2 promotes K48-linked ubiquitination and proteasomal degradation of Tax. (A) 293 cells were transfected with Tax in the presence or absence of Myc-PDLIM2, followed by IP using Myc antibody and IB using Tax antibody. The expression levels of Tax and Myc-PDLIM2 were analyzed by direct IB. (B) 293 cells transfected with Myc-PDLIM2 alone or together with Tax were subjected to IP using Tax antibody and IB using Myc antibody. The expression levels of Tax and Myc-PDLIM2 were also analyzed. (C) Jurkat and C8166 cells were subjected to IP using PDLIM2 antibody (lanes 1 and 3) or control IgG (lanes 2 and 4) and IB using Tax antibody. The input was monitored by direct IB using Tax, PDLIM2, or Hsp90 antibody. (D) 293 cells were transfected with Tax and GFP in the presence of increasing amounts of PDLIM2, followed by IB to detect expression levels of Tax, GFP, PDLIM2, and Hsp90. (E) 293 cells transfected with Tax in the presence or absence of PDLIM2 were CHX chased at the indicated time (in hours). In lanes 4 and 8, the cells were chased in the presence of 10 μM MG132. The protein levels of Tax, Myc-PDLIM2, and Hsp90 were analyzed by direct IB. (F) MT-4 cells stably expressing PDLIM2 or an empty vector were CHX chased as described in panel D. (G) 293 cells transfected with the indicated constructs were left untreated or treated with MG132 for 3 hours, followed by IP-IB to detect the ubiquitinated Tax in the nucleus (top). To better compare Tax ubiquitination, the similar amounts of Tax proteins were used in each lane for IP (middle). The expression levels of PDLIM2 and its LIM deletion mutants were analyzed by IB (bottom).
Notably, PDLIM2 coexpression always led to decrease in Tax protein level (Figure 2A), and the decrease was in a dose-dependent manner (Figure 2D). The effect of PDLIM2 seemed specific, because the coexpressed GFP and endogenous Hsp90 were insensitive to PDLIM2. Our CHX-chase assays using both 293 cells and HTLV-I–transformed T cells further demonstrated that PDLIM2 promoted Tax degradation, which could be suppressed by the proteasome inhibition (Figure 2E,F). These results indicated that Tax is a novel target of PDLIM2 for proteasomal degradation.
These findings prompted us to investigate whether PDLIM2 could induce Tax K48-linked polyubiquitination. To do so, we performed in vivo ubiquitination assays using the nuclear extracts from 293 cells transfected with Tax and PDLIM2 plus Ub-WT or its mutant Ub-K48 or Ub-K63. To better compare the ubiquitination level of Tax, we normalized the amounts of total Tax proteins for the assays. As shown in Figure 2G, a significantly higher level of Tax polyubiquitination was detected when PDLIM2 was coexpressed (lane 3). This difference was much more significant when the proteasome was inhibited (Figure 2G lane 4). Consistent with its role in Tax degradation, PDLIM2 promoted K48-linked, but not K63-linked, polyubiquitination of Tax (Figure 2G lanes 5-8). Interestingly, a PDLIM2 mutant that loses its ubiquitin E3 ligase activity due to lack of its LIM domain failed to promote Tax ubiquitination (Figure 2G lanes 9 and 10). Collectively, these studies indicated that PDLIM2 promotes Tax K48-linked polyubiquitination and subsequent proteasomal degradation.
PDLIM2 shuttles Tax into the nuclear matrix for proteasomal degradation
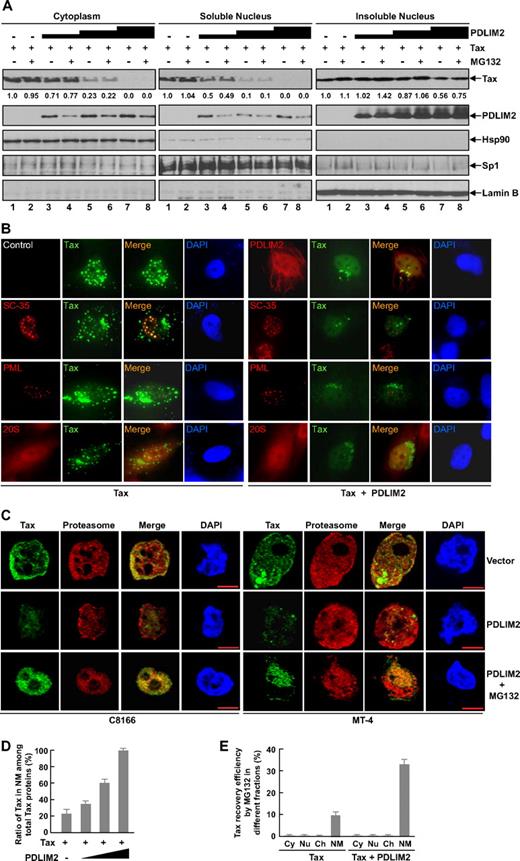
To identify the degradation site of Tax promoted by PDLIM2, we measured Tax protein levels in the cytoplasmic, soluble nuclear, and insoluble nuclear fractions of 293 cells transfected with Tax alone or Tax together with PDLIM2. As shown in Figure 3A, Tax proteins were detected in all 3 subcellular fractions when Tax was transfected alone (lanes 1). Importantly, PDLIM2 coexpression led to a dose-dependent decrease of Tax in all 3 fractions, although to different extents in the individual fractions (Figure 3A lanes 3-8). Notably, proteasome inhibition resulted in accumulation of Tax in the insoluble nuclear fraction but no significant change in the soluble nuclear fraction or the cytoplasmic fraction (Figure 3A even lanes). These data were also consistent with our previous finding that in the absence of exogenous PDLIM2, proteasomal inhibition blocks the basal degradation of Tax largely in the nucleus but only marginally in the cytoplasm (Figure 1A,B). Together, these results suggested that PDLIM2-induced Tax degradation occurs in the insoluble nuclear compartments. Interestingly, the proteasome inhibition resulted in dramatic decrease of the coexpressed PDLIM2 in both cytoplasmic and soluble nuclear fractions, but an increase in the insoluble nuclear fraction (Figure 3A). Given the strong interaction between Tax and PDLIM2, it was plausible that after delivering Tax into the insoluble nuclear compartments, PDLIM2 could not be released until Tax is degraded by the proteasome. Collectively, these data suggested that PDLIM2 shuttles Tax from the cytoplasm and the nucleoplasm into the insoluble nuclear compartments where Tax is sequestered and degraded by the proteasome.
PDLIM2 targets Tax into the nuclear matrix for proteasomal degradation. (A) 293 cells transfected with Tax in the presence of increasing amounts of PDLIM2 were left untreated or treated with MG132 for 3 hours, followed by cell fractions and IB to detect expression levels of the indicated proteins. The ratio of cytoplasm–soluble nuclear fraction–insoluble nuclear fraction used for IB assay was 1:2:2. The Tax levels were also quantitated by densitometry. (B) Hela cells transfected with Tax alone or together with PDLIM2 were analyzed by indirect immunofluorescence to visualize the indicated proteins. Tax was shown in green. SC-35, PML, and the proteasome were shown in red. Original magnification, ×1000. (C) C8166 or MT-4 cells stably expressing an empty vector (top panel) or PDLIM2 (middle and bottom panels) were analyzed by confocal microscopy to visualize the proteasome (red) and Tax (green). The cells in the bottom panel were treated with MG132 for 3 hours prior to the immunofluorescence assays. Bar represents 10 μM. (D) 293 cells were transfected with Tax alone or together with increasing amounts of PDLIM2, followed by nuclear matrix extraction and IB. The percentiles of Tax in the nuclear matrix (NM) among total Tax proteins in the cells were presented. Error bars indicate standard deviations (n = 3). (E) 293 cells transfected with Tax alone or together with PDLIM2 were treated with MG132 or DMSO for 3 hours, followed by cell fractions as described in “Subcellular fractionation, immunoblotting, and immunoprecipitation assays.” The data presented the percentiles of MG132-recovered Tax in the cytoplasm (Cy), nucleoplasm (Nu), chromatin (Ch), and nuclear matrix (NM) based on the Tax amounts in the respective fractions from the DMSO-treated cells. Error bars indicate standard deviations (n = 3).
PDLIM2 targets Tax into the nuclear matrix for proteasomal degradation. (A) 293 cells transfected with Tax in the presence of increasing amounts of PDLIM2 were left untreated or treated with MG132 for 3 hours, followed by cell fractions and IB to detect expression levels of the indicated proteins. The ratio of cytoplasm–soluble nuclear fraction–insoluble nuclear fraction used for IB assay was 1:2:2. The Tax levels were also quantitated by densitometry. (B) Hela cells transfected with Tax alone or together with PDLIM2 were analyzed by indirect immunofluorescence to visualize the indicated proteins. Tax was shown in green. SC-35, PML, and the proteasome were shown in red. Original magnification, ×1000. (C) C8166 or MT-4 cells stably expressing an empty vector (top panel) or PDLIM2 (middle and bottom panels) were analyzed by confocal microscopy to visualize the proteasome (red) and Tax (green). The cells in the bottom panel were treated with MG132 for 3 hours prior to the immunofluorescence assays. Bar represents 10 μM. (D) 293 cells were transfected with Tax alone or together with increasing amounts of PDLIM2, followed by nuclear matrix extraction and IB. The percentiles of Tax in the nuclear matrix (NM) among total Tax proteins in the cells were presented. Error bars indicate standard deviations (n = 3). (E) 293 cells transfected with Tax alone or together with PDLIM2 were treated with MG132 or DMSO for 3 hours, followed by cell fractions as described in “Subcellular fractionation, immunoblotting, and immunoprecipitation assays.” The data presented the percentiles of MG132-recovered Tax in the cytoplasm (Cy), nucleoplasm (Nu), chromatin (Ch), and nuclear matrix (NM) based on the Tax amounts in the respective fractions from the DMSO-treated cells. Error bars indicate standard deviations (n = 3).
We next visualized the effect of PDLIM2 on Tax subcellular distribution using indirect immunofluorescence staining assays. In agreement with previous studies,17-19 our immunofluorescence staining showed that Tax strongly localized to perinuclear aggregates and discrete nuclear bodies superimposed over a diffuse pattern in the cytoplasm and nucleus of both Hela cells and HTLV-I–transformed T cells (Figure 3B,C). In the nucleus, Tax colocalized with splicing factor 35 (SC-35, a component of interchromatin granules), but not with promyelocytic leukemia (PML), to form Tax foci dubbed as “Tax nuclear bodies” (Figure 3B). Consistent with its roles in Tax shuttling and degradation, PDLIM2 coexpression resulted in significantly less staining of Tax proteins in the cytoplasm and almost complete disappearance of Tax nuclear bodies (Figure 3B). Interestingly, the disappearance of Tax nuclear bodies was associated with decrease in the size of the SC-35 foci, reflecting the role of Tax in the recruitment of transcription-related components into the SC-35 foci.17,18 Surprisingly, the decrease of cytoplasmic Tax and disappearance of Tax nuclear bodies were not associated with Tax recruitment into PML nuclear bodies, the putative proteolytic centers in the nucleus.42 These results indicated that PML nuclear bodies were not the sites for Tax degradation. In further support of this, PML knockout did not alter overall subcellular location and stability of Tax (data not shown). Instead, PDLIM2 seemed to shuttle Tax into the “nucleoplasm” for degradation, because a diffuse nuclear costaining of Tax and the proteasome was detected (Figure 3B bottom panel; 3C middle panel), which was further enhanced by the proteasome inhibition (Figure 3C bottom panel).
On the surface, it seemed inconsistent between the indirect immunofluorescence and subcellular fraction studies. To clarify the paradox, we hypothesized that Tax is recruited into the nuclear matrix/reticulum for proteasomal degradation. The nuclear matrix is nuclear framework throughout the nucleoplasm but exists in the insoluble nuclear fraction rather than in the soluble nuclear fraction. In support of this hypothesis, it is known that in addition to colocalization with PML nuclear bodies, nuclear proteasomes are present throughout the nucleoplasm through association with the nuclear matrix.43 Moreover, PDLIM2 is found to preferentially interact with cell skeletal structures, including the nuclear matrix.25,28 To confirm the hypothesis, we isolated the nuclear matrix from other cellular compartments including chromatins and quantitated Tax amounts. As shown in Figure 3D, PDLIM2 could result in up to 100% of Tax in the nuclear matrix depending on the amounts of coexpressed PDLIM2. Further, proteasome inhibition could lead to Tax recovery only in the nuclear matrix but not in other fractions, which was much more significant when PDLIM2 was coexpressed with Tax (Figure 3E). Of note, PDLIM2 also decreased Tax amounts in the nuclear matrix in a dose-dependent manner, similar to Tax proteins in other cellular fractions (Figure 3A). Together, these studies suggested that PDLIM2 targets Tax from the cytoplasm, nucleoplasm, and Tax nuclear bodies into the nuclear matrix for ubiquitination-mediated proteasomal degradation.
PDLIM2 suppresses Tax-mediated signaling activation
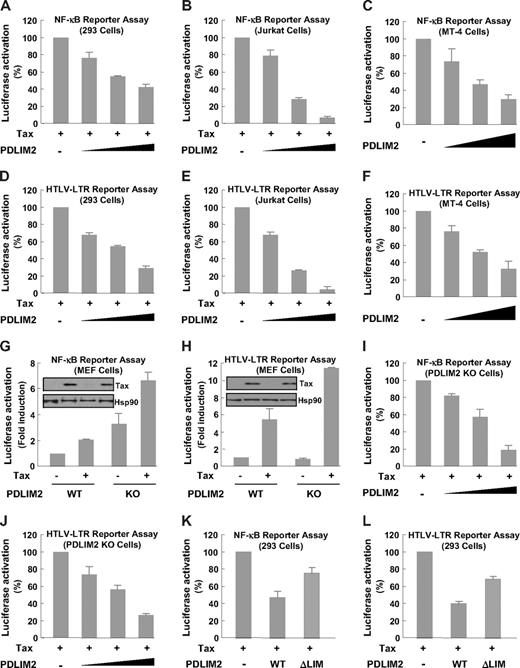
To investigate the significance of the PDLIM2 and Tax interaction, we examined the effect of PDLIM2 on Tax-mediated activation of the HTLV-I long-terminal repeat (LTR) and NF-κB transcription factor in luciferase gene report assays. Whereas HTLV-I-LTR transactivation by Tax is required for viral gene expression and virus replication,44 Tax-induced NF-κB activation is largely responsible for HTLV-I–mediated tumorigenesis.45 As shown in Figure 4A-F, PDLIM2 coexpression resulted in a dose-dependent suppression on the Tax-dependent NF-κB activation and HTLV-I transcription in 293, Jurkat, and HTLV-I–transformed T cells. Conversely, PDLIM2 knockout significantly augmented Tax's abilities in NF-κB and HLTV-I-LTR activation (Figure 4G,H). In addition, re-expression of PDLIM2 in these knockout cells suppressed the Tax functions in a dose-dependent manner (Figure 4I,J). Consistent with its inability to promote Tax ubiquitination and degradation, the LIM deletion mutant largely lost its ability to suppress Tax-mediated signaling (Figure 4K,L). These results agreed with the roles of PDLIM2 in Tax subcellular redistribution and degradation.
PDLIM2 prevents Tax-dependent NF-κB activation and HTLV-I viral transcription. (A-C) The indicated cells were transfected with Tax and κb driven luciferase reporter in the presence of increasing amounts of PDLIM2, followed by measure of luciferase activity. (D-F) The indicated cells were transfected with Tax and HTLV-I-LTR driven luciferase reporter in the presence of increasing amounts of PDLIM2, followed by measure of luciferase activity. (G,H) PDLIM2 wild-type (WT) or knockout (KO) MEFs were transfected with Tax and κb or HTLV-I-LTR driven luciferase reporter, followed by measure of luciferase activity. The protein expression levels of transfected Tax and endogenous Hsp90 were detected by direct IB. (I,J) PDLIM2 WT or KO MEFs were transfected with Tax and κb or HTLV-I-LTR driven luciferase reporter in the presence of increasing amounts of PDLIM2, followed by measure of luciferase activity. (K-L) 293 cells were transfected with Tax and κb or HTLV-I-LTR driven luciferase reporter in the presence of same amounts of PDLIM2 or its LIM deletion mutant, followed by measure of luciferase activity. The luciferase activities were presented as the percentile of that activated by Tax alone (denoted as 100) (A-F,I,J,K,L) or as fold induction relative to the basal level measured in WT MEFS (G,H). Error bars indicate standard deviations (n = 3).
PDLIM2 prevents Tax-dependent NF-κB activation and HTLV-I viral transcription. (A-C) The indicated cells were transfected with Tax and κb driven luciferase reporter in the presence of increasing amounts of PDLIM2, followed by measure of luciferase activity. (D-F) The indicated cells were transfected with Tax and HTLV-I-LTR driven luciferase reporter in the presence of increasing amounts of PDLIM2, followed by measure of luciferase activity. (G,H) PDLIM2 wild-type (WT) or knockout (KO) MEFs were transfected with Tax and κb or HTLV-I-LTR driven luciferase reporter, followed by measure of luciferase activity. The protein expression levels of transfected Tax and endogenous Hsp90 were detected by direct IB. (I,J) PDLIM2 WT or KO MEFs were transfected with Tax and κb or HTLV-I-LTR driven luciferase reporter in the presence of increasing amounts of PDLIM2, followed by measure of luciferase activity. (K-L) 293 cells were transfected with Tax and κb or HTLV-I-LTR driven luciferase reporter in the presence of same amounts of PDLIM2 or its LIM deletion mutant, followed by measure of luciferase activity. The luciferase activities were presented as the percentile of that activated by Tax alone (denoted as 100) (A-F,I,J,K,L) or as fold induction relative to the basal level measured in WT MEFS (G,H). Error bars indicate standard deviations (n = 3).
PDLIM2 prevents tumorigenicity of HTLV-I/Tax
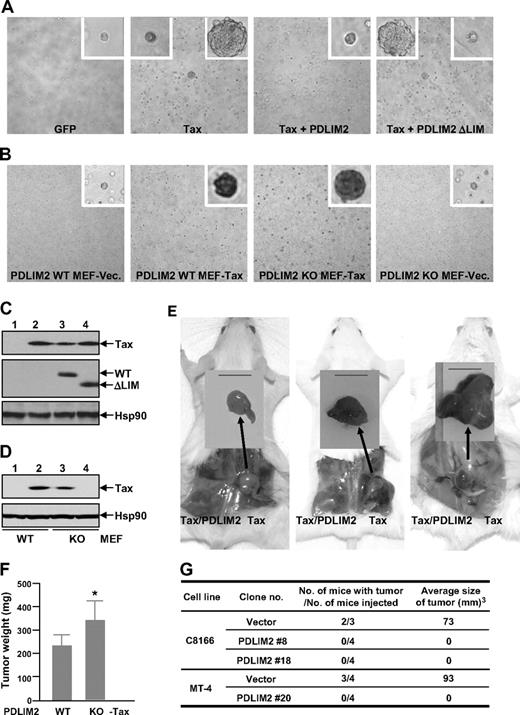
We next utilized 2 widely used model systems in the field to examine the effect of PDLIM2 on HTLV-I/Tax-mediated oncogenesis: rat-1 fibroblasts and HTLV-I–transformed T-cell lines. We also included PDLIM2 knockout and wild-type MEFs for these assays. When transduced with Tax, both Rat-1 and MEF cells acquired the ability to form foci in soft agar (Figure 5A,B second subpanel). In agreement with its negative role in Tax regulation, PDLIM2 coexpression sufficiently inhibited the anchorage-independent growth of the Tax-expressing Rat-1 cells (Figure 5A third subpanel), whereas its deficiency significantly increased the ability of the Tax-expressing MEFs (Figure 5B third subpanel). Of note, the LIM deletion mutant, which loses the ability to promote Tax ubiquitination and degradation, failed to block the in vitro transforming ability (Figure 5A fourth subpanel). These results suggested that PDLIM2 suppresses the transforming ability of Tax in vitro.
PDLIM2 suppresses tumorigenicity of HTLV-I/Tax both in vitro and in vivo. (A) Rat-1 cells stably expressing GFP, Tax, Tax, and PDLIM2, or Tax and PDLIM2 LIM deletion mutant (ΔLIM) were plated in soft agar for colony formation. Pictures shown here were taken at day 21 after plating. Original magnification: ×100. (B) PDLIM2 wild-type (WT) and knockout (KO) MEFs stably expressing Tax or an empty vector (Vec) were plated in soft agar for colony formation. Pictures shown here were taken at day 21 after plating. Original magnification: ×40. (C) Expression levels of Tax, exogenous PDLIM2 (WT), and LIM deletion mutant (ΔLIM) in Rat-1 stable cells used in panels A and E were examined by IB using Tax and Myc antibodies, respectively. The expression levels of Hsp90 were used as a loading control. (D) Expression levels of Tax in PDLIM2 wild-type or knockout stable cells used in panels B and F were examined by IB using Tax antibody. The expression levels of Hsp90 were used as a loading control. (E) Rat-1 cells stably expressing Tax or Tax/PDLIM2 were subcutaneously inoculated into the right and left hind-back of the same SCID mouse, respectively. Pictures (from left to right) here were taken at days 14, 21, and 28 after inoculation, respectively. Bar represents 1 cm. (F) PDLIM2 WT and KO MEFs stably expressing Tax were subcutaneously inoculated into the right and left hind-back of the same SCID mouse, respectively. The mice were killed at day 21 after inoculation and tumor weights were measured. The data presented are the mean plus or minus standard deviation (n = 15), and P value is .027 (Student t test). (G) The indicated HTLV-I–transformed T-cell lines stably expressing PDLIM2 or an empty vector were subcutaneously inoculated in the postauricular region of SCID mice. The mice were killed at day 21 of postinoculation for tumor evaluation.
PDLIM2 suppresses tumorigenicity of HTLV-I/Tax both in vitro and in vivo. (A) Rat-1 cells stably expressing GFP, Tax, Tax, and PDLIM2, or Tax and PDLIM2 LIM deletion mutant (ΔLIM) were plated in soft agar for colony formation. Pictures shown here were taken at day 21 after plating. Original magnification: ×100. (B) PDLIM2 wild-type (WT) and knockout (KO) MEFs stably expressing Tax or an empty vector (Vec) were plated in soft agar for colony formation. Pictures shown here were taken at day 21 after plating. Original magnification: ×40. (C) Expression levels of Tax, exogenous PDLIM2 (WT), and LIM deletion mutant (ΔLIM) in Rat-1 stable cells used in panels A and E were examined by IB using Tax and Myc antibodies, respectively. The expression levels of Hsp90 were used as a loading control. (D) Expression levels of Tax in PDLIM2 wild-type or knockout stable cells used in panels B and F were examined by IB using Tax antibody. The expression levels of Hsp90 were used as a loading control. (E) Rat-1 cells stably expressing Tax or Tax/PDLIM2 were subcutaneously inoculated into the right and left hind-back of the same SCID mouse, respectively. Pictures (from left to right) here were taken at days 14, 21, and 28 after inoculation, respectively. Bar represents 1 cm. (F) PDLIM2 WT and KO MEFs stably expressing Tax were subcutaneously inoculated into the right and left hind-back of the same SCID mouse, respectively. The mice were killed at day 21 after inoculation and tumor weights were measured. The data presented are the mean plus or minus standard deviation (n = 15), and P value is .027 (Student t test). (G) The indicated HTLV-I–transformed T-cell lines stably expressing PDLIM2 or an empty vector were subcutaneously inoculated in the postauricular region of SCID mice. The mice were killed at day 21 of postinoculation for tumor evaluation.
To further confirm these results in vivo, we subcutaneously injected the Rat-1 and MEF cells into SCID mice. Consistent with Tax's ability to promote tumorigenesis, these cells transduced with Tax, but not with the control vector or PDLIM2, developed tumors within the injected mice (Figure 5E,F, and data not shown). However, Tax-driven tumorigenesis was completely blocked by PDLIM2 coexpression (Figure 5E). On the other hand, PDLIM2 knockout significantly benefited Tax tumorigenicity in MEFs (Figure 5F). Although HTLV-I–transformed cell lines C8166 and MT-4 both formed tumors in injected mice, the tumors caused by inoculation of MT-4 were much larger (Figure 5G), which was inversely correlated with the expression levels of PDLIM2 in these cells (Figure 6). More importantly, PDLIM2 coexpression also sufficiently blocked the tumorigenicity of both cell lines (Figure 5G). These results indicated that PDLIM2 suppresses HTLV-I tumorigenicity by targeting Tax.
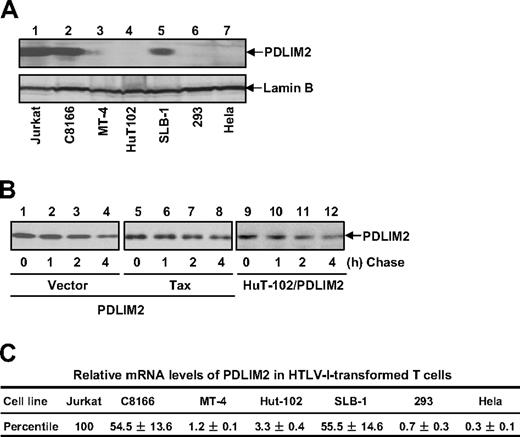
Both mRNA and protein levels of PDLIM2 were decreased in HTLV-I–transformed T cells. (A) Protein expression of PDLIM2 was analyzed in the indicated cell lines by IB. (B) CHX-chase assays were performed using 293 cells transfected with PDLIM2, PDLIM2/Tax, and HuT-102 cells stably expressing PDLIM2, followed by IB to detect PDLIM2 protein levels. (C) RNA expression of PDLIM2 was analyzed in the indicated cell lines by real-time PCR. The relative levels of PDLIM2 mRNA were normalized according to β-actin mRNA level and represented as percentile in Jurkat cells (arbitrarily set as 100). The data presented are the mean plus or minus standard deviation (n = 3).
Both mRNA and protein levels of PDLIM2 were decreased in HTLV-I–transformed T cells. (A) Protein expression of PDLIM2 was analyzed in the indicated cell lines by IB. (B) CHX-chase assays were performed using 293 cells transfected with PDLIM2, PDLIM2/Tax, and HuT-102 cells stably expressing PDLIM2, followed by IB to detect PDLIM2 protein levels. (C) RNA expression of PDLIM2 was analyzed in the indicated cell lines by real-time PCR. The relative levels of PDLIM2 mRNA were normalized according to β-actin mRNA level and represented as percentile in Jurkat cells (arbitrarily set as 100). The data presented are the mean plus or minus standard deviation (n = 3).
PDLIM2 expression is down-regulated in HTLV-I–transformed T cells
To investigate the mechanism by which Tax escapes from PLDLIM2-mediated suppression for cell transformation and ultimate oncogenesis in approximately 5% of HTLV-I carriers, we examined the expression levels of PDLIM2 in HTLV-I–transformed T cells. In agreement with previous studies showing a high expression of PDLIM2 in various hematopoietic cells under physiological conditions,25,28 we found relatively abundant PDLIM2 proteins in Jurkat T cells, an HTLV-I–negative T-cell line (Figure 6A). Remarkably, PDLIM2 proteins were significantly decreased in all the HTLV-I–transformed T-cell lines we examined as well as in 293 and Hela cells, although to different extents. Of note, the different expression levels of PDLIM2 were highly consistent, in an opposite way, with the different dynamics of Tax turnover in the cells (Figure 1). The different PDLIM2 expression also inversely correlated with the different tumorigenicity of HTLV-I/Tax-expressing cells (Figure 5).
To define the mechanism by which PDLIM2 is down-regulated, we performed the CHX-chase assays to check whether it occurs at the protein level. Although PDLIM2 significantly decreased Tax stability (Figure 2), the protein stability of PDLIM2 was not affected by Tax or HTLV-I (Figure 6B), excluding the possibility. We then examined the mRNA levels of PDLIM2 in HTLV-I–transformed T cells using real-time PCR. We found that compared with Jurkat cells, all HTLV-I–transformed T-cell lines expressed much lower levels of the PDLIM2 mRNA, though to different extents (Figure 6C). These data were highly consistent with the protein expression levels of PDLIM2 in those cells. It thus seemed that PDLIM2 down-regulation occurred at the mRNA level. Given the sufficient role of PDLIM2 expression in prevention/reverse of HTLV-I/Tax-mediated oncogenesis, these results suggested that down-regulation of PDLIM2 transcription might be one important mechanism of HTLV-I/Tax-mediated tumorigenesis.
Discussion
During last 25 years, extensive studies have been performed on how Tax usurps cellular regulatory mechanisms to facilitate HTLV-I viral replication and to initiate malignant transformation leading to the development of ATL. However, how the Tax protein is regulated by cellular mechanisms has been rarely studied. Due to lack of this knowledge, the prognosis for this acute and fatal disease is still extremely poor.46 Here we have shown that Tax is negatively regulated by PDLIM2. Importantly, PDLIM2 expression is able to prevent HTLV-I/Tax-mediated cell transformation and oncogenesis. These studies therefore suggest a potential therapeutic application for ATL.
PDLIM2 is a newly identified ubiquitin E3 ligase that could specifically polyubiquitinate the nuclear p65 and STAT (signal transducers and activators of transcription) proteins for proteasomal degradation.25,28 Our data have shown that PDLIM2 could promote polyubiquitination of both cytoplasmic and nuclear Tax proteins, leading to their proteasomal degradation (Figures 2,3). Interestingly, Tax proteins were recruited into the nuclear matrix for the degradation (Figure 3). Our data therefore suggest that the nuclear matrix might serve as an additional nuclear proteolytic site distinct from PML nuclear bodies, for both cytoplasmic and nuclear proteins.
Targeting Tax into the nuclear matrix is important not only for the proteasomal degradation but also for prompt turnoff of Tax-mediated signaling. Like any other protein, Tax requires proper subcellular localization and environment for its function. For instance, Tax perinuclear localization is essential for IKK activation and subsequent NF-κB nuclear translocation,38,39 whereas its colocalization with interchromatin speckles to form so-called “Tax nuclear bodies” is important for full NF-κB transcription activation.17,18 Thus, PDLIM2-mediated redistribution of Tax from the cytoplasm and interchromatin granules into the nuclear matrix provides a rapid and efficient, but possibly reversible, means of terminating Tax-mediated signaling (Figures 3,4). On the other hand, PDLIM2-promoted proteasomal degradation of Tax leads to the final and irreversible shutoff of Tax signaling.
Interestingly, our studies indicate that PDLIM2 was negatively regulated in HTLV-I–transformed T cells. However, neither overall subcellular distribution of PDLIM2 protein nor its stability was altered by Tax or HTLV-I (Figures 3 and 6). Rather, PDLIM2 expression was down-regulated at the mRNA level (Figure 6). Although it is not clear how the PDLIM2 transcription is down-regulated in HTLV-I–transformed cells, it seems clear that down-regulation of PDLIM2 is one important mechanism in HTLV-I/Tax-mediated cell transformation and tumorigenesis, since PDLIM2 reconstitution in HTLV-I–transformed T cells was able to reverse their tumor formation ability in animals (Figure 5). Furthermore, PDLIM2 coexpression is sufficient to prevent in vitro cell transformation and in vivo oncogenesis induced by Tax (Figure 5). On the other hand, complete knockout of PDLIM2 further enhances Tax-mediated cell transformation and oncogenesis (Figure 5).
Obviously Tax sequestration and degradation at the nuclear matrix plays a predominant and direct role in PDLIM2-mediated suppression of HTLV-I/Tax oncogenicity. Other activities of PDLIM2, such as promotion of p65 degradation, might also be involved. As we already know, p65 has been suggested to be involved in HTLV-I/Tax-mediated oncogenesis. However, p65 degradation should only play a minor role in PDLIM2 suppression of HTLV-I/Tax oncogenicity, since nuclear p65 induced by Tax was largely insensitive to PDLIM2-promoted degradation due to the occupation of PDLIM2 by Tax (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). Nevertheless, the nuclear p65 is not sufficient to reverse the tumor suppression function of PDLIM2.
In addition to HTLV-I–transformed T cells, PDLIM2 was down-regulated in many other kinds of human cancers including cervical and breast cancer cells (Figure 6, and data not shown), suggesting PDLIM2 down-regulation as a general mechanism in cancer progression and as a new potential cancer biomarker. Currently, the mechanism by which PDLIM2 gene is suppressed in cancer cells is unknown. One clue is the genomic location of PDLIM2, 8p21, which frequently undergoes allelic loss in ovarian and prostate cancers.47,48 Another possibility is epigenetic suppression of PDLIM2, as PDLIM4, a family member of PDLIM2 that shows tumor suppression function, is repressed by DNA methylation in many cancers.49
In conclusion, we have shown that HTLV-I and PDLIM2 are counterbalanced. Whereas HTLV-I suppresses PDLIM2 expression at the transcription level, PDLIM2 targets Tax proteins into the nuclear matrix for degradation. We have also shown that the counterbalance between Tax and PDLIM2 will determine the outcome of HTLV-I infection. These studies therefore suggest a novel therapeutic strategy for cancer and other diseases associated with HTLV-I infection and/or PDLIM2 deregulation. These studies also provide important insights into the leukemogenesis, long latency, and cancer health disparities of HTLV-I as well as a potentially general tumor suppression role of PDLIM2.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank N. Raab-Traub for Rat-1 cells, Z. Chen for ubiquitin constructs, and National Institutes of Health (NIH) AIDS Research & Reference Reagent Program (Germantown, MD) for various reagents.
This study was supported in part by NIH/National Cancer Institute (NCI; Bethesda, MD) grant R01 CA116616 and UPCI MVP research award (G.X.).
National Institutes of Health
Authorship
Contribution: P.Y., J.F., Z.Q., and S.L. performed research and analyzed data; T.T. and M.J.G. contributed vital new reagents; and G.X. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gutian Xiao, Hillman Cancer Center Research Pavilion, 5117 Centre Ave, Pittsburgh, PA 15213; e-mail: xiaog2@upmc.edu.
References
Author notes
*P.Y. and J.F. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal