Abstract
Dendritic cells (DCs) are the most potent antigen-presenting cells for naive T cells. In this study, scavenger receptor class A type I and type II (SR-A) were shown to be expressed by peripheral blood DCs (PBDCs) and monocyte-derived DCs (MDDCs). In addition, the binding of anti–SR-A antibody to these cells was lower in the presence of fucoidan, an SR-A agonist. Treatment of these DCs with fucoidan or anti–SR-A antibody markedly increased the surface expression of costimulatory molecules CD83 and major histocompatibility complex class II on the CD11chighCD123low myeloid subset of PBDCs. Furthermore, fucoidan-treated PBDCs produced tumor necrosis factor-α (TNF-α) but not IL-12p70. In addition, fucoidan-induced maturation was eliminated by pretreatment with TNF-α–neutralizing antibody. Finally, interferon-γ secretion and T-cell proliferation were enhanced by coculture of T cells with fucoidan-matured PBDCs. Specific inhibitors of p38 MAPK and glycogen synthase kinase 3 suppressed TNF-α production and maturation of fucoidan-treated PBDCs. Moreover, MDDCs lacking SR-A failed to up-regulate CD83 expression, TNF-α production, and phosphorylation of p38 MAPK and glycogen synthase kinase 3-β in the presence of fucoidan. Taken together, these results suggest that ligation of SR-A leads to induction of TNF-α, which subsequently induces PBDC maturation, thereby leading to enhanced T-cell stimulatory capacity.
Introduction
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) that originate from bone marrow and play major roles in the regulation of immune responses to various antigens.1 Human blood DCs include CD11chighCD123low myeloid DCs (mDCs) and CD11clowCD123high plasmacytoid DCs (pDCs).2,3 Circulating monocytes4 and cord blood CD34+ precursors5 can be used as a major source of mDCs precursors in culture. In addition, pDCs develop from hematopoietic stem cells in the presence of Flt3 ligand.6 DCs exist in at least 2 functionally and phenotypically distinct stages, immature and mature DCs.7 Immature DCs phagocytose antigens, after which they become activated and migrate to local lymph tissues to present the major histocompatibility complex (MHC)–bound antigenic peptide to T cells.8 In addition, DCs can become mature after treatment with exogenous or endogenous stimuli, such as lipopolysaccharide (LPS),9 tumor necrosis factor-α (TNF-α),8,10 bacterial nucleic acid,11 or CD40 ligand.12
Macrophages and DCs can directly sense pathogen components via pattern recognition receptors, such as Toll-like receptors (TLRs), scavenger receptors (SRs), C-type lectins, mannose receptors, and complement receptors.13 Class A SRs are composed of AI, AII, AIII, macrophage receptor with collagenous structure, and SR with C-type lectin.14 SR type AI and AII (SR-A), which are trimeric membrane glycoproteins, are generated by alternative splicing of one gene; however, there are no functional differences between the AI and AII receptors, whereas SR-A type III is nonfunctional.14 SR-A expression is primarily restricted to cells belonging to the myeloid lineage and is multifunctional, being responsible for macrophage adhesion, endocytosis of modified lipoproteins, and phagocytosis of apoptotic cells.15
It has been shown that SR-A is expressed on mouse splenic DCs and mouse bone marrow–derived DCs cultured with granulocyte macrophage-colony stimulating factor (GM-CSF), but not with Flt3 ligand.16 In addition, SR-A expression plays an important role in the internalization of Gram-negative bacteria.17 However, although engagement of various TLRs on DCs results in their maturation and migration to lymph nodes,18,19 the role that SR-A agonists play in the maturation or activation of DCs has not been investigated. The addition of the oxidized low-density lipoprotein, an SR-A binding lipoprotein, during monocyte differentiation was found to give rise directly to phenotypically mature DCs; however, this treatment did not trigger maturation of monocyte-derived DCs (MDDCs).20 In addition, Ge et al showed that advanced glycosylation end products up-regulated SR-A expression on DCs and induced DC maturation through receptor for advanced glycosylation end products, but that they did not provide direct evidence of the involvement of SR-A in DC maturation.21 Conversely, it has been shown that SR-A–deficient DCs exhibited a more mature phenotype after LPS stimulation,16,22 which suggests that SR-A acts as an inhibitory receptor that limits proinflammatory responses.
Fucoidan is a natural sulfated polysaccharide that is isolated from brown algae23 and is a well-defined nonlipoprotein ligand for SR-A.24 Although fucoidan has various biologic activities,25 its effects on DCs are not yet fully understood. Therefore, we evaluated whether human blood DCs isolated from blood ex vivo, as well as differentiated DCs from monocytes grown in culture, expressed SR-A receptor and could be induced to mature in response to fucoidan, an SR-A agonist.
Methods
Chemicals and antibodies
We purified fucoidans from the brown algae, Fucus evanescens, using a method that has been previously described,26 or Fucus vesiculosus fucoidan obtained from Sigma-Aldrich (St Louis, MO). The endotoxin levels in commercially obtained and purified fucoidans were evaluated using a Limulus amebocyte lysate assay kit (Lonza Walkersville, Walkersville, MD). Isotype control antibodies (Abs; IgG1 or IgG2b), anti-CD14 (IgG1, 61D3), anti-CD1a (IgG1, HI149), anti-CD64 (IgG1, 10.1), anti-CD80 (IgG1, 2D10.4), and anti-CD11c (IgG1, 3.9) Abs were obtained from eBioscience (San Diego, CA); anti-CD86 (IgG1, 2331), anti-CD83 (IgG1, HB15e), anti-CD40 (IgG1, 5C3), and anti-HLA-DR, -DP, -DQ (MHC class II) Abs (IgG1, clone Tu39) were purchased from BD Biosciences PharMingen (San Diego, CA); anti–SR-A Ab (goat polyclonal IgG1) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA); and neutralizing Ab against human TNF-α (IgG1, 6401) and CD40 (IgG1, 82102) were purchased from R&D Systems (Minneapolis, MN). Abs against phosphorylated form of p38 mitogen-activated protein kinase (MAPK), AKT, glycogen synthase kinase 3-α/β (GSK3), and phospho-IκB kinase (IKK) were obtained from Cell Signaling Technology (Danvers, MA). LPS (Escherichia coli strain O111:B4) and other chemicals were purchased from Sigma-Aldrich.
Generation and sorting of DCs
Peripheral blood DCs (PBDCs) were isolated from the peripheral blood mononuclear cells by magnetically activated cell sorting using a BDCA-1+ cell (CD1c+ mDC subset), a BDCA-2+ cell (pDC subset), or a blood DC isolation (BDCA-1+, -2+, -4+ cells) kit II from Miltenyi Biotec (Bergisch Gladbach, Germany).27 Informed consent was obtained from all volunteers in accordance with the Declaration of Helsinki, and the Institutional Review Board at Dong-A University Hospital approved this study. The purity of isolated PBDCs was found to be more than 90%. For some experiments, CD11chighCD123low or CD11clowCD123high cells were sort purified using a desktop cell sorter JSAN (Bay Science, Kobe, Japan), to gate out contaminating cells. MDDCs were generated according to an established method with minor modifications.9 Briefly, monocytes were purified from mononuclear cells by positive selection using a magnetic-activated cell sorting column containing microbeads conjugated with Abs to CD14 (Miltenyi Biotec). Immature MDDCs (iMDDCs) were then differentiated from the monocytes (106) by incubation with 15 ng/mL GM-CSF plus 10 ng/mL interleukin-4 (IL-4) for 6 days. The differentiation of the DCs was monitored by the up-regulation of CD1a expression and down-regulation of CD14 expression.
Flow cytometry
Cells were washed with phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin, preincubated for 15 minutes with unlabeled isotype control Abs, and then labeled with either fluorescein isothiocyanate– or phycoerythrin (PE)–conjugated Abs by incubation on ice for 30 minutes followed by washing with PBS. Cellular debris was then eliminated from the analysis using a gate on forward and side scatter. A viability gate was set using 7-aminoactinomycin D (7-AAD) staining. The 7-AAD–negative population was subsequently analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). As a control for nonspecific staining, isotype-matched irrelevant Abs were used.
Alexa Fluor labeling of anti–SR-A Ab
Alexa Fluor 488 dye–labeled anti–SR-A Ab was prepared using an Alexa Fluor 488 Ab labeling kit (Invitrogen, Carlsbad, CA). Briefly, anti–SR-A Ab solution containing sodium bicarbonate was incubated with reactive dye for 1 hour at room temperature. Alexa 488–labeled anti–SR-A Ab solution was then purified using a size-exclusion column.
siRNA transfection of SR-A
The siRNA sequence used for the targeted silencing of SR-A (GenBank accession no. NM-002445) was designed by Dharmacon RNA Technologies (Lafayette, CO).28 iMDDCs (106) were left unmanipulated, transfected with Dharmafect alone, with 200 nM nonsilencing siRNA plus Dharmafect or with 200 nM SR-A siRNA plus Dharmafect for 24 hours.
ELISA
The cells were cultured in RPMI-1640 medium containing LPS, fucoidan, or TNF-α for the indicated times. The IL-10, IL-12p40, IL-12p70, interferon-γ (IFN-γ), and TNF-α concentrations in the supernatants were then measured in triplicate using standard enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems or BD Biosciences PharMingen), with standard cytokine preparations being used as the internal controls.
Allogeneic MLR
CD4+ T cells were purified from the peripheral blood using a column containing microbeads conjugated with Abs to CD4. The activated DCs were treated with 50 μg/mL mitomycin C for 1 hour, after which they were added to 105 T cells in 96-well microtiter culture plates. The mixtures were then cultured for 5 days at 37°C in RPMI 1640 medium less than 5% CO2, after which they were pulsed for 18 hours with 1 μCi [3H]thymidine (PerkinElmer Life and Analytical Sciences, Waltham, MA). The cells were then harvested and the amount of radioactivity incorporated was determined. In another mixed leukocyte reaction (MLR) system, CD4+ T cells were labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen), and proliferation was then analyzed for CFSE dilution in proliferating cells.
Western blotting
Cultured cells (5 × 106) were suspended in a lysis buffer containing 20 mM Tris-HCl (pH 7.4), 50 mM NaCl, 1% Triton X-100, and protease inhibitors. The lysates were then subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, after which they were transferred to nitrocellulose membranes that were blocked for 1 hour at 25°C with a blocking buffer and then incubated with primary Abs in blocking buffer overnight at 4°C. The samples were treated with secondary Abs in a blocking buffer. Signals were detected by enhanced chemiluminescence (GE Healthcare, Little Chalfont, United Kingdom).
Confocal microscopic analysis
Cells were fixed for 15 minutes in 3% paraformaldehyde in PBS and then permeabilized for 10 minutes in 0.1% Triton X-100 in PBS. Next, the slides were incubated in blocking buffer at 25°C for 1 hour. The cells were washed repeatedly, after which they were incubated at 4°C for 1 hour with PE-conjugated anti-CD80 Ab and fluorescein isothiocyanate–conjugated MHC class II Ab, and then washed again in PBS. The samples were then mounted using glycerol and analyzed by confocal microscopy (Carl Zeiss LSM 510; Carl Zeiss, Thornwood, NY).
Statistical analysis
Results are presented as the mean plus or minus SD. A Student t test for unpaired samples was used to compare the means. A P value of less than .05 was considered significant.
Results
SR-A was detected on blood-derived DCs
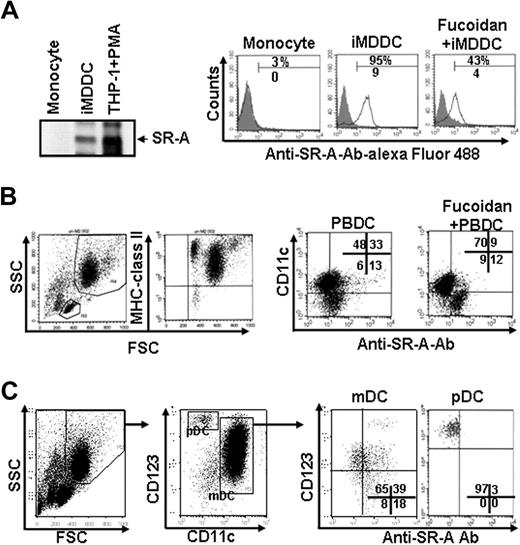
SR-A proteins were not detected in freshly isolated monocytes, but DC differentiation of monocytes with GM-CSF and IL-4 resulted in higher expression of SR-A (Figure 1A). Furthermore, when iMDDCs or monocytes were mixed with Alexa Fluor 488–conjugated anti–SR-A Abs, anti–SR-A Abs showed specific binding to iMDDCs but not monocytes. In addition, the binding of anti–SR-A Abs to iMDDCs decreased in response to the addition of either F vesiculosus or F evanescens fucoidan (82% ± 13% vs 42% ± 10%; Figure 1A). However, it is thought that iMDDCs produced with GM-CSF arise under inflammatory conditions and are not representative of DCs produced under steady-state conditions.29 Moreover, the presence of SR-As on blood DCs has not yet been reported. Therefore, we isolated blood BDCA+ DCs ex vivo and then used these cells directly for this study. These PBDCs were purity-checked and confirmed to be immature DCs (CD80−, CD83−, MHC class II+) by flow cytometry. The purified MHC class II+ cells were gated into either the CD11clow or CD11chigh population, and the binding of anti–SR-A Ab to each of these populations was then measured. CD11c+ cells were found to make up approximately 80% of the total isolated PBDCs, and Alexa-conjugated anti–SR-A Ab was bound primarily to CD11c+ cells (Figure 1B). In addition, fucoidan decreased the binding of anti–SR-A Ab to PBDCs, similar to the results observed when iMDDCs were evaluated. Furthermore, when the isolated PBDCs were gated into CD11c and CD123 because PBDCs contained the CD11chighCD123low mDC and CD11clowCD123high pDCs, Alexa-conjugated anti–SR-A Ab was bound to the CD11c+ mDC subtype but not in the CD123high pDC subtype (Figure 1C). These results suggest that blood mDCs express SR-A and that fucoidan is able to bind to the receptors on these cells. Therefore, we investigated the maturation of human blood DCs using fucoidan.
Expression of SR-A on blood DCs and inhibition of anti–SR-A Ab binding by fucoidan. (A) iMDDCs were prepared by culturing monocytes (106) with GM-CSF and IL-4 for 6 days. (Left panel) The cells were harvested and the cell lysates (30 μg/mL) were subjected to immunoblot analyses using an anti–human SR-A Ab. The cell lysates from PMA-stimulated THP-1 cells were used as a positive control. (Right panel) Monocytes and iMDDCs were pretreated with isotype-matched Ab for 1 hour and then incubated with Alexa Fluor 488–conjugated anti–SR-A Ab (5 μg/mL) in the presence or absence of fucoidan (50 μg/mL) for 30 minutes at 4°C, after which they were analyzed by fluorescence-activated cell sorter. (B) PBDCs were isolated using BDCA-1, -2, and -4–conjugated magnetic beads from peripheral blood mononuclear cells. The cells were incubated with Alexa-conjugated anti–SR-A Ab and PE-conjugated CD11c in the presence or absence of fucoidan for 30 minutes, and Ab binding was then analyzed. (C) CD11chighCD123low (mDC) and CD11clowCD123high (pDC) cells were gated and examined for anti–SR-A Ab binding. One representative experiment of 3 is shown.
Expression of SR-A on blood DCs and inhibition of anti–SR-A Ab binding by fucoidan. (A) iMDDCs were prepared by culturing monocytes (106) with GM-CSF and IL-4 for 6 days. (Left panel) The cells were harvested and the cell lysates (30 μg/mL) were subjected to immunoblot analyses using an anti–human SR-A Ab. The cell lysates from PMA-stimulated THP-1 cells were used as a positive control. (Right panel) Monocytes and iMDDCs were pretreated with isotype-matched Ab for 1 hour and then incubated with Alexa Fluor 488–conjugated anti–SR-A Ab (5 μg/mL) in the presence or absence of fucoidan (50 μg/mL) for 30 minutes at 4°C, after which they were analyzed by fluorescence-activated cell sorter. (B) PBDCs were isolated using BDCA-1, -2, and -4–conjugated magnetic beads from peripheral blood mononuclear cells. The cells were incubated with Alexa-conjugated anti–SR-A Ab and PE-conjugated CD11c in the presence or absence of fucoidan for 30 minutes, and Ab binding was then analyzed. (C) CD11chighCD123low (mDC) and CD11clowCD123high (pDC) cells were gated and examined for anti–SR-A Ab binding. One representative experiment of 3 is shown.
Maturation of iMDDCs by fucoidan
Culture of iMDDCs with fucoidans (50 μg/mL) from F evanescens and F vesiculosus for 24 hours resulted in a marked up-regulation of the surface expression of CD40, CD80, CD83, and MHC class II (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Immunofluorescence and morphologic studies using confocal microscopic analysis also showed morphologic changes and enhanced expression levels of CD80 and MHC class II in fucoidan-matured MDDCs compared with iMDDCs (Figure S1B). The appropriate dose of F evanescens and F vesiculosus fucoidans necessary to induce the maturation of iMDDCs was determined by performing dose-dependent experiments of CD83 expression on the cell surface (Figure S1C). We observed no difference in the maturation effect among fucoidans obtained from different sources.
Maturation of PBDCs by fucoidan
The expression level of CD83 after culture with fucoidan for 24 hours was measured as an indicator of maturation. When cultured in medium alone, the viable cell number was found to be decreased based on forward- and side-scatter characteristics and 7-AAD staining. However, the total viable cell number was higher in fucoidan-treated PBDCs than in untreated controls (data not shown). In addition, fucoidan, LPS, and TNF-α each significantly increased CD83 expression on CD11c+ DCs among purified total PBDCs (Figure 2A). The percentage of CD83+ cells in the population of CD11c+ cells was increased to 30% to 55% by fucoidan, whereas the number of CD83+ cells in the population of CD11clow cells was increased by either fucoidan (13% ± 6%) or LPS (16% ± 5%), but not to levels as high as those observed in the CD11c+ population. Fucoidan also induced strong up-regulation of CD80, CD86, and MHC class II on CD11c+ PBDCs. Next CD11chighCD123low DCs were purified using a cell sorter to gate out contaminating CD11c− cells and then treated with fucoidan. Similar results were obtained with fucoidan as with LPS when CD83 expression of the purified CD11c+ cells was evaluated (Figure 2B). The CD11c+CD1c+ subset has been shown to be the most active in phenotype among 5 different subtypes (CD1c+, CD16+, CD34+, BDCA3+, and CD123highCD11c−) of circulating blood DCs.30 Therefore, lineage−CD1c+ DCs were positively selected after depletion of CD19+ cells, and CD83 expression was measured after fucoidan treatment. As shown in Figure 2C, an increased CD83 expression was observed on purified CD11c+CD1c+ DCs after culture with fucoidan or LPS. Next, the possibility that the production of cytokines by pDCs on activation by fucoidan was responsible for expression of CD83 on CD123highCD11c− DCs was evaluated. To accomplish this, CD123highCD11c− pDCs were purified using anti-BDCA4+ magnetic beads or a cell sorter and then treated with fucoidan or LPS. Fucoidan and LPS stimulation of pDCs resulted in no significant up-regulation of CD83 (data not shown). Taken together, these results indicate that mDC subtype of PBDCs, but not pDCs, is able to be matured by fucoidan, an SR-A agonist.
Effects of fucoidan on the maturation of PBDCs. (A) Freshly isolated PBDCs (106) were treated with or without fucoidan (50 μg/mL), LPS (1 μg/mL), or TNF-α (10 ng/mL) for 24 hours as in Figure 1, and the surface expression of CD83, CD80, CD86, or MHC class II on CD11c+ DCs was then measured. (B) CD11chighCD123low mDCs were isolated using a BDCA isolation kit and further purified using a cell sorter and then treated as in panel A. (C) CD1c+ DCs were isolated using a BDCA-1 isolation kit and then treated as in panel A. Data represent the mean ± SD of 3 independent experiments. *P < .01 compared with none. **P < .001 compared with none.
Effects of fucoidan on the maturation of PBDCs. (A) Freshly isolated PBDCs (106) were treated with or without fucoidan (50 μg/mL), LPS (1 μg/mL), or TNF-α (10 ng/mL) for 24 hours as in Figure 1, and the surface expression of CD83, CD80, CD86, or MHC class II on CD11c+ DCs was then measured. (B) CD11chighCD123low mDCs were isolated using a BDCA isolation kit and further purified using a cell sorter and then treated as in panel A. (C) CD1c+ DCs were isolated using a BDCA-1 isolation kit and then treated as in panel A. Data represent the mean ± SD of 3 independent experiments. *P < .01 compared with none. **P < .001 compared with none.
Effects of SR-A knockdown and anti–SR-A Ab on the maturation of blood DCs by fucoidan
We analyzed the levels of the maturation markers on fucoidan-treated DCs in which SR-A was knocked down using siRNA. For this experiment, iMDDCs were used instead of PBDCs because the numbers of purified PBDCs were too low to perform knockdown experiments. As shown in Figure 3A, knockdown of SR-A in iMDDCs was demonstrated by decreased protein levels as analyzed by flow cytometry and Western blot. The CD83 expression of SR-A–deficient and control siRNA-treated iMDDCs that were treated with various concentrations of LPS was similar (Figure 3B). However, SR-A–deficient iMDDCs failed to express CD83 in the presence of fucoidan.
SR-A–mediated maturation of fucoidan-stimulated blood DCs. (A) iMDDCs (106) were left untreated (Untreated) or transfected with DharmaFECT alone (Shock), 200 nM siRNA of SR-A (SR-A siRNA), or 200 nM nontargeting siRNA (Non-siRNA) for 24 hours. Next, the cells were harvested and incubated with Alexa Fluor 488–conjugated anti–SR-A Ab for 30 minutes at 4°C and then analyzed by fluorescence-activated cell sorter. (Bottom panel) The expression of SR-A proteins in siRNA-transfected iMDDCs was then measured by Western blot. (B) iMDDCs were treated and harvested as in panel A. Control (Non-siRNA) and SR-A–deficient iMDDCs were then stimulated with the indicated concentrations of fucoidan or LPS for 24 hours, after which the expression level of CD83 was measured by flow cytometry. *P < .01 compared with Non-siRNA. (C) PBDCs were pretreated with control IgG or anti–SR-A Ab (5 μg) for 1 hour and further cultured in the presence or absence of fucoidan. After 24 hours, CD11chighCD123low and CD11clowCD123high cells were gated and examined for CD83 expression. (Top panel) The expression levels of CD123, CD11c, and CD83 on freshly isolated PBDCs. (D) CD11c+ mDCs were purified using a cell sorter as in Figure 2B and then treated with control IgG or anti–SR-A Ab for 24 hours. The results shown represent the mean ± SD of 3 independent experiments.
SR-A–mediated maturation of fucoidan-stimulated blood DCs. (A) iMDDCs (106) were left untreated (Untreated) or transfected with DharmaFECT alone (Shock), 200 nM siRNA of SR-A (SR-A siRNA), or 200 nM nontargeting siRNA (Non-siRNA) for 24 hours. Next, the cells were harvested and incubated with Alexa Fluor 488–conjugated anti–SR-A Ab for 30 minutes at 4°C and then analyzed by fluorescence-activated cell sorter. (Bottom panel) The expression of SR-A proteins in siRNA-transfected iMDDCs was then measured by Western blot. (B) iMDDCs were treated and harvested as in panel A. Control (Non-siRNA) and SR-A–deficient iMDDCs were then stimulated with the indicated concentrations of fucoidan or LPS for 24 hours, after which the expression level of CD83 was measured by flow cytometry. *P < .01 compared with Non-siRNA. (C) PBDCs were pretreated with control IgG or anti–SR-A Ab (5 μg) for 1 hour and further cultured in the presence or absence of fucoidan. After 24 hours, CD11chighCD123low and CD11clowCD123high cells were gated and examined for CD83 expression. (Top panel) The expression levels of CD123, CD11c, and CD83 on freshly isolated PBDCs. (D) CD11c+ mDCs were purified using a cell sorter as in Figure 2B and then treated with control IgG or anti–SR-A Ab for 24 hours. The results shown represent the mean ± SD of 3 independent experiments.
Next, the anti–SR-A Ab was used to determine whether it had an effect on the maturation of PBDCs by gating fucoidan- and/or anti–SR-A Ab-treated PBDCs into 2 different subsets (CD11clowCD123high and CD11chighCD123low) and then measuring the expression level of CD83. CD83 was not detected on freshly isolated CD11clowCD123high PBDCs (Figure 3C). However, anti–SR-A Ab treatment significantly increased the CD83 expression on the CD11chighCD123low subtype, but not on the CD11clowCD123high subtype, compared with the isotype control IgG, although the expression levels in the CD11chighCD123low subtype were much lower than that in cells that were exposed to fucoidan (Figure 3C). Furthermore, pretreatment of PBDCs with anti–SR-A Ab did not prevent the expression of CD83 that was induced by fucoidan. In addition, the percentage of CD83+ cells in the population of sorted CD11c+ mDCs was increased by anti–SR-A Ab (Figure 3D). Taken together, these data suggest that ligation of Ab to SR-A exerts activation of PBDCs rather than inhibition of the effect of the SR-A agonist. In addition, we tested the effect of neutralizing Abs specific for receptors other than SR-A, including CD62L, CD11a, CD11b, CD15, CD18, CD32, CD36, RAGE, and TLR-2, on fucoidan-induced maturation of iMDDCs. However, none of the other Abs evaluated had an effect on the expression of CD80, CD83, CD86, and MHC class II molecules in cells that were cultured in the presence or absence of fucoidan (data not shown). Taken together, these data indicate that the binding of fucoidan to SR-A is able to induce the maturation of blood DCs.
Fucoidan-induced maturation of PBDCs via TNF-α production
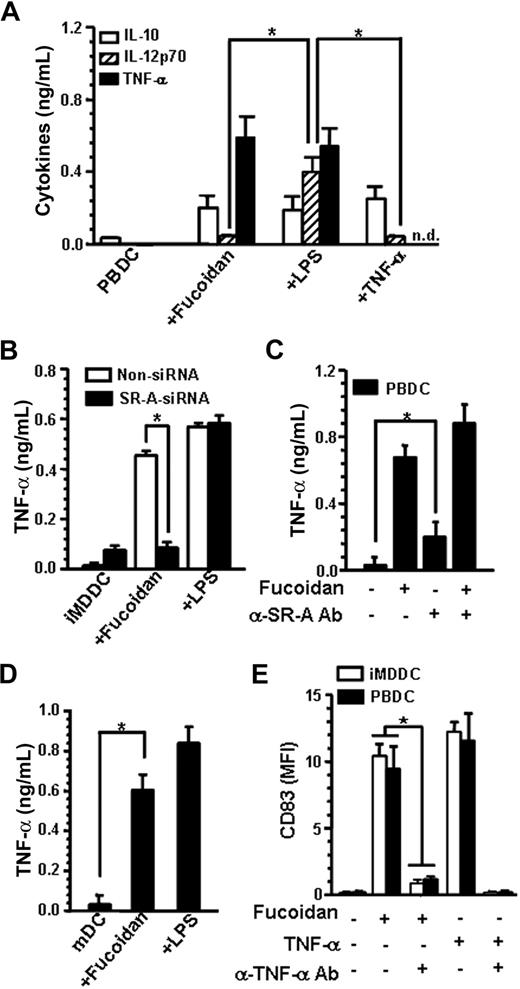
The ability of fucoidan-treated DCs to produce cytokines was evaluated. Unlike LPS-matured DCs, fucoidan-matured PBDCs and MDDCs were unable to produce IL-12p70 (Figures 4A, S2A). In addition, fucoidan-matured MDDCs and PBDCs, but not untreated controls, were able to produce a significantly high amount of TNF-α, which is known for its contribution to DC maturation.10 Furthermore, when PBDCs and iMDDCs were incubated with exogenous TNF-α alone for 24 hours, IL-12p70 was not produced. In addition, SR-A–deficient iMDDCs failed to secrete TNF-α in the presence of fucoidan (Figure 4B). Next, we investigated whether ligation of Ab to SR-A on PBDCs affected the TNF-α production induced by fucoidan. TNF-α production by PBDCs in the presence of fucoidan was not affected by anti–SR-A Ab (Figure 4C). The concentrations of TNF-α produced by PBDCs cultured with anti–SR-A Ab alone were significantly greater than those of untreated cells, even though the secretion of TNF-α by Abs was lower than that of fucoidan-matured PBDCs. Similarly, fucoidan stimulation resulted in the production of TNF-α by purified CD11c+ mDC using a cell sorter (Figure 4D). However, pDCs that were stimulated with LPS or anti–SR-A Ab produced no or little TNF-α, respectively, compared with untreated pDCs (data not shown). Finally, a neutralizing Ab against TNF-α significantly abrogated the effects of fucoidan on CD83 expression by both PBDCs and iMDDCs (Figure 4E). It has been suggested that SR-A down-regulates the maturation response of mouse bone marrow–derived DCs to LPS.16 However, we found that fucoidan was unable to inhibit the up-regulation of iMDDC surface makers by LPS (Figure S2B). Moreover, IL-12p70 production by LPS was not suppressed in the presence of fucoidan, which indicates that fucoidan treatment may not inhibit IL-12p70 production in the presence or absence of LPS (Figure S2B). Taken together, these results demonstrate that SR-A–mediated TNF-α plays an important role in the maturation of blood DCs by fucoidan.
TNF-α mediates fucoidan-induced maturation of blood DCs. (A) After PBDCs (106) were treated with or without fucoidan, LPS, or TNF-α for 24 hours, the supernatant of the cultures was collected, and the concentrations of cytokines were measured by ELISA. (B) iMDDCs were treated with SR-A siRNA as in Figure 3A and then incubated with or without fucoidan (50 μg/mL) or LPS (1 μg/mL). After 24 hours, TNF-α secretion was measured by ELISA. (C) PBDCs were incubated with or without 5 μg/mL anti–SR-A Ab for 1 hour, then further cultured in the presence or absence of fucoidan for 24 hours. (D) CD11c+ mDCs were purified from lin−BDCA+ cells using a cell sorter and treated with or without fucoidan or LPS as in Figure 2B. After 24 hours, TNF-α secretion was measured by ELISA. (E) PBDCs and iMDDCs were then pretreated with neutralizing Ab against TNF-α (1 μg) for 1 hour, then further incubated in the presence of fucoidan or TNF-α for 24 hours. The results shown represent the mean ± SD of 3 independent experiments. *P < .01; n.d. indicates not determined.
TNF-α mediates fucoidan-induced maturation of blood DCs. (A) After PBDCs (106) were treated with or without fucoidan, LPS, or TNF-α for 24 hours, the supernatant of the cultures was collected, and the concentrations of cytokines were measured by ELISA. (B) iMDDCs were treated with SR-A siRNA as in Figure 3A and then incubated with or without fucoidan (50 μg/mL) or LPS (1 μg/mL). After 24 hours, TNF-α secretion was measured by ELISA. (C) PBDCs were incubated with or without 5 μg/mL anti–SR-A Ab for 1 hour, then further cultured in the presence or absence of fucoidan for 24 hours. (D) CD11c+ mDCs were purified from lin−BDCA+ cells using a cell sorter and treated with or without fucoidan or LPS as in Figure 2B. After 24 hours, TNF-α secretion was measured by ELISA. (E) PBDCs and iMDDCs were then pretreated with neutralizing Ab against TNF-α (1 μg) for 1 hour, then further incubated in the presence of fucoidan or TNF-α for 24 hours. The results shown represent the mean ± SD of 3 independent experiments. *P < .01; n.d. indicates not determined.
Role of p38 MAPK, PI3-K, and GSK3 in fucoidan-induced cytokine production and CD83 expression in PBDCs
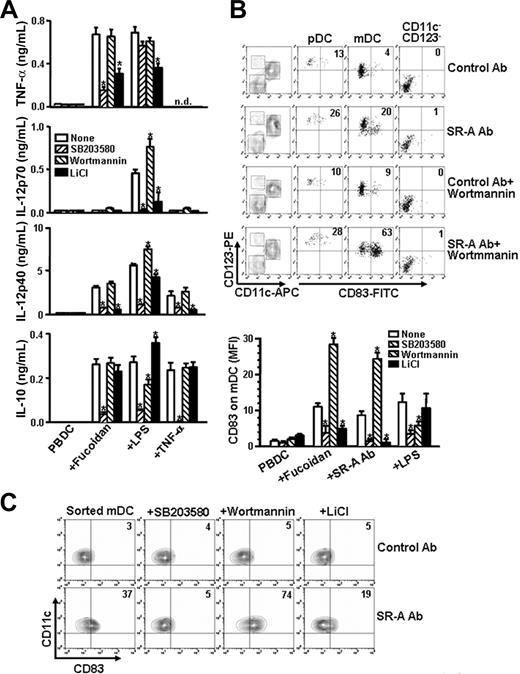
We investigated whether the difference of the cytokine release by SR-A and TLR-4 agonists was reflective of distinctive signaling characteristics of their receptors. It has been shown that MAPK signaling pathways are activated in DCs that have been induced to mature by LPS or TNF-α.31 A low concentration of SB203580 (1 μM), a p38 MAPK inhibitor, was found to inhibit the effect of fucoidan on the expression of CD83 and MHC class II, but to inhibit only slightly the effect of LPS (Figure S3A[b]). In contrast, inhibitors of c-Jun N-terminal kinase (SP600125) and MAPK/extracellular signal-regulated kinase kinase (PD98059) did not abrogate the up-regulation of CD83 and MHC class II, which was induced by fucoidan. Furthermore, pretreatment with SB203580 resulted in almost complete inhibition of TNF-α production in fucoidan-treated iMDDCs and PBDCs; however, this effect was not observed in LPS-treated iMDDCs and PBDCs (Figures 5A, S3B).
The effects of SB203580, wortmannin, and LiCl on fucoidan- or anti–SR-A Ab–induced cytokine production and CD83 expression in PBDCs. (A) PBDCs were pretreated with SB203580 (5 μM), wortmannin (1 μM), or LiCl (10 mM) for 1 hour and then further cultured in the presence or absence of fucoidan, LPS, or TNF-α. After 24 hours, cytokine secretion was measured by ELISA. (B) In the top panel, PBDCs were pretreated with wortmannin (1 μM) for 1 hour and then further cultured in the presence or absence of anti–SR-A Ab (5 μg) or control Ab (5 μg). After 24 hours, mDC, pDC, and CD11c−CD123− cells were gated and examined for CD83 expression. (Bottom panel) PBDCs were pretreated with kinase inhibitors as in panel A. The results represent the expression levels of CD83 on CD11chighCD123low mDCs. (C) The sorted CD11c+ mDCs were pretreated with kinase inhibitors as in panel A for 1 hour, after which they were further cultured with anti–SR-A Ab or control Ab for 24 hours. The results shown represent the mean ± SD of 3 independent experiments. *P < .05 compared with none; n.d. indicates not determined.
The effects of SB203580, wortmannin, and LiCl on fucoidan- or anti–SR-A Ab–induced cytokine production and CD83 expression in PBDCs. (A) PBDCs were pretreated with SB203580 (5 μM), wortmannin (1 μM), or LiCl (10 mM) for 1 hour and then further cultured in the presence or absence of fucoidan, LPS, or TNF-α. After 24 hours, cytokine secretion was measured by ELISA. (B) In the top panel, PBDCs were pretreated with wortmannin (1 μM) for 1 hour and then further cultured in the presence or absence of anti–SR-A Ab (5 μg) or control Ab (5 μg). After 24 hours, mDC, pDC, and CD11c−CD123− cells were gated and examined for CD83 expression. (Bottom panel) PBDCs were pretreated with kinase inhibitors as in panel A. The results represent the expression levels of CD83 on CD11chighCD123low mDCs. (C) The sorted CD11c+ mDCs were pretreated with kinase inhibitors as in panel A for 1 hour, after which they were further cultured with anti–SR-A Ab or control Ab for 24 hours. The results shown represent the mean ± SD of 3 independent experiments. *P < .05 compared with none; n.d. indicates not determined.
Phosphorylation of GSK3-α/β results (Ser21/Ser9) in its inhibition and is enhanced by AKT,32 and PI3-K down-regulates the production of IL-12p70 through GSK3 inhibition.33-35 As shown in Figure 5A, wortmannin, a PI3-K inhibitor, induced increases in IL-12p40 and IL-12p70 production after LPS stimulation but did not induce the production of measurable levels of IL-12p70 in fucoidan- or TNF-α–treated PBDCs. TNF-α production by fucoidan- or LPS-treated PBDCs was suppressed by LiCl, an inhibitor of GSK3, but was unaffected by wortmannin. In addition, LiCl inhibited IL-12p40 production but had little effect on IL-10 production by fucoidan- or TNF-α–treated PBDCs. We obtained a similar result with SB216763 and SB415286, which are also specific inhibitors of GSK3-β in iMDDCs (Figure S4). Taken together, these results suggest that fucoidan-induced TNF-α production is mediated by p38 MAPK- and GSK3-dependent pathways.
We also examined the effects of kinase inhibitors on SR-A agonist-induced expression of CD83 in PBDCs. Anti–SR-A Ab-induced expression of CD83 on PBDCs was significantly enhanced by wortmannin, but its expression levels were not affected by wortmannin in pDC and CD123−CD11c− cells (Figure 5B). Similar results were obtained when fucoidan was used as PBDCs were treated with anti–SR-A Ab and wortmannin (Figure 5B bottom panel). However, wortmannin inhibited the expression of CD83 induced by LPS in mDCs. Conversely, LiCl blocked the effects of anti–SR-A Ab and fucoidan on CD83 expression in PBDCs, whereas LiCl had little effect on CD83 expression by LPS in PBDCs. Similar results were obtained with SB203580, wortmannin, and LiCl as in PBDCs when CD83 expression in the sorted CD11c+ mDCs was evaluated (Figure 5C). Taken together, these data suggest that fucoidan-induced maturation of PBDCs is up-regulated by p38 MAPK and GSK3 but down-regulated by PI3-K.
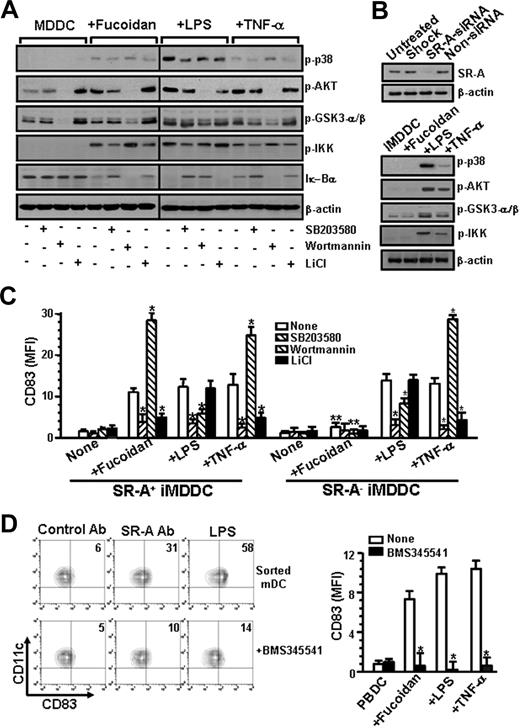
The ability of fucoidan to induce the activation of kinases was evaluated by measuring the increase in their phosphorylation state using antiphospho-p38 MAPK, AKT, and GSK3-α/β Abs. Fucoidan, TNF-α, and LPS increased the phosphorylated form of p38 MAPK, AKT, and GSK3-β (Figure 6A). However, SR-A–deficient iMDDCs failed to phosphorylate the kinases in the presence of fucoidan, but not in the presence of LPS and TNF-α (Figure 6B), which indicates that fucoidan-induced phosphorylation of the kinases is mediated by SR-A. Next, we investigated the effects of specific inhibitors of the kinases on the phosphorylation of kinases. SB203580 partially blocked the phosphorylation of AKT but failed to block the phosphorylation of GSK3-β in fucoidan-, LPS-, and TNF-α–treated iMDDCs. Moreover, wortmannin completely blocked the phosphorylation of AKT but only partially blocked the phosphorylation of GSK3-β in fucoidan-, LPS-, and TNF-α–treated iMDDCs. LiCl increased the phosphorylation of GSK3 but suppressed the phosphorylation of p38 MAPK in fucoidan- or TNF-α-treated iMDDCs. These findings indicate that fucoidan-induced phosphorylation of GSK3-β is p38 MAPK-independent and is mediated by both PI3-K–dependent and –independent pathways. Treatment of iMDDCs with fucoidan, TNF-α, or LPS induced phosphorylation of IKK and degradation of Iκ-Bα, which indicates that ligation of SR-A and TRL-4 activates the nuclear factor-κB pathway in DC maturation. In addition, the phosphorylation of IKK and degradation of Iκ-Bα in fucoidan- and TNF-α–treated iMDDCs were suppressed by SB203580 and LiCl but enhanced by wortmanninn. Conversely, LPS-induced phosphorylation of IKK and degradation of Iκ-Bα were suppressed by SB203580 and wortmannin but unaffected by LiCl.
Phosphorylation of p38 MAPK, PI-3K, and GSK3-α/β during fucoidan-induced maturation of iMDDCs. (A) iMDDCs (5 × 106) were pretreated with or without SB203580 (5 μM), wortmannin (1 μM), or LiCl (10 mM) for 1 hour and then incubated with fucoidan (50 μg/mL), LPS (1 μg/mL), or TNF-α (10 ng/mL) for 30 minutes. The samples were then subjected to Western blot using anti–Iκ-Bα and antiphosphorylated p38 MAPK (p-p38), AKT (p-AKT), GSK3-α/β (p-GSK3-α/β), and IKK (p-IKK) Abs. One representative experiment of 3 is shown. Vertical lines have been inserted to indicate a repositioned gel lane. (B) iMDDCs (106) were treated with DharmaFECT alone (Shock), siRNA of SR-A (SR-A siRNA), or nontargeting siRNA (Non-siRNA) as in Figure 3A. iMDDCs were harvested and subjected to Western blot as in panel A. (C) Non-siRNA–treated (SR-A+ iMDDC) or SR-A siRNA-treated iMDDCs (SR-A− iMDDC) were pretreated with kinase inhibitors for 1 hour and then cultured with or without fucoidan, LPS, or TNF-α. After 24 hours, the expression levels of CD83 were determined. (D) In the left panel, CD11c+ mDCs were purified as in Figure 2B and then pretreated with BMS345541 (10 μM) and further cultured with control IgG, anti–SR-A Ab, or LPS for 24 hours. CD83 expression is indicated as percentages of positive cells among total cells. In the right panel, PBDCs were pretreated with BMS345541 (10 μM) for 1 hour and further cultured with or without fucoidan, LPS, or TNF-α for 24 hours. The results shown represent the mean ± SD of 3 independent experiments. *P < .05 compared with that without inhibitors. **P < .05 compared with SR-A+ iMDDCs.
Phosphorylation of p38 MAPK, PI-3K, and GSK3-α/β during fucoidan-induced maturation of iMDDCs. (A) iMDDCs (5 × 106) were pretreated with or without SB203580 (5 μM), wortmannin (1 μM), or LiCl (10 mM) for 1 hour and then incubated with fucoidan (50 μg/mL), LPS (1 μg/mL), or TNF-α (10 ng/mL) for 30 minutes. The samples were then subjected to Western blot using anti–Iκ-Bα and antiphosphorylated p38 MAPK (p-p38), AKT (p-AKT), GSK3-α/β (p-GSK3-α/β), and IKK (p-IKK) Abs. One representative experiment of 3 is shown. Vertical lines have been inserted to indicate a repositioned gel lane. (B) iMDDCs (106) were treated with DharmaFECT alone (Shock), siRNA of SR-A (SR-A siRNA), or nontargeting siRNA (Non-siRNA) as in Figure 3A. iMDDCs were harvested and subjected to Western blot as in panel A. (C) Non-siRNA–treated (SR-A+ iMDDC) or SR-A siRNA-treated iMDDCs (SR-A− iMDDC) were pretreated with kinase inhibitors for 1 hour and then cultured with or without fucoidan, LPS, or TNF-α. After 24 hours, the expression levels of CD83 were determined. (D) In the left panel, CD11c+ mDCs were purified as in Figure 2B and then pretreated with BMS345541 (10 μM) and further cultured with control IgG, anti–SR-A Ab, or LPS for 24 hours. CD83 expression is indicated as percentages of positive cells among total cells. In the right panel, PBDCs were pretreated with BMS345541 (10 μM) for 1 hour and further cultured with or without fucoidan, LPS, or TNF-α for 24 hours. The results shown represent the mean ± SD of 3 independent experiments. *P < .05 compared with that without inhibitors. **P < .05 compared with SR-A+ iMDDCs.
We also observed similar effects of kinase inhibitors on CD83 expression in untreated control iMDDCs as observed in PBDCs (Figure 6C). However, fucoidan failed to induce the expression of CD83, and kinase inhibitors did not influence the effect of fucoidan on CD83 expression in SR-A–deficient iMDDCs. In addition, BMS345541, an inhibitor of IKK, blocked the effects of anti–SR-A Ab, fucoidan, and TNF-α on CD83 expression in sorted mDCs or PBDCs (Figure 6D). These results suggest that SR-A–mediated maturation of PBDCs is regulated by p38 MAPK, PI3-K, GSK3, and nuclear factor-κB.
T-cell proliferation by fucoidan-matured blood DCs
We also investigated fucoidan-matured PBDCs to determine whether they produced IL-12p70 in response to stimulation with CD40L (CD154) expressed by stimulated CD4+ T cells. As shown in Figures 7A and S5A, coculture of fucoidan-matured PBDCs and MDDCs with CD4+ T cells resulted in a high level of secretion of IL-12p70. In addition, the production of IFN-γ in the coculture condition was correlated with the measured levels of IL-12p70 (Figures 7B, S5A). The production of IL-12p70 and IFN-γ and T-cell proliferation in the cocultured condition decreased in response to the addition of anti-CD40-neutralizing Abs (Figures 7A,B, S5A,B), which suggests that IL-12p70 production was induced by activated T cells. Next, PBDCs were pretreated with or without anti–TNF-α Abs and then cultured in the presence or absence of fucoidan or LPS for 24 hours. These cells were then cocultured with naive CD4+ T cells. As shown in Figure 7C, IFN-γ secretion and T-cell proliferation were significantly diminished by treatment with fucoidan-treated and TNF-α–treated PBDCs that had been pretreated with anti–TNF-α–neutralizing Abs. Taken together, these results indicate that there was a clear difference in inducing T-cell response between immature and fucoidan-matured blood DCs.
Production of IL-12p70 and activation of CD4+ T cells in coculture of fucoidan-matured PBDCs and CD4+ T cells. (A) Fucoidan-, LPS-, or TNF-α–matured PBDCs (F-PBDC, L-PBDC, or T-PBDC) were harvested and washed twice with media. These DCs were then pretreated with mitomycin C and mixed with CD4+ T cells (105) in a 1:25 ratio. CD4+ T cells alone (T cells) and mixtures of the cells were then cultured in the presence or absence of neutralizing Abs against CD40 (5 μg) for an additional 1 or 5 days to determine the concentrations of IL-12p70 and IFN-γ, respectively. The concentrations of cytokines in the supernatants were determined by ELISA. (B) CD4+ T cells were labeled with CFSE and cocultured with fucoidan-, LPS-, or TNF-α–matured PBDCs in the presence or absence of neutralizing Abs against CD40. After 5 days, proliferation of CD4+ T cells was analyzed for CFSE dilution using flow cytometry. (C) PBDCs were pretreated with or without neutralizing Abs against TNF-α and then stimulated with fucoidan, LPS, or TNF-α for 24 hours. After coculture of the DCs and T cells in a 1:25 ratio for 5 days, the concentrations of IFN-γ in the supernatants were measured as in panel A. *P < .05 compared with PBDC alone. **P < .05 compared with that without anti-CD40 neutralizing Abs. ***P < .01.
Production of IL-12p70 and activation of CD4+ T cells in coculture of fucoidan-matured PBDCs and CD4+ T cells. (A) Fucoidan-, LPS-, or TNF-α–matured PBDCs (F-PBDC, L-PBDC, or T-PBDC) were harvested and washed twice with media. These DCs were then pretreated with mitomycin C and mixed with CD4+ T cells (105) in a 1:25 ratio. CD4+ T cells alone (T cells) and mixtures of the cells were then cultured in the presence or absence of neutralizing Abs against CD40 (5 μg) for an additional 1 or 5 days to determine the concentrations of IL-12p70 and IFN-γ, respectively. The concentrations of cytokines in the supernatants were determined by ELISA. (B) CD4+ T cells were labeled with CFSE and cocultured with fucoidan-, LPS-, or TNF-α–matured PBDCs in the presence or absence of neutralizing Abs against CD40. After 5 days, proliferation of CD4+ T cells was analyzed for CFSE dilution using flow cytometry. (C) PBDCs were pretreated with or without neutralizing Abs against TNF-α and then stimulated with fucoidan, LPS, or TNF-α for 24 hours. After coculture of the DCs and T cells in a 1:25 ratio for 5 days, the concentrations of IFN-γ in the supernatants were measured as in panel A. *P < .05 compared with PBDC alone. **P < .05 compared with that without anti-CD40 neutralizing Abs. ***P < .01.
Discussion
SR-A expression is up-regulated during differentiation from monocytes to DCs. This finding is consistent with those of studies conducted on bone marrow-derived DC differentiation with GM-CSF and IL-4.17 In this study, SR-A proteins were found to be expressed on both subtypes of PBDCs and CD1a+ iMDDCs. Previously, it has been shown that monocyte-derived immature DCs express SR-A-II and that maturation induces a switch in isoform expression to SR-AI.36 The amount of SR-A detected in fucoidan-matured MDDCs was similar to that in iMDDCs. Moreover, the expression levels of the surface molecules, including lineage markers such as CD1a and CD14, were not changed by exposure to fucoidan. Taken together, these findings suggest that fucoidan does not alter the differentiation of monocytes into immature DCs, at least in terms of surface marker phenotypes.
Becker et al16 observed the surface expression of SR-A on the subpopulation of CD11c+ DCs, but not on B220+ pDCs, in mouse spleen. Our study showed that SR-A expression was high in human CD11c+ mDCs but it was not detected on human blood pDCs, in line with the mouse spleen data. In addition, we found that CD83 was not expressed on fucoidan- or LPS-treated pDCs, whereas fucoidan-induced up-regulation of CD83 was primarily detected on the CD11c+ DC subtype. In addition, in this study, iMDDCs that lacked SR-A did not mature in the presence of fucoidan, and the binding of anti–SR-A Ab to PBDCs or iMDDCs was decreased by fucoidan. These results suggest that fucoidan-induced maturation of the blood DCs occurred through SR-A and that down-modulation of SR-A by fucoidan results in decreased surface expression and Ab staining. Furthermore, fucoidan-treated PBDCs and iMDDCs demonstrated similar phenotypic and functional characteristics of mature DCs, even though phenotypic and functional differences have been reported between CD11c+ mDCs and iMDDCs stimulated by TLR agonists.37 CD1a is used as a marker for iMDDCs, and these CD1a-expressing blood cells were induced to mature using SR-A agonist. Tissue DCs, such as Langerhans cells, are also CD1a+ cells.38 Therefore, further work should be conducted to determine whether tissue DCs that express SR-A can be activated by its agonists.
We compared LPS-treated DCs and fucoidan-treated DCs in their mature state, but we did not find any significant differences in the DC phenotype and function except for their cytokine profiles and signaling pathways. Furthermore, the ability of LPS and fucoidan to induce alloreactive T-cell proliferation and IFN-γ secretion was equal, but major differences were observed in DCs that were pretreated with TNF-α–neutralizing Abs. LPS is also recognized by SR-A in macrophages.39 Fucoidans from F vesiculosus and F evanescens used in these experiments contained less than 0.1 endotoxin unit/mL. Our comparison of cytokine production and the effects of kinase inhibitors on CD83 expression and TNF-α secretion between fucoidan- and LPS-treated DCs indicated that the response to fucoidan was not the result of contamination with LPS. Moreover, the surface marker expression, cytokine production, and the phosphorylation of kinases in SR-A–deficient DCs observed in this study suggest that fucoidan and LPS responses were specific to binding to their respective receptor and not the result of cross-reactivity.
Our study also showed that fucoidan-induced maturation of blood DCs was abrogated by treatment with neutralizing TNF-α Abs. It has been reported that mouse bone marrow–derived DCs treated with TNF-α achieved only a partially matured phenotype, whereas those that were treated with LPS and anti-CD40 matured fully.40 However, in our study, treatment with TNF-α or fucoidan alone effectively induced the maturation of PBDCs and iMDDCs in a manner similar to LPS. Furthermore, TNF-α from innate lymphocytes has been identified as an inducer of DC maturation.41 Hsu et al42 observed TNF-α production by the murine macrophage line, J744A, after stimulation with fucoidan. Therefore, the secreted TNF-α may trigger blood DC maturation in either an autocrine or paracrine fashion.
Proinflammatory cytokines, such as IL-12, are essential for inducing Th1 response.40 The results of this study indicated that fucoidan-matured PBDCs and MDDCs were unable to produce IL-12p70, although CD40L signaling by activated T cells caused IL-12p70 production and the induction of IFN-γ in the MLR assay when fucoidan-matured DCs were used as stimulators. Consistent with these findings, others have reported differences in the cytokine production of LPS-matured and TNF-α–matured DCs.43 In addition, the exposure of immature DCs to TNF-α has been found to prevent the secretion of IL-12p70.40 In comparison, IL-12p40 production was similar between cells that were treated with fucoidan and LPS. Furthermore, the suppression of IL-12p70 production is known to be mediated by ligation of SR-A expressed on macrophages.44 Taken together, these results indicate that endogenous TNF-α is a crucial factor in the matured blood DCs through SR-A engagement.
This study demonstrates that differential regulation of TNF-α and IL-12p70 production through different signaling pathways may also be pivotal in DC activation by SR-A and TRL ligation. However, even though the involvement of PI3-K and GSK3 in regulating cytokine production has been studied in detail,33-35 the ability of these kinases to regulate the maturation of PBDCs has not been fully investigated. The results of this study suggest that SR-A ligand-induced maturation of PBDCs is regulated by GSK3 and that GSK3 activity is down-regulated by AKT. In this process, GSK3 inhibitors blocked the effects of fucoidan and anti–SR-A Ab on CD83 expression in PBDCs and the phosphorylation of IKK in iMDDCs. Next, wortmannin enhanced expression levels of CD83 and the phosphorylation of IKK in fucoidan and anti–SR-A–treated PBDCs or iMDDCs. Finally, wortmannin decreased and LiCl increased the phosphorylation of GSK3-β in fucoidan-treated iMDDCs, respectively.
In conclusion, human blood DCs can be activated or induced to mature in response to endogenously or exogenously generated SR-A ligands, which suggests that SR-A plays an important role in determining the adaptive immune responses that follow innate recognition. SR-A stimulation can indirectly elicit maturation of PBDCs through production of TNF-α by activated DCs and indirectly produce IL-12p70 after interaction with the activated T cells. Further work should be conducted to develop SR-A agonists to be used as immune modulators and to identify the role of SR-A in antigen presentation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Ken Shortman (The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) for his critical review.
This work was supported by the Korea Science and Engineering Foundation (grant R13-2002-044-04001-0) funded by the Korean government.
Authorship
Contribution: J.-Y.K. designed and performed the majority of the experiments and wrote the manuscript; J.-O.J. performed MDDC and PBDC experiments; H.-Y.P. performed PBDC experiments; Q.X. helped with the MDDC experiments; J.-I.P. helped design the experiments; and T.Z. and V.A.S. purified fucoidan.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jong-Young Kwak, Department of Biochemistry, School of Medicine and Medical Research Center for Cancer Molecular Therapy, Dong-A University, Seo-Ku, Busan 602-714, Korea; e-mail: jykwak@dau.ac.kr.