Managing patients with myelofibrosis (MF), either those with primary MF or those whose MF has evolved from antecedent polycythemia vera or essential thrombocythemia, presents many challenges to the hematologist. MF patients have a range of debilitating disease manifestations (eg, massive splenomegaly, cytopenias, constitutional symptoms, and transformation to a treatment-refractory blast phase). Cure is potentially achievable through allogeneic stem cell transplantation; however, this therapy is either inappropriate or not feasible for the majority of patients. Therefore, remaining therapies are palliative but can be of significant value to some MF patients. In particular, management of symptomatic splenomegaly remains one of the most perplexing aspects of MF clinical care. Using medications is the simplest approach for reducing splenomegaly, yet achieving symptomatic response without undue myelosuppression is challenging. Splenectomy or radiotherapy offers benefit, but careful patient selection and close monitoring are required because both have the potential for dangerous adverse effects. Experimental medical therapies, such as JAK2 inhibitors, show promise and may soon play an important role in the management of symptomatic splenomegaly in MF patients. Future care of MF patients, including splenomegaly management, will continue to require the hematologist to select therapeutic options carefully in the context of realistic, achievable goals.
Introduction
Myelofibrosis (MF) includes primary MF and MF that has arisen from an antecedent polycythemia vera or essential thrombocythemia.1 MF can affect patients in various ways, including progressive splenomegaly, cytopenias (particularly anemia), and troublesome constitutional symptoms (ie, night sweats, fevers, unintentional weight loss, debilitating fatigue).2 MF can also result in blastic transformation3 and death.4
Splenomegaly can be a major independent source of morbidity and detriment to quality of life when it develops in MF patients.5,6 First, splenomegaly (sometimes massive, > 10 kg) can lead to pain, early satiety, bloating, or even portal hypertension.7 Second, the bulk of the spleen can result in areas of ischemia and painful episodes of splenic infarction. Finally, splenomegaly can result in development (or exacerbation) of cytopenias from splenic sequestration.
Management of MF-associated splenomegaly can be difficult, and the questions that arise represent a microcosm of the management issues for the entire condition. What are the treatment goals for an individual patient: cure or palliation? Who should receive an allogeneic stem cell transplantation? What are the benefits of current medical therapies? How do I manage the myelosuppression brought about by medical therapy? Which patients should be considered for splenectomy or splenic radiation? What is the impact of experimental drugs (particularly the JAK2 inhibitors) on splenomegaly, and would my patient benefit from treatment with one of these agents? These are the key challenges for hematologists involved in treating patients with MF (they also comprise a large proportion of the patient-related inquiries in my e-mail inbox), and each of these questions is addressed herein.
MF management strategies: prognosis and treatment goals
Deciding on a treatment plan for patients with MF requires both an assessment of the disease impact at diagnosis and an accurate assessment of prognosis. The International Working Group for Myelofibrosis Research and Treatment recently published an International Prognostic Scoring System (IPSS-MF; Table 1) to aid in assessing MF prognosis. The IPSS-MF defines 4 risk groups with projected survival medians for each group, ranging from 27 months for high-risk disease to 135 months for low-risk disease.4
The International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) International Prognostic Scoring System for Primary Myelofibrosis (IPSS-PMF)4
| Risk group . | No. of risk factors* . | Median survival, mo (95% CI) . |
|---|---|---|
| Low | 0 | 135 (117-181) |
| Intermediate 1 | 1 | 95 (79-114) |
| Intermediate 2 | 2 | 48 (43-59) |
| High | ≥ 3 | 27 (23-31) |
| Risk group . | No. of risk factors* . | Median survival, mo (95% CI) . |
|---|---|---|
| Low | 0 | 135 (117-181) |
| Intermediate 1 | 1 | 95 (79-114) |
| Intermediate 2 | 2 | 48 (43-59) |
| High | ≥ 3 | 27 (23-31) |
Risk factors for primary myelofibrosis (present at diagnosis): (1) age > 65 years, (2) hemoglobin < 10 g/dL, (3) leukocytes > 25 × 109/L, (4) peripheral blood blasts ≥ 1%, and (5) constitutional symptoms (weight loss > 10%, night sweats, or fevers).
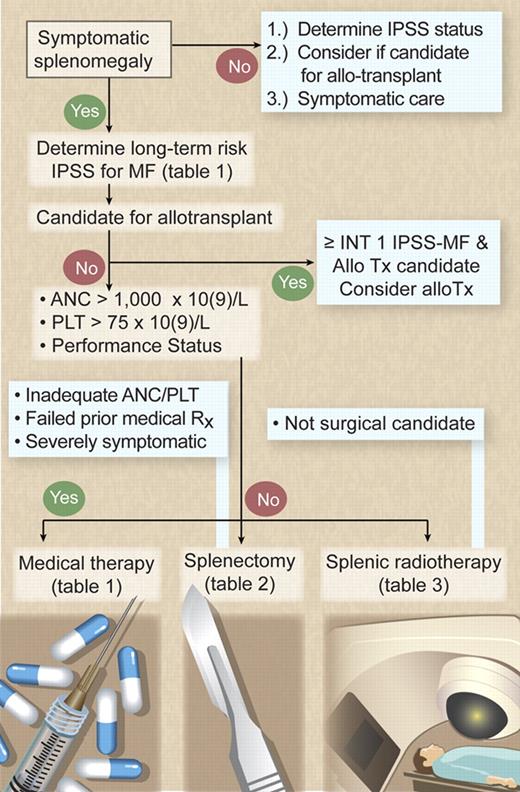
Currently, there is only one curative therapy for MF: allogeneic stem cell transplantation, which offers a 58% 3-year survival rate with a 32% relapse-free mortality rate.8 More favorable outcomes have been reported with reduced-intensity transplantation (85% survival rate at a median of 31 months), but long-term outcomes, including the burden of chronic graft-versus-host disease, need to be assessed.9 Therefore, the key question after MF diagnosis is whether the patient is a suitable candidate for allogeneic transplantation (Figure 1). Given that no other therapy has been found curative or has been proved to confer a survival advantage in MF, all other nontransplantation forms of therapy must be considered palliative in nature and intent.
Management algorithm for symptomatic splenomegaly in MF. Illustration by Debra T. Dartez.
Management algorithm for symptomatic splenomegaly in MF. Illustration by Debra T. Dartez.
Which patients with MF require treatment for splenomegaly?
The first decision made in managing splenomegaly in MF patients is whether the patient has sufficient symptoms (ie, pain, mechanical symptoms, splenic infarcts, or refractory cytopenias) to merit therapy (Figure 1). Splenomegaly and cytopenias are sometimes present concurrently (ie, splenic sequestration exacerbates ineffective hematopoiesis from the marrow). Frequently, however, splenomegaly may exist with adequate peripheral blood counts in those patients whose marrow remains very proliferative. The presence or absence of concurrent cytopenias is essential in the decision-making process because many therapies have a detrimental impact on any cytopenias present. Nontransplantation (ie, palliative) therapeutic options are judged by their efficacy in achieving symptom relief balanced against toxicity. Assessing the patient's performance status and peripheral blood counts to determine the ability to tolerate the potential myelosuppression of medical therapy is central in therapy selection (Figure 1).
Medical therapy for splenomegaly in MF
Medical therapy is the cornerstone therapeutic option for symptomatic splenomegaly in patients with MF (Table 2). In MF, sequestration of immature circulating myeloid progenitors leads to accumulation and proliferation in the splenic cords.19 Current medical therapies can ameliorate splenomegaly in MF mainly through nonspecific myelosuppression, thereby decreasing the immature circulating myeloid pool accumulating in the spleen.
Medical therapy for symptomatic splenomegaly in myelofibrosis (available medications)
| Agent . | Administration route . | Dose/schedule . | Response rate* . | Additional efficacy . | Toxicities . | Reference . |
|---|---|---|---|---|---|---|
| Tier 1 | ||||||
| Hydroxyurea | Oral | 500-3000 mg daily | 40%-50% | None | Myelosuppression, skin ulcers | 10 |
| Busulfan | Oral | 2-4 mg daily | Variable | None | Myelosuppression, leukemia | 11 |
| Melphalan | Oral | 2.5 mg 3 times weekly | 67% | None | Myelosuppression, leukemia | 12 |
| Interferon-2α | Subcutaneous | 0.5-1.0 × 106 units 3 times weekly | Limited data; 75% with small spleens, less with larger | None | Myelosuppression, depression | 13 |
| Thalidomide with or without prednisone taper | Oral | 50 mg daily | 19% | Anemia, thrombocytopenia | Neuropathy, sedation, myeloproliferation | 14 |
| Lenalidomide with or without prednisone taper | Oral | 5-10 mg daily | 33% | Anemia, thrombocytopenia | Myelosuppression, rash, diarrhea | 15 |
| Tier 2 | ||||||
| Cladribine (2-CdA) | Intravenous | 5 mg/m2 per day × 5 days per month | 56% | None | Myelosuppression | 16 |
| Daunorubicin | Intravenous | 60 mg/m2 on days 1-3 | NA | None | Myelosuppression, cardiotoxicity | None |
| 5-Azacytidine | Subcutaneous | 75 mg/m2 on days 1-7 per month | 21% | Anemia | Myelosuppression, gastrointestinal | 17,18 |
| Decitabine | Subcutaneous | 20 mg/m2 on days 1-5 per month | NA | Myelosuppression | None |
| Agent . | Administration route . | Dose/schedule . | Response rate* . | Additional efficacy . | Toxicities . | Reference . |
|---|---|---|---|---|---|---|
| Tier 1 | ||||||
| Hydroxyurea | Oral | 500-3000 mg daily | 40%-50% | None | Myelosuppression, skin ulcers | 10 |
| Busulfan | Oral | 2-4 mg daily | Variable | None | Myelosuppression, leukemia | 11 |
| Melphalan | Oral | 2.5 mg 3 times weekly | 67% | None | Myelosuppression, leukemia | 12 |
| Interferon-2α | Subcutaneous | 0.5-1.0 × 106 units 3 times weekly | Limited data; 75% with small spleens, less with larger | None | Myelosuppression, depression | 13 |
| Thalidomide with or without prednisone taper | Oral | 50 mg daily | 19% | Anemia, thrombocytopenia | Neuropathy, sedation, myeloproliferation | 14 |
| Lenalidomide with or without prednisone taper | Oral | 5-10 mg daily | 33% | Anemia, thrombocytopenia | Myelosuppression, rash, diarrhea | 15 |
| Tier 2 | ||||||
| Cladribine (2-CdA) | Intravenous | 5 mg/m2 per day × 5 days per month | 56% | None | Myelosuppression | 16 |
| Daunorubicin | Intravenous | 60 mg/m2 on days 1-3 | NA | None | Myelosuppression, cardiotoxicity | None |
| 5-Azacytidine | Subcutaneous | 75 mg/m2 on days 1-7 per month | 21% | Anemia | Myelosuppression, gastrointestinal | 17,18 |
| Decitabine | Subcutaneous | 20 mg/m2 on days 1-5 per month | NA | Myelosuppression | None |
NA indicates not applicable.
When considering current medical therapies for splenomegaly in MF patients, I categorize them into 2 distinct tiers. Tier 1 consists predominantly of oral agents with (usually) modest toxicity; tier 2 includes more aggressive approaches suitable for patients with severe splenomegaly or involvement of other organs by extramedullary hematopoiesis (EMH; eg, lungs, abdominal cavity, pericardium). In those patients, a more intensive, frequently parenteral (intravenous or subcutaneous), myelosuppressive approach may be warranted. The choice of both initial and subsequent therapies should be based on toxicity profile and specifically the ability to withstand myelosuppression (Figure 2).
Medical therapy algorithm for symptomatic splenomegaly in MF. Illustration by Debra T. Dartez.
Medical therapy algorithm for symptomatic splenomegaly in MF. Illustration by Debra T. Dartez.
Tier 1
Hydroxyurea.
Hydroxyurea, the most common initial medical therapy used to decrease splenomegaly in MF patients (estimated response rate of < 50% for splenomegaly, although very little prospective clinical trial data exist),10 is an oral myelosuppressive agent. There are, however, several limitations to the use of this agent in MF. First, hydroxyurea rarely induces a complete resolution of splenomegaly or even an International Working Group for Myelofibrosis Research and Treatment clinical improvement for splenomegaly (ie, > 50% improvement sustained for at least 2 months).20 Nevertheless, more modest reductions in splenomegaly may benefit some patients with MF. A second limitation is that splenomegaly, compared with thrombocytosis, is less responsive to hydroxyurea and might require a higher dose (ie, 2-3 g/day). Third, hydroxyurea therapy may potentially exacerbate cytopenias, particularly at higher doses. Responses to hydroxyurea for splenomegaly usually occur within 2 to 3 months, and the dose must be titrated to achieve the benefit for splenomegaly by lowering the leukocyte count as best tolerated or to the lower limits of the normal range.
Oral alkylators.
Alkylators, such as melphalan and busulfan, can alleviate splenomegaly in some MF patients. However, myelosuppression and the increased risk of blastic transformation are potential adverse effects for both medications. In one study,12 melphalan (2.5 mg orally 3 times per week) reduced spleen size in 66% of patients; however, 26% of the study cohort developed acute leukemia. Busulfan can be used to treat splenomegaly,21 although it was used more classically in the treatment of chronic myeloid leukemia in the pre-imatinib era.22
Immunomodulatory drugs.
The immunomodulatory drugs known collectively as IMiDs (ie, thalidomide, lenalidomide, pomalidomide) inhibit cytokines and have antiangiogenic properties. IMiDs have been used primarily for palliation in MF patients with cytopenias (anemia and thrombocytopenia) but may also be used to reduce splenomegaly. This combination of potential effects should lead to consideration of IMiD therapy for patients with concurrent cytopenias and splenomegaly.
Thalidomide produced responses for cytopenias in MF patients but was not well tolerated.23 Low-dose (50 mg/day) thalidomide combined with a prednisone taper (thalidomide-prednisone regimen)14 achieves significant responses for anemia (67%), thrombocytopenia (75%), and splenomegaly (33%) in MF patients and was better tolerated than single-agent thalidomide. Subsequently, lenalidomide (a second-generation IMiD) was evaluated in 68 patients with symptomatic MF, achieving overall response rates of 22% for anemia, 33% for splenomegaly, and 50% for thrombocytopenia.15 Lenalidomide was effective in patients where prior treatment with thalidomide had failed and, given its more significant myelosuppressive properties, was better tolerated if patients' pretreatment peripheral blood neutrophil and platelet counts were adequate. In addition, as in del(5q) myelodysplastic syndromes,24 MF patients with an abnormality of chromosome 5 seem to respond best to lenalinomide.25
Currently, I recommend thalidomide-prednisone as first-line treatment for MF patients with cytopenias and splenomegaly, with lenalidomide as a second-line treatment (first-line treatment if a del5[q] is present in the marrow karyotype).
Interferon-α.
Therapy with interferon-α (IFN-α) has been used in MF patients based on its cytoreductive properties and has been effective in patients with polycythemia vera.26 However, clinical trials of IFN-α in MF with both standard preparations27 and pegylated IFN-α (PEG-IFN-α)28 have demonstrated poor patient tolerance and negligible response rates. In one study,28 subcutaneous PEG-IFN-α-2b (PEG-intron) was administered weekly to 36 patients with BCR-ABL-negative myeloproliferative neoplasms, and none of the patients with MF responded. Therefore, I do not recommend use of IFN-α in advanced MF at present.
Tier 2
Cladribine.
Palliative benefit from the purine nucleoside analog 2-chlorodeoxyadenosine (2-CdA) has been reported in MF patients.16 2-CdA has been administered as 4 to 6 once-monthly cycles of treatment with either 0.1 mg/kg per day intravenously by continuous infusion for 7 days or 5 mg/m2 intravenously over 2 hours for 5 consecutive days. In the Mayo Clinic experience, we observed responses in 55%, 50%, 55%, and 40% of patients for splenomegaly, thrombocytosis, leukocytosis, and anemia, respectively.16 Responses were frequently durable and lasted for a median of 6 months after discontinuation of treatment. Myelosuppression is the primary toxicity of this regimen, with occasional additional adverse effects of gastrointestinal disturbances. I think that 2-CdA is the most helpful of the tier 2 agents and should be given on monthly cycles (up to 4 cycles) to achieve maximal response for refractory splenomegaly.
Hypomethylating agents.
The 2 hypomethylating agents approved for myelodysplastic syndromes, azacytidine and decitabine, have been tested in MF patients with a goal of improving cytopenias and splenomegaly or delaying blastic transformation. Recent trials of azacytidine (75 mg/m2 per day for 5 or 7 days)17,18 showed a 21% response rate for splenomegaly in MF using the 7-day regimen only.17 The lack of success for the 5-day regimen in the trial leads me to suggest the use of only the 7-day regimen at this time. Decitabine use in MF is in the early stages of testing, and trials are under way. The frequent visits required for administration and myelosuppression, the most common adverse effect, limit the use of these therapies in MF patients.
Low-intensity therapy for acute leukemia.
A range of myelosuppressive therapies most typically used for acute leukemia or higher-risk myelodysplastic syndromes have been used anecdotally to treat MF patients with refractory splenomegaly. Low-dose subcutaneous cytarabine and daunorubicin administered as a single agent (60 mg/m2 per day for 3 days) are included in this group. These strategies, however, have not been vetted through structured clinical trials, and I use them only in difficult cases in which clinical trial participation is not an option.
Splenectomy
Therapeutic splenectomy in MF
The experience with splenectomy in MF patients dates back to the beginning of the 20th century (Table 3).29 Progressive surgical series demonstrated a significant but slowly decreasing (because of improvements in surgical technique, antimicrobials, and patient selection) perioperative mortality rate. Although splenectomy can be helpful for improving symptoms in MF patients, it seems to have no clear effect on patient survival, disease course, or intramedullary manifestations of the disease.
Indications and outcomes for elective splenectomy in myelofibrosis: Mayo Clinic experience29
| Variable . | Result . |
|---|---|
| Preoperative | |
| Surgical indications | |
| Anemia | 78 (25%) |
| Symptomatic splenomegaly | 156 (49%) |
| Portal hypertension | 47 (15%) |
| Severe thrombocytopenia | 33 (11%) |
| Median intervals, mo (range) | |
| Diagnosis to splenectomy (n = 314) | 26.3 (0-389) |
| Follow-up period from diagnosis (n = 314) | 54.6 (0.6-487) |
| Follow-up period from splenectomy (n = 314) | 18.6 (0.2-151) |
| Perioperative | |
| Median spleen mass, g (range) | 2700 (380-11 750) |
| Complications, no. (%) | 87 (27.7) |
| Bleeding | 44 (14.0) |
| Thrombosis | 31 (9.9) |
| Infection | 31 (9.9) |
| Fatal complications, no. (%) | 21 (6.7) |
| Infection | 8 (2.6) |
| Hemorrhage | 7 (2.2) |
| Thrombosis | 2 (0.6) |
| Other | 5 (1.6) |
| Median hospital stay, d (range) | 9 (1-63) |
| Laboratory values at discharge, median (range) | |
| Hemoglobin, g/dL | 10 (7-16.3) |
| White blood cell count, ×109/L | 21.2 (1.2-217) |
| Platelet count, ×109/L | 256 (10-1968) |
| Patients (n, %) with > 450 × 109/L | 90 (28.7%) |
| Patients (n, %) with > 1000 × 109/L | 17 (5.4%) |
| Postsplenectomy outcomes | |
| Leukemic transformation | 45 (14.3%) |
| Accelerated hepatomegaly | 32 (10.2%) |
| Long-term improvements | |
| Symptomatic splenomegaly | 121/156 (48.8%) |
| Anemia | 39/78 (50.0%) |
| Portal hypertension | 19/47 (40.4%) |
| Severe thrombocytopenia | 10/33 (30.3%) |
| Survival after splenectomy (by indication) | |
| Overall | 19 mo (95% CI, 14-22) |
| Anemia | 70.1% (at 1 y) |
| Symptomatic splenomegaly | 66% (at 1 y) |
| Portal hypertension | 59.5% (at 1 y) |
| Severe thrombocytopenia | 66.7% (at 1 y) |
| Variable . | Result . |
|---|---|
| Preoperative | |
| Surgical indications | |
| Anemia | 78 (25%) |
| Symptomatic splenomegaly | 156 (49%) |
| Portal hypertension | 47 (15%) |
| Severe thrombocytopenia | 33 (11%) |
| Median intervals, mo (range) | |
| Diagnosis to splenectomy (n = 314) | 26.3 (0-389) |
| Follow-up period from diagnosis (n = 314) | 54.6 (0.6-487) |
| Follow-up period from splenectomy (n = 314) | 18.6 (0.2-151) |
| Perioperative | |
| Median spleen mass, g (range) | 2700 (380-11 750) |
| Complications, no. (%) | 87 (27.7) |
| Bleeding | 44 (14.0) |
| Thrombosis | 31 (9.9) |
| Infection | 31 (9.9) |
| Fatal complications, no. (%) | 21 (6.7) |
| Infection | 8 (2.6) |
| Hemorrhage | 7 (2.2) |
| Thrombosis | 2 (0.6) |
| Other | 5 (1.6) |
| Median hospital stay, d (range) | 9 (1-63) |
| Laboratory values at discharge, median (range) | |
| Hemoglobin, g/dL | 10 (7-16.3) |
| White blood cell count, ×109/L | 21.2 (1.2-217) |
| Platelet count, ×109/L | 256 (10-1968) |
| Patients (n, %) with > 450 × 109/L | 90 (28.7%) |
| Patients (n, %) with > 1000 × 109/L | 17 (5.4%) |
| Postsplenectomy outcomes | |
| Leukemic transformation | 45 (14.3%) |
| Accelerated hepatomegaly | 32 (10.2%) |
| Long-term improvements | |
| Symptomatic splenomegaly | 121/156 (48.8%) |
| Anemia | 39/78 (50.0%) |
| Portal hypertension | 19/47 (40.4%) |
| Severe thrombocytopenia | 10/33 (30.3%) |
| Survival after splenectomy (by indication) | |
| Overall | 19 mo (95% CI, 14-22) |
| Anemia | 70.1% (at 1 y) |
| Symptomatic splenomegaly | 66% (at 1 y) |
| Portal hypertension | 59.5% (at 1 y) |
| Severe thrombocytopenia | 66.7% (at 1 y) |
CI indicates confidence interval.
Current outcomes.
We recently analyzed 3 decades of experience with palliative splenectomy in MF at the Mayo Clinic30,31 to determine whether better control of postsplenectomy thrombocytosis and modern operative techniques and supportive care have diminished morbidity and mortality. Patients and outcomes are summarized in Table 2.32 Although meaningful improvement in symptoms was observed in 30% to 50% of patients, complication rates (27.7% overall, 6.7% fatal) are sobering and indicate that candidates for surgery should be chosen carefully and monitored closely in the perioperative period.
Candidates for splenectomy.
Given the potential dangers of splenectomy, the procedure should be considered only for patients who meet several criteria: substantial and refractory splenic symptoms (ie, requiring narcotics or significantly interfering with quality of life); status as adequate surgical candidates without decompensated coagulopathy or significant comorbidities; treated unsuccessfully with at least one medical therapy for splenomegaly (if eligible and able to enroll into a JAK2 inhibitor trial, “JAK2 inhibitors,” this should be considered); and have an adequate performance status and life expectancy (ie, > 1 year) to consider this surgical procedure. I would consider patients meeting these criteria with refractory cytopenias candidates for splenectomy, but they should be aware that the cytopenias may not improve. In addition, many of the constitutional symptoms of the disease (ie, fever, night sweats, and fatigue) are not linked to splenomegaly; therefore, improvement in these symptoms should not be expected. Splenectomy preceding allogeneic stem cell transplant speeds engraftment but without a significant improvement in outcomes; given the risks outlined in Table 3, routine pretransplantation splenectomy is not recommended.33
Perioperative management.
The major risk with splenectomy remains perioperative thrombosis and hemorrhage; given that fact, our practice at Mayo Clinic has evolved to aggressively control postoperative platelet counts. Indeed, in our analysis, perioperative thrombohemorrhagic complications decreased in the last decade because we use platelet apheresis as necessary and promptly administer cytoreductive agents to counteract postsplenectomy thrombocytosis.32 I now use hydroxyurea to treat patients with postoperative platelet counts rising above 300 × 109/L and plateletpheresis to treat those with counts exceeding 450 × 109/L, within postoperative weeks 1 and 2, although these guidelines have not been vetted by a randomized trial.
Splenic radiotherapy
Radiotherapy for MF
Radiotherapy has several uses in MF (Table 4). EMH is exquisitely sensitive to external beam radiotherapy in patients with MF, regardless of location. In patients with MF, the lungs41 (where EMH can contribute to pulmonary hypertension), paraspinal masses,6 and spleen are frequently irradiated sites. Splenomegaly can be treated with external beam radiotherapy for palliation, but the benefit is typically transient and myelosuppression can be severe.
Published experience with splenic radiotherapy for myelofibrosis
| Reference . | Patients, n . | Dose, cGy . | Fractions . | Response/comments . |
|---|---|---|---|---|
| Silverstein34 | 39 | NR | NR | 63% response rate |
| Greenberger et al35 | 14 | 600 | 9 | 95% response rate; significant cytopenias in 57% |
| Slanina et al36 | 25 | NR | NR | 88% with symptomatic decrease in splenomegaly |
| Wagner et al37 | 10 | 150-300 | 6-10 | Responses in 90%; cytopenias common |
| Elliott et al38 | 23 | 277 | 7.5 | 93.9% decrease in spleen size; median duration of response 6 months; toxicities: cytopenias, complicated subsequent splenectomy |
| Bouabdallah et al39 | 15 | 980 | 22 | 59% response rate; toxicities: cytopenias |
| McFarland et al40 | 4 | 450 | 9 | All patients with a palliative response; toxicities: cytopenias |
| Reference . | Patients, n . | Dose, cGy . | Fractions . | Response/comments . |
|---|---|---|---|---|
| Silverstein34 | 39 | NR | NR | 63% response rate |
| Greenberger et al35 | 14 | 600 | 9 | 95% response rate; significant cytopenias in 57% |
| Slanina et al36 | 25 | NR | NR | 88% with symptomatic decrease in splenomegaly |
| Wagner et al37 | 10 | 150-300 | 6-10 | Responses in 90%; cytopenias common |
| Elliott et al38 | 23 | 277 | 7.5 | 93.9% decrease in spleen size; median duration of response 6 months; toxicities: cytopenias, complicated subsequent splenectomy |
| Bouabdallah et al39 | 15 | 980 | 22 | 59% response rate; toxicities: cytopenias |
| McFarland et al40 | 4 | 450 | 9 | All patients with a palliative response; toxicities: cytopenias |
NR indicates not reported.
Candidates for splenic radiotherapy.
Splenic radiation is effective for palliating MF-associated splenomegaly, but the effects are transient. In addition, the abscopal effects of the radiotherapy can lead to significant myelosuppression and profound thrombocytopenia. Splenic radiotherapy is of greatest utility for patients with considerable symptoms and an adequate platelet count who, because of age or comorbidities, probably would not undergo splenectomy in the future.
Reported outcomes for splenic radiotherapy.
Several reports (Table 3) have described the palliative benefit of external beam radiotherapy for improving symptomatic splenomegaly in MF.38,39 The Mayo Clinic experience38 included a group of 23 MF patients (who received a total of 49 courses of radiotherapy) who received a median radiation course of 277 cGy in a median of 8 fractions. An objective decrease in spleen size was noted after 94% of radiotherapy courses; however, 44% of patients experienced posttreatment cytopenias (26% were severe, 13% fatal). Splenic radiation also seemed to increase morbidity and mortality of subsequent splenectomy when undertaken. This latter effect seems related to the development of splenic adhesions to the abdominal wall and surrounding viscera, leading to greater complexity with subsequent attempts at surgical extirpation. In addition, in irradiated areas, delayed hemorrhage was common, resulting in blunt dissection of the spleen away from other structures.
Experimental medical therapy
JAK2 inhibitors
The discovery of several key myeloproliferative disorder-associated mutations has broadened the therapeutic horizons forMF significantly (Table 5).42 Discovery of the JAK2V617F mutation in the 14th exon of JAK2 was followed by the 10 mutations currently described in the 12th exon of JAK2. In addition, 5 constitutively activating mutations thus far have identified in the thrombopoietin receptor, MPL, which signals through JAK2. All of these mutations seem to feed into a final common pathway of cellular activation through the PI3 kinase pathway, the STAT pathway, and the MAP kinase pathway.42
Experimental therapies for myelofibrosis with reported efficacy for symptomatic splenomegaly
| . | Anemia . | Splenomegaly . | Constitutional symptoms . | Pruritus . | Reference . |
|---|---|---|---|---|---|
| INCB018424 | < 10% | X | X | X | 43 |
| CEP-701 | < 10% | X | — | X | 47 |
| XL019 | — | X | X | X | 46 |
| TG101348 | — | X | X | X | 45 |
| ITF2357 | — | X | X | X | 48 |
| . | Anemia . | Splenomegaly . | Constitutional symptoms . | Pruritus . | Reference . |
|---|---|---|---|---|---|
| INCB018424 | < 10% | X | X | X | 43 |
| CEP-701 | < 10% | X | — | X | 47 |
| XL019 | — | X | X | X | 46 |
| TG101348 | — | X | X | X | 45 |
| ITF2357 | — | X | X | X | 48 |
— indicates not applicable; and X, instance.
The best-studied clinical experience for a JAK2 inhibitor is for INCB018424 (Incyte, Wilmington, DE), which is selective against JAK1 and JAK2; this agent leads to significant reduction in splenomegaly and dramatic improvement in constitutional symptoms43 with toxicities of thrombocytopenia and anemia43 (Table 5). In addition, the adverse increase in inflammatory cytokines observed in myelofibrosis seem to improve with INCB018424 therapy and correlate with symptom improvement.44 Other drugs currently being tested include TG101348, a selective JAK2 inhibitor (TarGen, San Francisco, CA)45 ; XL019, a selective JAK2 inhibitor (Exelexis, San Francisco, CA)46 ; CEP-701, a tyrosine kinase inhibitor of JAK2 and FLT3 (Cephalon, Frazer, PA)47 ; and ITF2357, a histone deacetylase inhibitor (Italfarmaco, Milan, Italy).48 With each agent, preliminary results also report improvements in splenomegaly and symptoms in MF patients (Table 4), although various toxicities seen (ie, gastrointestinal, neuropathy; XL019) all share the potential for causing anemia and/or thrombocytopenia. To date, no JAK2 inhibitor has reported a significant ability to improve cytopenias, fibrosis, or histologic changes associated with MF. It should be mentioned that the JAK2 inhibitor trials are all in the preliminary stages of reporting (ie, abstracts from the last American Society of Hematology meeting), with all trials remaining active and results not published in manuscript form at this time.
Patients with symptomatic splenomegaly who qualify for and have access to a JAK2 inhibitor trial should be considered for enrollment. This is particularly the case for MF patients without baseline anemia or thrombocytopenia, who have constitutional symptoms.
Future directions
Current management of symptomatic splenomegaly in MF patients requires thoughtful consideration of prognosis and treatment goals. For those who are not candidates for transplantation, the palliative benefit of a specific therapeutic choice must be balanced against its potential risks. For those treating MF patients, the JAK2 inhibitors have brought great excitement because of their targeted approach. Preliminary data indicate a meaningful response for MF patients in terms of symptomatic splenomegaly. However, although the JAK2 inhibitors have provided a valuable and incremental benefit over existing options, particularly for symptoms and quality of life, they have not yet demonstrated a significant impact for anemia or more advanced disease features. Future advances for MF patients may require further vetting of current and future JAK2 inhibitors, trials of JAK2 inhibitors combined with other agents (ie, such as an IMiD against anemia), or further advances in our understanding of the pathogenesis of the disorder to open additional avenues of targeted therapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The author acknowledges the longstanding collaboration and mentorship of Dr Ayalew Tefferi and the collaboration of his colleagues at Mayo Clinic during the author's 18 years there in the care and clinical investigation of patients with myelofibrosis.
Authorship
Contribution: R.A.M. wrote the manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Ruben A. Mesa, Division of Hematology-Oncology, Mayo Clinic Arizona, 13400 E Shea Blvd, Scottsdale, AZ 85259; e-mail: mesa.ruben@mayo.edu.