Abstract
HIV infection remains a worldwide threat. HIV-1 transactivator protein Tat is one of the retroviral proteins identified as a key immunomodulator in AIDS pathogenesis. Although the primary function of Tat is to regulate HIV-1 replication in the infected cell, it also dysregulates cytokine production resulting in perturbation of the host immune response and enhancement of the retrovirus survival. Because interferon-γ (IFNγ) is a pleiotropic cytokine with potent antiviral and immunoregulatory effects, we investigated whether Tat interferes with the IFNγ signal transduction in primary monocytes. We demonstrated that Tat impaired the IFNγ-receptor signaling pathway at the level of STAT1 activation, possibly via Tat-dependent induction of suppressor of cytokine signaling-2 (SOCS-2) activity. We delineated the inhibitory role of SOCS-2 in IFNγ signaling pathway by overexpression of exogenous SOCS-2 in HEK293 cell. The results showed that SOCS-2 suppressed the IFNγ-activated STAT1 phosphorylation and consequent IFNγ-regulated transcription of specific genes. To confirm the role of SOCS2 in the Tat-induced process, we demonstrated that SOCS-2 siRNA in human blood monocytes abrogated the Tat-dependent inhibition of IFNγ signaling. Our data suggested a possible mechanism implicating the role of SOCS-2 in mediating HIV-1–induced immune evasion and dysregulation of IFNγ signaling in primary human monocytes.
Introduction
AIDS is characterized by a profound reduction of CD4+ T lymphocytes, immune defects, and cytokine dysregulation, leading to opportunistic infections and tumorgenesis.1 HIV-1 has been identified to be the primary etiologic agent. Among HIV-1 gene products, transactivator protein (Tat) is required for efficient viral gene expression by interacting with the HIV long terminal repeat to enhance transcription and RNA processing.2 Apart from its effects on viral replication, Tat is secreted extracellularly by the infected cells and exerts its paracrine effects on neighboring cells.3
It has been shown that extracellular Tat may play a diverse role in dysregulating the host immune response via the modulation of cellular gene expression.4 For example, previous reports including ours showed Tat induces the overexpression of interleukin-10 (IL-10), which is a well known immunosuppressive cytokine capable of down-regulating TH1 cell function and IL-2 synthesis.5 We recently demonstrated Tat induces IL-10 expression via cellular kinase PKR activity and the activation of transcription factor Ets-1.6,7 PKR, one of the key genes in the mediation of interferon-induced activities, is a prototype kinase modulated by Tat in HIV-1 perturbation of the cytokine systems.8
Infection with HIV-1 marks the onset of changes in the microenvironment of the host cell. Throughout all stages of HIV-1 infection, chronic immune activation and dysfunctional cytokine production have been observed.9 Soluble mediators, including cytokines and viral products such as Tat, produced by the infected cells may enhance the progression of HIV-1 infection either by direct effects or through deregulation of cytokine expression such as interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα).7,10 Therefore, elucidating the transcriptional regulation of host genes including cytokines and their regulated pathways upon HIV-1 viral protein stimulation can be used as a tool to delineate the mechanisms of host-virus interactions, and to understand the molecular basis of AIDS pathogenesis.
Interferon-γ is a pleiotropic cytokine produced primarily by T lymphocytes and natural killer (NK) cells in response to viral infection. It is an important mediator with multiple biological activities including macrophage activation, and antimicrobial, antiproliferative, and immunomodulatory effects.11 It has been shown that IFNγ receptor–deficient mice are significantly impaired in their abilities to resist infection by microbes including virus, bacteria, and protozoa.12 Central to IFNγ-induced cellular responses is the activation of Janus kinase-1 (Jak1) and Jak2 and consequent phosphorylation of STAT1. The phosphorylated STAT1 (pSTAT1) undergoes dimerization and translocation into the nucleus, where it binds to IFNγ-activated sequence (GAS) elements present in the promoter of IFNγ-regulated genes, and consequently leads to initiation of transcription.13
Previous studies of IFN-induced signaling cascade suggested overexpression of suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 inhibits antiproliferative and antiviral activities of IFNα/β and IFNγ.14,15 The SOCS family consists of 8 members (SOCS-1 to -7 and CIS). The structure of these proteins includes a central SH2 domain and a conserved SOCS box. The inducible SOCS proteins are key negative feedback regulators of the Jak/STAT signaling cascade16 that suppress inflammation.
To understand the mechanisms underlying HIV-1 dysregulation of the IFNγ signaling system, we examined whether HIV-1 Tat acts through the induction of SOCS family of proteins to suppress IFNγ activation of Jak/STAT pathways. Our results demonstrated the IFNγ signaling cascade was inhibited proximally at the level of STAT1 phosphorylation by Tat. This Tat-induced inhibition of IFNγ signaling can be abrogated by the expression of SOCS2 siRNA. Taken together, our data showed HIV-1 Tat induces SOCS2 expression, which results in the suppression of IFNγ-mediated antimicrobial response.
Methods
Reagents and plasmids
Recombinant HIV-1 Tat protein was obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program (Germantown, MD).17 Endotoxin levels of Tat protein were measured by Pyrotell assay kit (Associates of Cape Cod, East Falmouth, MA). The biological activities of the Tat protein were confirmed by HIV-LTR luciferase activities (data not shown). Recombinant human IFNγ was purchased from R&D Systems (Minneapolis, MN). The sources of expression vectors used in this study are listed in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Pseudotyped HIV-1 encoding a luciferase reporter was produced by cotransfection of the pNL.Luc.E-R- and pADA-Env plasmids according to the manufacturer's instructions (SuperFect; QIAGEN, Valencia, CA). The supernatants containing the HIV-pseudovirus were collected for further infection of primary blood macrophages.
CD14+ primary blood monocytes isolation and cell culturing
Primary blood monocytes (PBMos) with CD14 surface marker expression were isolated from the buffy coat of heparinized blood samples donated by healthy volunteers (source: Hong Kong Red Cross Blood Transfusion Service), using Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) density gradient centrifugation.7,18 CD14+ PBMos were purified from these cells by magnetic-activated cell sorting (MACS) system using anti-CD14 magnetic beads (Miltenyi Biotec, Auburn, CA). Cell viability was more than 99% as measured by trypan blue exclusion assay and the purity of monocytes was 90% to 95% as verified by fluorescence-activated cell sorting (FACS) using PE-conjugated anti-CD14 antibody (Beckman Coulter, Miami, FL). In addition, the cell viability was examined using immunofluorescence imaging (Thermo Scientific Cellomics ArrayScan VTI Live; Pittsburgh, PA) as described in Document S1. Purified CD14+ PBMos were resuspended in RPMI containing 5% autologous plasma before culture. For cell-line culturing, HEK293 cells were routinely grown at 37°C in 5% CO2/95% air in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin.
Extraction of RNA, RT-PCR, and quantitative real-time RT-PCR
Total cellular RNA extraction and the cDNA synthesis were as described in our previous reports.18,19 The cDNA produced was then subjected to quantitative reverse transcription–polymerase chain reaction (QRT-PCR) assays.19 TaqMan probes including SOCS2 (Hs00374416_m1) and 18S rRNA (reference gene) were purchased from Applied Biosystems (Foster City, CA). Results of QRT-PCR were analyzed with reference to the comparative Cτ (cycle number to threshold) according to the manufacturer's instructions. All samples were run in triplicates along with no template controls in ABI Prism 7700 Sequence Detector (Applied Biosystems, Foster City, CA) and LightCycler 480II Real-Time PCR System (Roche Diagnostics, Mannheim, Germany). The primer sequences for the conventional PCR used in this study are summarized in Table 1.
Primer sequences used in this study
| Gene name . | Primer sequences . |
|---|---|
| SOCS-1 | Forward: 5′-GAGCTGCTGGAGCACTACG-3′ |
| Reverse: 5′-AGGGGAAGGAGCTCAGGTAG-3′ | |
| SOCS-2 | Forward: 5′-CTCGGTCAGACAGGTTGGTA-3′ |
| Reverse: 5′-ACAGAGATGCTGCAGAGATG-3′ | |
| SOCS-3 | Forward: 5′-GCCACCTACTGAACCCTCCT-3′ |
| Reverse: 5′-ACGGTCTTCCGACAGAGATG-3′ | |
| OAS p40/46 | Forward: 5′-GAAGCCTGTCAAAGAGAGAG-3′ |
| Reverse: 5′-TGTGTTTTCATGCTCCCTCG-3′ | |
| OAS p69/71 | Forward: 5′-CAATCAGCGAGGCCAGTAATC-3′ |
| Reverse: 5′-CTTGACGATTTTGTGCCGCT-3′ | |
| HLA-DRA | Forward: 5′-AAGAAGGAGACGGTCTGG-3′ |
| Reverse: 5′-TGGTCCCAATAATGATGC-3′ | |
| HLA-DQA1 | Forward: 5′-AGGTTCCTGAGGTCACAGTG-3′ |
| Reverse: 5′-ACCTTGACAGACAAGAAAGCATC-3′ | |
| GAPDH | Forward: 5′-ACCACAGTCCATGCCATCAC-3′ |
| Reverse: 5′-TCCACCACCCTGTTGCTGTA-3′ |
| Gene name . | Primer sequences . |
|---|---|
| SOCS-1 | Forward: 5′-GAGCTGCTGGAGCACTACG-3′ |
| Reverse: 5′-AGGGGAAGGAGCTCAGGTAG-3′ | |
| SOCS-2 | Forward: 5′-CTCGGTCAGACAGGTTGGTA-3′ |
| Reverse: 5′-ACAGAGATGCTGCAGAGATG-3′ | |
| SOCS-3 | Forward: 5′-GCCACCTACTGAACCCTCCT-3′ |
| Reverse: 5′-ACGGTCTTCCGACAGAGATG-3′ | |
| OAS p40/46 | Forward: 5′-GAAGCCTGTCAAAGAGAGAG-3′ |
| Reverse: 5′-TGTGTTTTCATGCTCCCTCG-3′ | |
| OAS p69/71 | Forward: 5′-CAATCAGCGAGGCCAGTAATC-3′ |
| Reverse: 5′-CTTGACGATTTTGTGCCGCT-3′ | |
| HLA-DRA | Forward: 5′-AAGAAGGAGACGGTCTGG-3′ |
| Reverse: 5′-TGGTCCCAATAATGATGC-3′ | |
| HLA-DQA1 | Forward: 5′-AGGTTCCTGAGGTCACAGTG-3′ |
| Reverse: 5′-ACCTTGACAGACAAGAAAGCATC-3′ | |
| GAPDH | Forward: 5′-ACCACAGTCCATGCCATCAC-3′ |
| Reverse: 5′-TCCACCACCCTGTTGCTGTA-3′ |
Enzyme-linked immunosorbent assay
Culture supernatants of the treated PBMos were collected and stored at −70°C until use. The levels of IL-10 and TNFα in the culture supernatants were measured in triplicates by enzyme-linked immunosorbent assay (ELISA) using the respective assay kits (R&D Systems).
TCA-DOC/acetone protein precipitation
TCA-DOC/acetone method was used to reduce the volume and to concentrate the proteins in the cell lysates. Briefly, for 1 volume of protein lysates, 2% sodium deoxycholate (Na-DOC; Sigma-Aldrich, St Louis, MO) was added to a final concentration of 0.02%. After mixing, samples were incubated at room temperature for 15 minutes and followed by adding 100% trichloroacetic acid (TCA; Sigma-Aldrich) to a final concentration of 10%. After centrifugation at 16 110g at 4°C for 10 minutes, the supernatant was removed completely. The protein pellet was mixed thoroughly with 200 μL ice-cold acetone and kept on ice for 15 minutes. After another centrifugation at 16 110g speed at 4°C for 10 minutes, the protein pellets were air-dried to eliminate the residual acetone. Finally, the concentrated protein pellets were resuspended in a minimal volume of the sample buffer, and then titrated with 1 M Tris-Cl (pH 8.5) to neutralize the acidic medium.
Western blot analysis
Before the protein extraction, PBMos were washed with 1 mM sodium orthovanadate in PBS. For the cytoplasmic and nuclear protein isolation, the cells were incubated with buffer A for 15 minutes followed by buffer C for another 5 minutes (please refer to Document S1).7,18 Western analysis assays were preformed as in our previous reports and the source of antibodies was listed in Document S1.7
Cloning, expression, and purification of recombinant SOCS-2
The full-length human SOCS-2 cDNA was amplified by PCR from THP-1 monocytic cell and cloned into BamHI and EcoRI sites of pET28a(+) expression vector (Novagen, Darmstadt, Germany) containing a His6-tag at N terminus. The details of the procedure for the production of recombinant SOCS-2 protein was described in Document S1.
Transcription reporter assay
For the luciferase reporter assay, HEK293 cells (0.2 × 106) in a 24-well cell-culture plate were transiently transfected with expression plasmids (pGL3-8xGAS, pcDNA-SOCS-2, and pRL-TK) for 24 hours using Lipofectamine 2000 Reagent (Invitrogen, Frederick, MD) according to the manufacturer's instructions. Total amount of DNA for transfection was normalized with the parental pcDNA3.0 vector (Invitrogen). Luciferase activities in the transfectants were determined by Dual-Luciferase reporter assay system (Promega, Madison, WI) using FusionTM-αFP microplate reader (Packard, Downers Grove, IL).
SOCS-2 siRNA study
SOCS-2 knockdown in CD14+ PBMos was accomplished using SOCS-2–specific siRNA (Stealth; Invitrogen; 5′-GCACCAGAAGGAACUUUCUUGAUUA-3′; 5′-UAAUCAAGAAAGUUCCUUCUGGUGC-3′) and the Stealth RNAi Negative Control Duplexes (Low GC Duplex; Invitrogen). These sequences contain no cross-homology to other sequences in the GenBank database. Transfection of the primary cells was conducted using jetPEI reagent (polyethyleneimine; PolyPlus). CD14+ PBMos (106 cells/mL in 1 mL) were transfected with 100 μL jetPEI-siRNA complex (2 μL: 100 nM) for 24 hours and then treated with 10 nM Tat for 5 hours. For the protein harvest, 100 U/mL IFNγ was added for 10 minutes after 5 hours of Tat pretreatment.
HIV-1 viral load assay
HIV-1 RNA copy number in the viral stock was determined by a quantitative SYBR Green real-time PCR assay. Briefly, 50 μL viral particles was lysed with equal amount of lysis buffer (PBS containing 2% Triton-100). Released viral RNA was subsequently subjected to cDNA synthesis with random hexamers using Superscript III RNA polymerase (Invitrogen). Each 25-μL reaction mixture contained 12.5 μL 2× SYBR Green PCR mix (SuperArray Bioscience, Frederick, MD), 1 μL of each 20 μM primer, and 5 μL cDNA products. The reactions were run in an ABI 7500 Fast system (Applied Biosystems) with 1 cycle at 94°C (10 minutes) and then 45 cycles at 94°C (15 seconds) followed by 60°C (1 minute). Absolute viral RNA copy number was calculated against a standard curve. The real-time PCR primers include 5-CCCTCAGATGCTGCATATAAGC (forward); and 5′-GCACTCAAGGCAAGCTTTATTG (reverse), targeting the HIV LTR region. Each sample was tested in triplicates.
Statistical analysis
The statistical analyses were done by comparison between the treatment and control groups using the paired Student t test. Data were presented as mean values plus or minus standard error of the mean (SEM) of at least 3 independent experiments from different donors. A P value of less than .05 was considered statistically significant.
Results
Tat impairs IFNγ-induced STAT1 phosphorylation in CD14+ blood monocytes
To enhance their own survival, several viruses known to encode viral proteins can inhibit interferon signaling pathways.11 To examine whether HIV-1 Tat protein interferes with IFNγ signal transduction, we evaluated the STAT1 phosphorylation level of IFNγ-stimulated human CD14+ peripheral blood monocytes (PBMos) in the presence of Tat. PBMos (0.5 × 106 cells) isolated from healthy blood donors were pretreated with indicated concentrations of Tat for 4 hours and followed by treatment with IFNγ (100 U/mL) for 10 minutes (Figure 1). Western blotting of total cell lysates indicated Tat inhibited early signal transduction of IFNγ by impairing STAT1 tyrosine (Tyr701) phosphorylation in a dose-dependent manner (Figure 1A). In addition, pretreatment of Tat abrogated the time-dependent IFNγ activation of STAT1, as shown with a decrease in the level of phosphorylated STAT1 (pSTAT1; Figure 1B). However, the presence of Tat did not affect the phosphorylation levels of serine residue (Ser727) of STAT1 (Figure S1A).
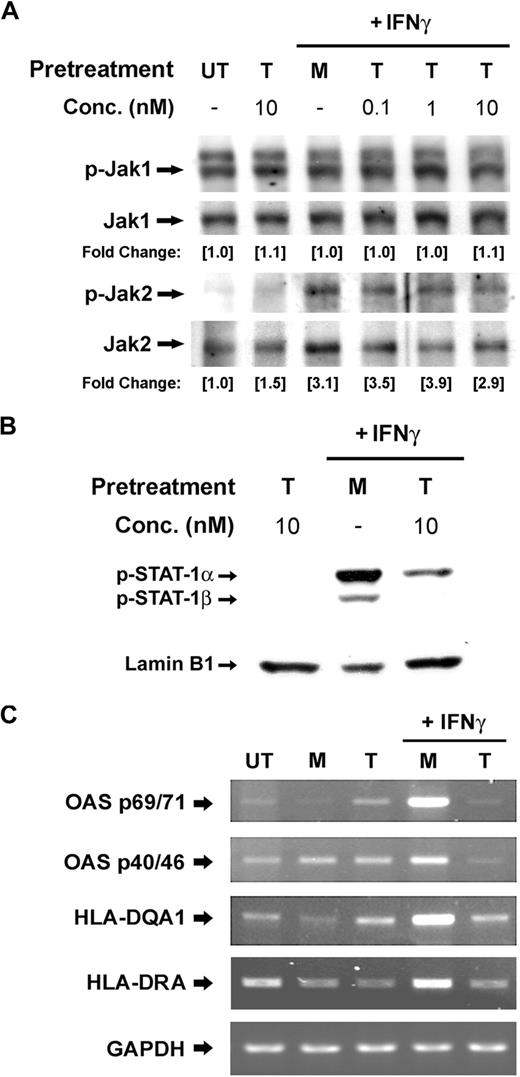
Tat impairs IFNγ-induced phosphorylation of STAT1. (A) PBMos were untreated (UT), mock treated (M), or treated with indicated concentrations of Tat (T) for 4 hours and followed by the treatment of IFNγ (100 U/mL) for 10 minutes. The tyrosine phosphorylation of STAT1 was probed with specific antibodies. Total STAT1 was probed as loading control. The levels of phosphorylation were normalized with total STAT1, whereas the fold changes of individual treatment with reference to the untreated were indicated. Fold change refers to measurement of the indicated band by densitometry. Vertical line has been inserted to indicate a repositioned gel lane. (B) PBMos were untreated (UT), treated with mock (M), or treated with 10 nM Tat (T) for 4 hours and followed by the treatment of IFNγ (100 U/mL) at indicated time intervals (minutes) as shown on top of each panel. Cell lysates were examined by Western blotting using anti-pSTAT1 or anti-STAT1 antibodies as indicated. (C,D) To evaluate the effects of Tat on the expression levels of IFNγ receptors, PBMos were treated with mock or Tat (10 nM) at indicated time intervals. Total RNA was extracted and examined for the mRNA expression levels of (C) IFNγR1 and (D) IFNγR2 by QRT-PCR. All data presented were plotted as mean values ± SEM of at least 3 independent experiments in duplicates.
Tat impairs IFNγ-induced phosphorylation of STAT1. (A) PBMos were untreated (UT), mock treated (M), or treated with indicated concentrations of Tat (T) for 4 hours and followed by the treatment of IFNγ (100 U/mL) for 10 minutes. The tyrosine phosphorylation of STAT1 was probed with specific antibodies. Total STAT1 was probed as loading control. The levels of phosphorylation were normalized with total STAT1, whereas the fold changes of individual treatment with reference to the untreated were indicated. Fold change refers to measurement of the indicated band by densitometry. Vertical line has been inserted to indicate a repositioned gel lane. (B) PBMos were untreated (UT), treated with mock (M), or treated with 10 nM Tat (T) for 4 hours and followed by the treatment of IFNγ (100 U/mL) at indicated time intervals (minutes) as shown on top of each panel. Cell lysates were examined by Western blotting using anti-pSTAT1 or anti-STAT1 antibodies as indicated. (C,D) To evaluate the effects of Tat on the expression levels of IFNγ receptors, PBMos were treated with mock or Tat (10 nM) at indicated time intervals. Total RNA was extracted and examined for the mRNA expression levels of (C) IFNγR1 and (D) IFNγR2 by QRT-PCR. All data presented were plotted as mean values ± SEM of at least 3 independent experiments in duplicates.
Because extracellular Tat has been known to exert cellular effects,4 we next evaluated whether down-regulation of IFNγ receptor expression may play a role in the Tat-induced suppression of STAT1 phosphorylation. Results of QRT-PCR revealed no change in the expression level of IFNγR1 and IFNγR2 transcripts over 8 hours, suggesting the early inhibition (within 4 hours) of IFNγ signaling by Tat is not due to the down-regulation of IFNγ receptor expression (Figure 1C,D). Blocking of ligand-receptor interaction by specific viral protein had been reported previously.20 However, there is no evidence suggesting Tat can block IFNγ ligand-receptor interactions directly. In addition, the endotoxin level in the recombinant Tat was found to be less than 0.3 ng/mL as measured by Pyrotell assay (Figure S1B), whereas propidium iodide staining indicated the Tat-treated monocytic cells remained healthy with increases in their metabolic rate (Figure S1C). As a result, it is unlikely cytotoxicity of Tat is responsible for the inhibition of IFNγ-induced STAT1 phosphorylation.
Tat inhibits IFNγ-induced pSTAT1 nuclear translocation and gene expression
Because the IFNγ signaling pathway was suppressed proximally at the stage of STAT1 phosphorylation, we next evaluated whether phosphorylation of Janus kinases was also affected in the presence of Tat. PBMos (0.5 × 106 cells) were pretreated with indicated concentrations of Tat for 4 hours and followed by treatment with IFNγ (100 U/mL) for 10 minutes (Figure 2). Western blotting of total cell lysates demonstrated the phosphorylation levels of both Jak1 and Jak2 were not affected by Tat (Figure 2A), suggesting the IFNγ-activated signaling pathway was impaired upstream of STAT1 phosphorylation, but downstream of receptor aggregation and Janus kinases phosphorylation.
Tat inhibits the nuclear translocation of IFNγ-activated pSTAT1 and the expression of ISGs. (A) PBMos were untreated (UT), mock treated (M), or treated with indicated concentrations of Tat (T) for 4 hours and followed by the treatment of IFNγ (100 U/mL) for 5 minutes. Cell lysates were examined by Western blotting using anti-pJak1 or -pJak2 antibodies. Total Janus kinases were probed as loading control. The levels of phosphorylation were normalized with total Jak1 and Jak2, respectively, whereas the fold changes of individual treatment with reference to the untreated were indicated. (B) PBMos were pretreated with mock (M) or Tat (T) for 4 hours and then stimulated with IFNγ (100 U/mL) as indicated on top of each panel. Nuclear extracts harvested were examined by Western blotting using anti-pSTAT1 antibodies. Lamin B1 was probed as loading control. (C) PBMos were untreated (UT), mock treated (M), or Tat treated (T, 10 nM) for 4 hours and followed by the treatment of IFNγ (100 U/mL) for 16 hours. The expression of IFNγ-inducible genes was examined using RT-PCR. GAPDH was used as a loading control.
Tat inhibits the nuclear translocation of IFNγ-activated pSTAT1 and the expression of ISGs. (A) PBMos were untreated (UT), mock treated (M), or treated with indicated concentrations of Tat (T) for 4 hours and followed by the treatment of IFNγ (100 U/mL) for 5 minutes. Cell lysates were examined by Western blotting using anti-pJak1 or -pJak2 antibodies. Total Janus kinases were probed as loading control. The levels of phosphorylation were normalized with total Jak1 and Jak2, respectively, whereas the fold changes of individual treatment with reference to the untreated were indicated. (B) PBMos were pretreated with mock (M) or Tat (T) for 4 hours and then stimulated with IFNγ (100 U/mL) as indicated on top of each panel. Nuclear extracts harvested were examined by Western blotting using anti-pSTAT1 antibodies. Lamin B1 was probed as loading control. (C) PBMos were untreated (UT), mock treated (M), or Tat treated (T, 10 nM) for 4 hours and followed by the treatment of IFNγ (100 U/mL) for 16 hours. The expression of IFNγ-inducible genes was examined using RT-PCR. GAPDH was used as a loading control.
Tyrosine phosphorylation of STAT1 is essential for nuclear translocation of STAT1 homodimers and subsequent STAT-DNA complex formation.13 Therefore, we next investigated the nuclear translocation of IFNγ-induced pSTAT1 in the presence of Tat. PBMos (0.5 × 106 cells) pretreated with Tat for 4 hours were stimulated with IFNγ for 30 minutes. Western blotting of the nuclear extracts indicated Tat significantly inhibited IFNγ-induced STAT1 phosphorylation and its consequent nuclear translocation (Figure 2B).
In addition, the Tat-induced suppression of IFNγ signaling in PBMos can be assessed by studying the expression of IFNγ-stimulated genes (ISGs) including 2′,5′-oligoadenylate synthetase (OAS) and human leukocyte antigen (HLA). As shown, compared with untreated and mock-treated cells, the induction of the indicated ISGs was severely inhibited when cells were pretreated with Tat (10 nM) for 4 hours and followed by IFNγ (100 U/mL) for 16 hours (Figure 2C). IFNγ responses are mediated through the Jak/STAT1 signaling cascade, which is known to have highly specific functions in regulating crucial immune responses. Thus the impairment of IFNγ activities and its downstream effectors may lead to viral persistence and contribute to disease pathogenesis.
Tat induces SOCS-2 expression in human CD14+ blood monocytes
Recently, regulatory effects of cytokine-inducible SOCS on tyrosine phosphorylation and activation of STATs have been investigated.21 We measured the mRNA levels of the SOCS family members SOCS-1, -2, and -3; and only SOCS-2 was found to be induced by Tat (Figure S2). As evaluated by QRT-PCR, we showed Tat up-regulated SOCS-2 mRNA expression in a dose- and time-dependent manner in PBMos (Figure 3A,B). The maximum induction of SOCS-2 transcripts occurred at 6 to 8 hours after Tat treatment and persisted for 18 hours before returning to normal range (Figure S2). We also confirmed Tat-induced SOCS2 mRNA expression was not due to the endotoxin or other bacterial products. Because Tat at 10 nM contains less than 0.3 ng/mL endotoxin, we treated the PBMos with 0.25 ng/mL LPS and did not find SOCS2 mRNA expression with the treatment (Figure S1D).
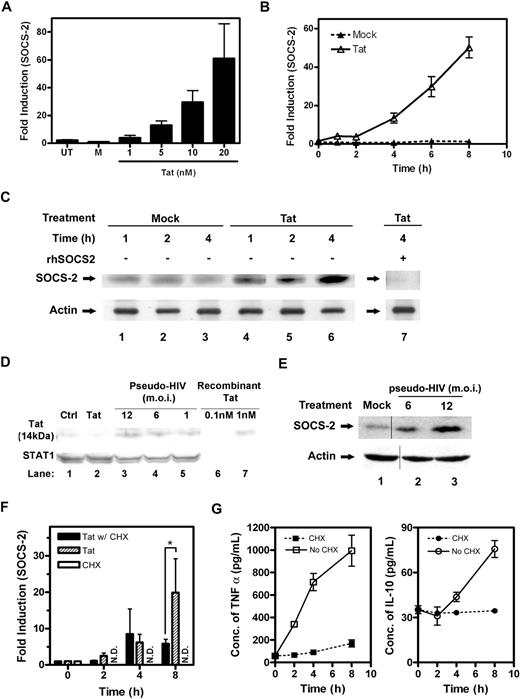
Tat induces SOCS-2 expression in CD14+ PBMos. The mRNA expression levels of SOCS-2 in response to Tat were verified by QRT-PCR using SOCS-2 TaqMan probe. (A) PBMos were untreated (UT), mock treated (M), or Tat treated at indicated concentrations for 4 hours. (B) PBMos were treated with 10 nM Tat at indicated time intervals. (C) The expression levels of SOCS-2 protein were confirmed by Western blotting using specific antibodies. PBMos were mock treated or Tat treated (10 nM) at indicated time intervals. After blotting to PVDF membrane, SOCS-2 protein was probed with specific anti–SOCS-2 antibodies. The SOCS-2 protein band was further verified by competitive Western blotting, of which cell lysates treated with Tat for 4 hours were probed with anti–SOCS-2 antibodies in the presence of purified recombinant SOCS-2 (rhSOCS-2, lane 7). Actin was used as a loading control. (D) HIV Tat expression in HIV-pseudovirus–treated macrophages. Primary blood macrophages were treated with control plasmid, HIV-pseudovirus, or Tat for 48 hours. Total cellular protein (70 μg) was collected and examined by Western blotting using anti-Tat antibodies and anti-STAT1 antibodies. Total STAT1 was probed as a loading control. A representative figure of independent experiments performed on macrophages from 3 different donors is shown. (E) Primary blood macrophages were treated with HIV-pseudovirus or with plasmids alone (mock) for 48 hours and total cellular protein was collected. The SOCS2 expression was measured by Western blot and probed against anti-SOCS2 and actin antibodies. Vertical lines have been inserted to indicate a repositioned gel lane. (F) PBMos were cycloheximide treated (CHX), or Tat treated (10 nM) in the absence or presence of CHX (40 μg/mL) at indicated time intervals. The expression levels of SOCS-2 mRNA were measured by QRT-PCR. ND refers to not detected. The statistical analysis was done by comparison between groups using the Student t test. *P < .05 was considered statistically significant. (G) The expression levels of TNFα and IL-10 from the cell-culture supernatants of PBMos, which were treated with Tat (10 nM) in the absence or presence of cycloheximide (40 μg/mL) at indicated time intervals, were analyzed using specific ELISA kit. All data presented were plotted as mean values ± SEM of at least 3 independent experiments in triplicates.
Tat induces SOCS-2 expression in CD14+ PBMos. The mRNA expression levels of SOCS-2 in response to Tat were verified by QRT-PCR using SOCS-2 TaqMan probe. (A) PBMos were untreated (UT), mock treated (M), or Tat treated at indicated concentrations for 4 hours. (B) PBMos were treated with 10 nM Tat at indicated time intervals. (C) The expression levels of SOCS-2 protein were confirmed by Western blotting using specific antibodies. PBMos were mock treated or Tat treated (10 nM) at indicated time intervals. After blotting to PVDF membrane, SOCS-2 protein was probed with specific anti–SOCS-2 antibodies. The SOCS-2 protein band was further verified by competitive Western blotting, of which cell lysates treated with Tat for 4 hours were probed with anti–SOCS-2 antibodies in the presence of purified recombinant SOCS-2 (rhSOCS-2, lane 7). Actin was used as a loading control. (D) HIV Tat expression in HIV-pseudovirus–treated macrophages. Primary blood macrophages were treated with control plasmid, HIV-pseudovirus, or Tat for 48 hours. Total cellular protein (70 μg) was collected and examined by Western blotting using anti-Tat antibodies and anti-STAT1 antibodies. Total STAT1 was probed as a loading control. A representative figure of independent experiments performed on macrophages from 3 different donors is shown. (E) Primary blood macrophages were treated with HIV-pseudovirus or with plasmids alone (mock) for 48 hours and total cellular protein was collected. The SOCS2 expression was measured by Western blot and probed against anti-SOCS2 and actin antibodies. Vertical lines have been inserted to indicate a repositioned gel lane. (F) PBMos were cycloheximide treated (CHX), or Tat treated (10 nM) in the absence or presence of CHX (40 μg/mL) at indicated time intervals. The expression levels of SOCS-2 mRNA were measured by QRT-PCR. ND refers to not detected. The statistical analysis was done by comparison between groups using the Student t test. *P < .05 was considered statistically significant. (G) The expression levels of TNFα and IL-10 from the cell-culture supernatants of PBMos, which were treated with Tat (10 nM) in the absence or presence of cycloheximide (40 μg/mL) at indicated time intervals, were analyzed using specific ELISA kit. All data presented were plotted as mean values ± SEM of at least 3 independent experiments in triplicates.
The protein expression levels of Tat-induced SOCS-2 were confirmed by Western blotting (Figure 3C). After Tat treatment at indicated time intervals, total cell lysates were precipitated and concentrated using the TCA-DOC/acetone method. Total protein (200 μg/lane) was loaded for Western analysis. As probed with specific anti-SOCS2 antibodies, the results revealed Tat induced a protein of approximately 22 kDa in a time-dependent fashion (Figure 3C lanes 4-6). The specificity of the protein band was verified using competitive Western blotting. Cell lysates harvested at 4 hours after Tat treatment were probed with specific anti-SOCS2 antibodies in the presence of recombinant human SOCS-2 (rhSOCS-2), which was previously expressed and purified from Escherichia coli, BL21(DE3)pLyS (Figure S3A lanes 2-4, 8,9). In the presence of purified rhSOCS-2, probing of SOCS-2 protein by its specific antibodies was completely abolished, suggesting the protein band recognized by the antibodies was specific (Figure 3C lane 7 vs lane 6).
It is interesting to note that SOCS-2 protein was not constitutively expressed in CD14+ PBMos, but rapidly induced by Tat at approximately 2 hours and sustained throughout a period of 4 hours (Figure 3C lanes 4-6 vs lanes 1-3). Moreover, in line with the QRT-PCR results (Figure 3B), Tat-induced SOCS-2 transcript and protein expression had a similar pattern suggesting SOCS-2 activity may be regulated at the transcriptional level. In addition, the level of Tat-induced SOCS-2 protein in PBMos was very low as reflected from the amount of proteins required for loading. As shown, no SOCS-2 protein could be detected from 30 μg total lysates after 6 hours of Tat treatment, compared with 200 μg lysates at the same time point (Figure S3B lane 5 vs lane 3). It is unclear whether the low abundance of SOCS-2 protein expression is due to rapid protein degradation associated with SOCS box-mediated proteasomal activity.22
We also measured the SOCS2 protein level in the Tat-encoding HIV-pseudovirus–treated primary blood macrophages. At first, we measured the protein expression of Tat in HIV-pseudovirus–treated macrophages. The results indicated that there were approximately 0.1 nM to 1.5 nM Tat produced (Figure 3D). We also quantified the Tat mRNA expression level in HIV-pseudovirus–treated macrophages. The expression level was approximately 500 to 2700 copies/mL in a million macrophages (Figure S1G). The results also showed at 48 hours after HIV-pseudovirus treatment that SOCS2, but not SOCS1 and SOCS3, protein expression was up-regulated. The data from these experiments provided additional information on SOCS2 expression in cells treated by the HIV-pseudovirus (Figures 3E, S2C).
To determine whether de novo protein synthesis is required for SOCS-2 induction, cycloheximide (CHX) pretreatment experiments were conducted to exclude a potential role of other secreted factors. PBMos (0.5 × 106 cells) were treated with Tat in the absence or presence of CHX (40 μg/mL) for indicated time intervals (Figure 3F). Results of QRT-PCR revealed CHX pretreatment did not affect the induction of SOCS-2 by Tat over the 8-hour test period. However, with CHX pretreatment, the induction of SOCS-2 transcripts peaked at 4 hours and leveled off throughout the test period, suggesting concomitant protein synthesis may be required for further increases of SOCS-2 transcripts at later time points (Tat with CHX treatment, Figure 3F). Because Tat is well known to induce TNFα and IL-10 production in monocytes,7 the effectiveness of CHX in inhibiting protein synthesis was confirmed by ELISA. The results indicated the production of TNFα and IL-10 was completely abolished in the presence of CHX (40 μg/mL) throughout the test period of 8 hours (Figure 3G). Taken together, our data suggested de novo synthesis of other proteins was not required during the initial stage of SOCS-2 transcription in response to Tat, but concomitant synthesis of as yet unidentified proteins may be helpful in producing more SOCS-2 transcripts.
Overexpression of SOCS-2 inhibits GAS-driven luciferase expression and STAT1 phosphorylation
To understand the role of SOCS-2 in Tat-dependent inhibition of IFNγ signaling, luciferase reporter assays on HEK293 cells were performed to delineate the ability of SOCS-2 in attenuating IFNγ signal transduction. The luciferase reporter vector (pGL3-8xGAS), which contains multiple IFNγ-activated site (GAS) elements for pSTAT1 binding, was used to transfect HEK293 cells in the presence of either the pcDNA (parental) or pcDNA-SOCS2 expression vector. Twenty-four hours after transfection, the transfectants were treated with indicated concentrations of IFNγ for 16 hours. As shown, treatment of IFNγ resulted in a dose-dependent increase in GAS-driven luciferase activities, which peaked at 20 U/mL IFNγ (Figure 4A). In contrast, with SOCS-2 expression, the IFNγ-induced enhancement of luciferase activities was significantly reduced (Figure 4A).
SOCS-2 abrogates IFNγ-induced GAS luciferase reporter activity. (A) HEK293 cells were transfected with pGL3-8xGAS reporter vector, together with either parental pcDNA vector (w/o SOCS-2) or pcDNA-SOCS-2 expression vector (SOCS-2) for 24 hours. HEK transfectants were then stimulated with indicated concentrations of IFNγ for 16 hours. The luciferase activities were determined by Dual-Luciferase reporter assay system using FusionTM-αFP microplate reader. (B) HEK293 cells were transfected with pGL3-8xGAS reporter vector and either pcDNA (parental) or pcDNA-SOCS-2 expression vector at indicated ratio for 24 hours. Cells were then stimulated with mock (Ctrl) or IFNγ (10 U/mL) for 16 hours. The luciferase activities were determined as described for panel A. All data were plotted as mean values ± SEM of at least 3 independent experiments in duplicates. The statistical analysis was done by comparison between groups using the Student t test. *P < .05 was considered statistically significant. (C) The overexpression of SOCS-2 protein in HEK transfectants was verified by Western blotting using specific anti–SOCS-2 antibodies. Actin was shown as loading control. Vertical line has been inserted to indicate a repositioned gel lane. (D) The role of SOCS-2 in inhibition of STAT1 phosphorylation was verified by overexpression study. HEK cells transiently transfected with pcDNA (lane 3, Ctrl) or pcDNA-SOCS-2 (lane 4, S2) were subjected to IFNγ treatments (20 U/mL) as indicated. Untreated (lane 1, UT) and lipofectamine treated (lane 2, LF) were shown as control. Overexpression of SOCS-2 transcripts was confirmed by RT-PCR (D top panel). Cell lysates were examined by Western blotting using anti-pSTAT1 antibodies, whereas the overexpression of SOCS-2 was verified using anti–SOCS-2 antibodies (D bottom panel). Total STAT1 was probed as a loading control. (E) To further confirm the role of SOCS-2 in inhibition of IFNγ signal transduction, CD14+ PBMos were transfected with either control siRNA or SOCS-2 siRNA, and later treated with Tat for 5 hours as indicated. The siRNA-mediated SOCS-2 knockdown in PBMos was verified using RT-PCR (lane 4 vs lane 3). (F) PBMos transfected with either control siRNA or SOCS-2 siRNA were pretreated with Tat for 5 hours and followed by IFNγ treatment for 10 minutes. The phosphorylation levels of STAT1 were examined by Western blotting using anti-pSTAT1 antibodies (lane 4 vs lanes 3 and 5). Total STAT1 was probed as a loading control. The levels of phosphorylation were normalized with total STAT1, whereas the fold changes of individual treatment with reference to the untreated were indicated. Fold change refers to measurement of the indicated band by densitometry. A representative figure of 6 independent experiments on PBMos from different donors was shown. Vertical lines have been inserted to indicate a repositioned gel lane.
SOCS-2 abrogates IFNγ-induced GAS luciferase reporter activity. (A) HEK293 cells were transfected with pGL3-8xGAS reporter vector, together with either parental pcDNA vector (w/o SOCS-2) or pcDNA-SOCS-2 expression vector (SOCS-2) for 24 hours. HEK transfectants were then stimulated with indicated concentrations of IFNγ for 16 hours. The luciferase activities were determined by Dual-Luciferase reporter assay system using FusionTM-αFP microplate reader. (B) HEK293 cells were transfected with pGL3-8xGAS reporter vector and either pcDNA (parental) or pcDNA-SOCS-2 expression vector at indicated ratio for 24 hours. Cells were then stimulated with mock (Ctrl) or IFNγ (10 U/mL) for 16 hours. The luciferase activities were determined as described for panel A. All data were plotted as mean values ± SEM of at least 3 independent experiments in duplicates. The statistical analysis was done by comparison between groups using the Student t test. *P < .05 was considered statistically significant. (C) The overexpression of SOCS-2 protein in HEK transfectants was verified by Western blotting using specific anti–SOCS-2 antibodies. Actin was shown as loading control. Vertical line has been inserted to indicate a repositioned gel lane. (D) The role of SOCS-2 in inhibition of STAT1 phosphorylation was verified by overexpression study. HEK cells transiently transfected with pcDNA (lane 3, Ctrl) or pcDNA-SOCS-2 (lane 4, S2) were subjected to IFNγ treatments (20 U/mL) as indicated. Untreated (lane 1, UT) and lipofectamine treated (lane 2, LF) were shown as control. Overexpression of SOCS-2 transcripts was confirmed by RT-PCR (D top panel). Cell lysates were examined by Western blotting using anti-pSTAT1 antibodies, whereas the overexpression of SOCS-2 was verified using anti–SOCS-2 antibodies (D bottom panel). Total STAT1 was probed as a loading control. (E) To further confirm the role of SOCS-2 in inhibition of IFNγ signal transduction, CD14+ PBMos were transfected with either control siRNA or SOCS-2 siRNA, and later treated with Tat for 5 hours as indicated. The siRNA-mediated SOCS-2 knockdown in PBMos was verified using RT-PCR (lane 4 vs lane 3). (F) PBMos transfected with either control siRNA or SOCS-2 siRNA were pretreated with Tat for 5 hours and followed by IFNγ treatment for 10 minutes. The phosphorylation levels of STAT1 were examined by Western blotting using anti-pSTAT1 antibodies (lane 4 vs lanes 3 and 5). Total STAT1 was probed as a loading control. The levels of phosphorylation were normalized with total STAT1, whereas the fold changes of individual treatment with reference to the untreated were indicated. Fold change refers to measurement of the indicated band by densitometry. A representative figure of 6 independent experiments on PBMos from different donors was shown. Vertical lines have been inserted to indicate a repositioned gel lane.
To further investigate the effects of SOCS-2 expression on GAS reporter activities, HEK293 cells were cotransfected with a fixed amount of pGL3-8xGAS reporter and higher concentrations of pcDNA-SOCS2 expression vector. As shown, treatment of the cells with IFNγ (10 U/mL) resulted in a marked increase in luciferase activities compared with that of the untreated controls (Figure 4B bar 2 vs 1). However, in the presence of higher concentrations of SOCS-2, IFNγ-induced luciferase activities were reduced (Figure 4B bars 3-6), and the nadir of activity (∼ 60.2% inhibition) occurred at 1:1 ratio of the reporter to SOCS-2 vector (Figure 4B bar 4). No further inhibition in luciferase activities was observed with higher concentrations of SOCS-2 used in the cotransfection. The levels of SOCS-2 overexpression were confirmed by Western blotting probed with polyclonal anti-SOCS2 antibodies (Figure 4C). Meanwhile, to ascertain whether other suppressor proteins were not involved, expression of SOCS-1 and -3 transcripts was monitored by RT-PCR, of which the endogenous expression levels of SOCS-1 and -3 were barely detectable upon Tat treatment (Figure S4A).
To document the effects of SOCS-2 in abrogating STAT1 phosphorylation, SOCS-2 was overexpressed in HEK293 cells for 24 hours, and followed with IFNγ treatment (20 U/mL) for 10 minutes. As shown, results of Western analysis demonstrated overexpression of SOCS-2 significantly inhibited IFNγ-induced STAT1 phosphorylation compared with controls (Figure 4D lane 3 vs lane 4). The overexpression of SOCS-2 was confirmed by RT-PCR and Western blotting (Figure 4D). Taken together, all these observations suggested SOCS-2 is a significant inhibitor of STAT1 signal transduction, with its overexpression causing a remarkable impairment of IFNγ signaling cascade.
SOCS-2 siRNA abrogates Tat inhibition of IFNγ signaling in blood monocytes
To further confirm the role of SOCS-2 in inhibiting IFNγ-induced STAT1 phosphorylation, SOCS-2–specific siRNA was used to transfect CD14+ blood monocytes. PBMos (106 cells/mL) were transfected with either SOCS-2 siRNA or control siRNA for 24 hours, and followed by treatment with Tat (10 nM) for 5 hours. The efficiency of siRNA-mediated SOCS-2 knockdown of Tat-induced SOCS-2 mRNA was verified by RT-PCR compared with that of the controls (Figure 4E lane 4 vs lane 3). For Western analysis of STAT1 phosphorylation, Tat-pretreated siRNA transfectants were stimulated with IFNγ (100 U/mL) for 10 minutes. As shown, the results demonstrated Tat effectively inhibited the IFNγ-induced STAT1 phosphorylation in mock- and control siRNA–transfected cells (Figure 4F lanes 3 and 5 vs lane 2). Furthermore, concomitant with knockdown of SOCS-2 mRNA by its specific siRNA, there was restoration of the IFNγ effects on STAT1 phosphorylation, compared with mock- and control siRNA–transfected cells (Figure 4F lane 4 vs lanes 3 and 5). Taken together, these results demonstrated Tat-induced inhibition of IFNγ signaling including suppression of STAT1 phosphorylation can be reversed by SOCS-2–specific siRNA, signifying the potential role of SOCS-2 in modulating Tat-induced cytokine effects in human CD14+ blood monocytes.
Discussion
Peripheral blood monocytes/macrophages are major cellular targets of HIV-1 infection and play an important role in the pathogenesis of AIDS.23 Because they can serve as virus reservoirs during infection, these cells are good targets of virus-against-host action through the production of viral and cellular factors to deregulate immune response. Among the viral proteins of HIV-1, Tat has been known to be an important factor in the pathogenesis of AIDS. Apart from its effect on HIV-1 transcription in the infected cell, Tat is secreted into the microenvironment and taken up by neighboring cells including the uninfected ones.3 Tat also dysregulates the production of cytokines, which may contribute to HIV evasion of the immune system.6,7 In addition, Tat has been shown to induce apoptosis in neurons and may mediate neurotoxin effects that lead to HIV-associated dementia.24,25 These dementia and neurologic symptoms are commonly seen in AIDS patients. In a previous report, the level of circulating Tat in AIDS patients' serum is from 0.2 nM to 4 nM.26 In that paper, these authors also suggested the figures could be underestimated due to the poor binding efficiency of Tat with the detection antibodies and no defined sampling site from AIDS patients. In cultured supernatants of HIV-1–infected cells, there is approximately 10 to 100 pM Tat produced.27 This concentration is similar to the level of cytokines or chemokines produced in stimulated or infected cells. Different dosages of extracellular Tat protein (1 nM to 100 nM) have been shown to induce cytokines in monocytes and macrophages in previous in vitro studies including ours.7,28 It is believed that in an in vitro cell culture model, the cell required higher concentrations of a stimulant for the activation of cellular response. Thus, Tat may exert dysregulatory effects on both infected and uninfected cells, thus contributing its role in HIV-1 pathogenesis.
In our previous studies, we demonstrated Tat serves as an important factor in perturbing the host immune system by overproduction of cytokines including IL-6, IL-10, and TNF-α.6,7 After this, we would like to explore the role of Tat in dysregulating the host antimicrobial responses that are regulated by IFNγ. In AIDS progression, IFNγ plays a dual role in the host immune system. Several studies showed IFNγ enhances the Tat and gp120 protein–induced neurotoxicity, thus implicating its role in the pathogenesis of HIV-associated dementia.29,30 On the other hand, other reports showed IFNγ could slow down the Tat-mediated HIV-1 replication rate in the infected cell.31,32 Here, we showed Tat interferes with the IFNγ-activated Jak/STAT1 signaling pathway. Furthermore, Tat can impair IFNγ-induced STAT1 tyrosine phosphorylation, nuclear translocation, and its consequent GAS-driven gene transcription in PBMos (Figures 1,2). We also demonstrated Tat impaired the expression of HLA and IFNγ–activated OAS, a key enzyme responsible for the mediation of IFN-induced activation of latent ribonuclease L.11 These results suggest an interplay between Tat and IFNγ may affect viral pathogenicity, clearance, and immunity. At present, it is unknown whether inhibition of STAT1 signaling observed in primary blood monocytes in vitro here would be the same as natural HIV-1 infection in vivo. However, given the importance of IFNγ in regulating immunity, it is conceivable that such impairment of IFNγ activities would further cripple the immune system and contribute to HIV-1 persistence and development of opportunistic infections including mycobacteria and herpes viruses.33
To delineate the mechanisms of Tat-IFNγ interaction, we investigated the involvement of negative regulators in the IFNγ system. The activated Jak/STAT signaling pathway is negatively modulated by distinct regulatory proteins, including protein inhibitor of activated STAT (PIAS),34 protein tyrosine phosphatase (PTP),35 and suppressor of cytokine signaling protein (SOCS).36 Two members of PIAS family, PIAS-1 and PIAS-Y, were shown to be capable of interacting with STAT1 signal transduction.37,38 However, both proteins mediated their effects after the phosphorylation of STAT1, which was not observed throughout the experiments here. Besides, PTP family members, including SHP-1, -2, PTP1B, and TCPTP, were found interfering with IFNγ signaling by Jak-1 and -2 dephosphorylation.35,39,40 However, the phosphorylation levels of Janus kinases were not affected as shown in our results, suggesting PTPs are unlikely to be the negative regulators involved in the Tat inhibition of IFNγ signaling.
SOCS proteins have been known to be effective negative regulators of cytokine signaling. Among the SOCS family members, SOCS-1 and SOCS-3 have been reported to be the most potent inhibitors of the IFNγ-induced STAT1 signal transduction.14 The role of SOCS-2 in regulating signal transduction remains controversial. SOCS-2 was suggested to be a negative regulator of the leukemia inhibitory factor– and growth hormone–activated signaling pathways.41,42 However, it did not abrogate the effects of granulocyte-colony stimulating factor, prolactin, or interferon in other studies.14,43 In these systems, overexpression of SOCS1, SOCS2, or SOCS3 protein using a tet-inducible system or constitutive expression of the SOCS proteins was used in HeLa or in MCF7 cells, respectively.14 Because SOCS2 is a low abundance protein, the overexpression system may produce a higher level of SOCS2 than the physiologic state. This may lead to different cellular responses compared with cells in the normal host.44 In addition to the different experimental design of gene overexpression, our cell model used is different from the other reports. Here, we measured the SOCS2 activity in primary human blood monocytes. The phenotypes, cellular responses, surface receptor expression, and other cellular functions in monocytes are also different from HeLa and MCF7 cells. Thus, potential discrepancy in results between ours and others may due to the different cell types used and experimental designs.
Moreover, studies using SOCS-2 overexpressing or knockout mice revealed a dual role of the suppressor in mediating growth hormone and insulin-like growth factor signaling.44,45 These studies demonstrated the functions of SOCS2 are related to its concentration in the host. Recently, the role of SOCS-2 in the antiinflammatory actions of lipoxin was reported.46 And here, when we overexpressed the SOCS-2 protein, it resulted in inhibition of IFNγ-induced STAT1 phosphorylation and its consequent signaling cascade as demonstrated by Western blotting and GAS-driven luciferase reporter assay (Figure 4). These observations suggest SOCS-2 may play a role in the regulation of IFNγ-induced STAT1 signaling.
In the presence of Tat, we found only SOCS-2, but not SOCS-1 and SOCS-3 (Figure S2), was induced. We also demonstrated the Tat-encoding HIV-pseudovirus (MOI = 6 and MOI = 12) can up-regulate SOCS2 expression in primary human blood macrophages (Figures 3D,E and S1G) and inhibit phosphorylation of STAT1 on tyrosine 701 induced by IFNγ (Figure S2D). The SOCS-2 protein induced by Tat perturbed the early stage of IFNγ-induced signaling pathway at the tyrosine phosphorylation of STAT1 in CD14+ PBMos. To confirm the role of SOCS2 in Tat-suppressed IFNγ signaling, we knocked down the SOCS-2 expression by transfecting SOCS-2–specific siRNA into CD14+ PBMos. The results showed the Tat-dependent inhibition of IFNγ signaling was abrogated by SOCS2 siRNA (Figure 4).
In addition, Tat did not affect the phosphorylation levels of the serine residue (Ser727) of STAT1 suggesting SOCS-2 may not play a role in regulating the phosphatidylinositol 3-kinase cascade. From the literature, there was no study showing that SOCS-2 could specifically inhibit the phosphorylation of STAT1 on tyrosine 701. The phosphorylation and activation of STAT1 Tyr701 and Ser727 were induced by different pathways. IFNγ activated AKT and its downstream effector PI3K to phosphorylate STAT1 at Ser727 position. For the Tyr701 phosphorylation of STAT1, it involves mainly the JAK pathways.47 It has been shown that cerebral ischemia is associated with phosphorylation of STAT1 at Tyr701 but not Ser727.48 In addition, a JAK2 inhibitor could inhibit hydrogen peroxide–induced STAT1 Tyr701 phosphorylation.49 Therefore, the detailed mechanisms and functions underlying Tat abrogation of IFNγ–induced STAT1 phosphorylation remain to be investigated.
We also examined whether the Tat-induced IL-10 inhibits the IFNγ-activated STAT1 phosphorylation. The IL-10 gene in PBMos was knocked down by IL-10–specific siRNA oligo. Our results showed IL-10 did not interfere with IFNγ-activated STAT1 phosphorylation (Figure S1E,F). Taken together, these results suggest SOCS-2 plays multiple and yet specific roles in the modulation of cytokine- and growth factor–regulated Jak/STAT signaling pathways.
As revealed from our results, high concentrations of IFNγ still cannot abrogate the inhibitory effects of SOCS-2 on STAT1 signal transduction in SOCS-2–overexpressing cells (Figure 4A). This observation indicates SOCS-2 may act as a competitive inhibitor for the docking site of activated IFNγ receptor similar to the case of growth hormone receptor,41 thus fine-tuning the signaling strength via SOCS box-mediated proteasomal degradation.22 Further investigations are needed to delineate the detailed mechanisms of Tat-dependent SOCS-2 induction, and SOCS-2–mediated inhibition of signal transduction including the targets of SOCS-2 and its mode of actions in human blood monocytes.
Previous studies reported SOCS-2 is not involved in regulating IFNγ-induced activities.14,50 However, these studies of SOCS were conducted in mice model or tumor cell lines, which may behave totally different from human primary monocytic cells. For instance, in contrast to CD14+ PBMos, we observed there are basal levels of SOCS-2 transcripts in monocytic cell lines including THP-1 and U937 (Figure S4B). Therefore, experimental studies on HIV-1 interactions with the immune system require the proper choice of cells from relevant tissue origin and host species.
In summary, our data showed HIV-1 Tat induces SOCS-2 resulting in the suppression of IFNγ-mediated antimicrobial responses. Such perturbation of IFNγ signal transduction may have clinical significance. It provides a window of opportunity for opportunistic microbes including cytomegalovirus, herpes simplex virus, and mycobacteria to invade the HIV-infected host. These results may provide insights into designing novel therapeutics that can restore or augment the host response to IFNγ treatment. Moreover, understanding of the mechanisms of viral interference with the IFNγ-activated Jak/STAT pathway in primary cells may help us to clarify the role of signaling inhibition by specific viral proteins in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr M. Shong (Laboratory of Endocrine Cell Biology, Chungnam National University College of Medicine, Daejeon, South Korea) for providing the plasmid containing 8X GAS responsive elements. We are grateful to our colleague Dr B. K. Cheung (Cytokine Biology Group-Paediatrics, University of Hong Kong) for his helpful discussion and suggestions.
This project was supported by grants to A.S.Y.L. from the Hong Kong Research Grants Council (HKU 7594/06M and HKU 7685/07M) and the Research Fund for the Control of Infectious Diseases (06060612), Department of Health, Hong Kong. Additional support to the AIDS Institute was provided by the University Development Fund, University of Hong Kong, Hong Kong SAR, China.
Authorship
Contribution: S.M.C., J.C.B.L., and S.S.L. performed experiments; S.M.C., J.C.B.L., D.C.W.L., and A.S.Y.L. analyzed results; L.L. and Z.C. contributed some of the viral reagents and performed the relevant experiments; S.M.C., J.C.B.L., and A.S.Y.L. designed the research and wrote the paper; and the original concept of the HIV-SOCS project was designed by A.S.Y.L.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Allan S. Y. Lau, Cytokine Biology Group, Department of Paediatrics and Adolescent Medicine, Li Ka Shing Faculty of Medicine, University of Hong Kong, Pokfulam, Hong Kong; e-mail: asylau@hku.hk.
References
Author notes
*S.M.C. and J.C.B.L. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal