Abstract
Autologous stem cell transplantation (ASCT) has been successfully used in HIV-related lymphoma (HIV-Ly) patients on highly active antiretroviral therapy. We report the first comparative analysis between HIV-Ly and a matched cohort of HIV− lymphoma patients. This retrospective European Group for Blood and Marrow Transplantation study included 53 patients (66% non-Hodgkin and 34% Hodgkin lymphoma) within each cohort. Both groups were comparable except for the higher proportion of males, mixed-cellularity Hodgkin lymphoma and patients receiving granulocyte colony-stimulating factor before engraftment and a smaller proportion receiving total body irradiation-based conditioning within the HIV-Ly cohort. Incidence of relapse, overall survival, and progression-free survival were similar in both cohorts. A higher nonrelapse mortality within the first year after ASCT was observed in the HIV-Ly group (8% vs 2%), predominantly because of early bacterial infections, although this was not statistically significant and did not influence survival. Thus, within the highly active antiretroviral therapy era, HIV patients should be considered for ASCT according to the same criteria adopted for HIV− lymphoma patients.
Introduction
Human immunodeficiency virus (HIV) infection is associated with an increased incidence of non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma (HL).1,2 Furthermore, HIV-associated lymphomas (HIV-Ly) frequently show high-risk features.3,4
Because of the better performance and immunologic status of HIV-Ly patients on highly active antiretroviral therapy (HAART), the use of intensive chemotherapy has resulted in a higher rate of complete remission (CR) and overall survival (OS) rates.5-7 Nevertheless, refractory or relapsed HIV-Ly patients still show poor survival results.8,9
Throughout the HAART era, autologous stem cell transplantation (ASCT) has been reported as a feasible, safe, and useful approach to either rescue or consolidate HIV-Ly patients.10-14 However, comparative studies according to the HIV status have only been preliminarily reported.15
To further evaluate the usefulness of ASCT in HIV-Ly, a retrospective matched comparative analysis with patients who received HIV− lymphoma autotransplants was designed within the European Group for Blood and Marrow Transplantation (EBMT).
Methods
Study design
Following the EBMT study14 on HIV-Ly patients undergoing peripheral blood ASCT, a registry-based, retrospective, individual-matched, case-controlled study according to HIV serologic status (positive vs negative) was designed. Among the EBMT centers reporting patients who received HIV-Ly autotransplants, the best matched HIV− lymphoma autotransplant recipient (control-Ly) was identified according to the following mandatory matching criteria: (1) histology (HL, diffuse large B-cell or plasmablastic NHL, Burkitt or Burkitt-like NHL, peripheral T-cell NHL); (2) Ann Arbor stage at diagnosis (I or II vs III or IV); (3) non–age-adjusted IPI at diagnosis, except for HL and Burkitt lymphoma patients (1 or 2 vs ≥ 3); and (4) disease status at ASCT (1st CR, 2nd or subsequent CR, partial remission, or chemosensitive [Chemo-S] relapse; primary refractory or chemoresistant [Chemo-R] relapse). In addition, 3 optional matching criteria were considered: (5) age at ASCT plus or minus 5 years, (6) year of ASCT plus or minus 2 years, and (7) country of ASCT. The institutional review boards of all participating centers approved this study, and informed consent was obtained in accordance with the Declaration of Helsinki.
Definitions and statistical analysis
The EBMT guidelines were used to define CR, partial response (PR), relapse (after achievement of CR), progression (after PR), neutrophil and platelet engraftment, OS, and progression-free survival (PFS). Relapse was considered to be Chemo-S if at least PR was achieved after salvage treatment; otherwise, it was considered Chemo-R. The latter also included primary refractory patients. Nonrelapse mortality (NRM) was defined as death from any cause without a previous disease relapse or progression.
Estimates of engraftment, NRM, and relapse or progression after ASCT were calculated using cumulative incidence (CI) rates to accommodate competing risks and compared by univariate Cox regression test. Estimates of PFS and OS were calculated using the Kaplan-Meier method and compared by the 2-tailed log-rank test.
Results
Fifty-three HIV-Ly patients who received autotransplants since 1999 and 53 control-Ly from 18 EBMT institutions in 7 countries were included. Patient features for both cohorts are shown in Table 1. Both groups were comparable for all clinical and transplantation characteristics except for the higher proportion of male sex (P = .05), mixed-cellularity HL subtype (P = .01), granulocyte colony-stimulating factor (G-CSF) use before engraftment (P = .001), and the lower proportion of total body irradiation–based conditioning regimen (P = .04) and of patients with nodular sclerosis HL subtype (P = .03) within the HIV-Ly cohort.
Patient features for HIV-Ly and control-Ly cohorts
| Feature . | HIV-Ly cohort . | Control-Ly cohort . | P . | ||
|---|---|---|---|---|---|
| n . | % . | n . | % . | ||
| Patients | 53 | 100 | 53 | 100 | NS |
| Median age, y (range) | 40.7 (29-62.5) | 44.3 (19.5-64) | NS | ||
| Male sex | 43 | 81 | 33 | 62 | .05 |
| Non-Hodgkin lymphoma | 35 | 66 | 35 | 66 | NS |
| Diffuse large B-cell/plasmablastic | 28 | 80 | 28 | 80 | NS |
| Burkitt/Burkitt-like | 3 | 8.5 | 3 | 8.5 | NS |
| Peripheral T-cell | 4 | 11.5 | 4 | 11.5 | NS |
| IPI at diagnosis > 2 (except Burkitt) | 18 | 62 | 21 | 65.5 | NS |
| Hodgkin lymphoma | 18 | 34 | 18 | 34 | NS |
| Mixed cellularity | 8 | 61.5 | 3 | 17.5 | .01 |
| Nodular sclerosis | 3 | 23 | 11 | 65 | .03 |
| Ann Arbor stage at diagnosis > II | 11 | 61 | 11 | 61 | NS |
| Treatment before ASCT | |||||
| Median no. of treatment lines (range) | 2 (1-5) | 2 (1-4) | NS | ||
| Patients undergoing rituximab treatment | 18 | 35.6 | 22 | 41.5 | NS |
| Disease status at ASCT | |||||
| Complete remission | 25 | 47 | 25 | 47 | NS |
| First complete remission | 12 | 22.5 | 12 | 22.5 | |
| Partial remission/chemosensitive relapse | 10/13 | 19/24.5 | 13/10 | 24.5/19 | NS |
| Primary induction failure/chemoresistant relapse | 5 | 9.5 | 5 | 9.5 | NS |
| Conditioning regimen | |||||
| TBI-based regimens | 3 | 5.5 | 6 | 11.5 | .04 |
| BEAM/variants | 50 | 94.5 | 43 | 81 | NS |
| Other conditioning schemes | 0 | 0 | 4 | 7.5 | NS |
| Median CD34+ infused cells × 106/kg (range) | 5.1 (1.6-21) | 3.9 (0.9-17) | NS | ||
| Patients on G-CSF before engraftment | 47 | 89 | 31 | 58.5 | .001 |
| Post-ASCT antitumor preemptive treatment | 9 | 17 | 6 | 11 | NS |
| Local radiotherapy | 8 | 15 | 4 | 7.5 | NS |
| Rituximab | 1 | 2 | 2 | 3.5 | NS |
| Median months of follow-up from ASCT (range) | 30 (3-81) | 29.5 (3-98) | NS | ||
| Median months to ASCT from diagnosis (range) | 14.5 (4.5-106) | 17 (4-173) | NS | ||
| Patients with follow-up censored at second ASCT | 1 | 2 | 5 | 9.5 | NS |
| Feature . | HIV-Ly cohort . | Control-Ly cohort . | P . | ||
|---|---|---|---|---|---|
| n . | % . | n . | % . | ||
| Patients | 53 | 100 | 53 | 100 | NS |
| Median age, y (range) | 40.7 (29-62.5) | 44.3 (19.5-64) | NS | ||
| Male sex | 43 | 81 | 33 | 62 | .05 |
| Non-Hodgkin lymphoma | 35 | 66 | 35 | 66 | NS |
| Diffuse large B-cell/plasmablastic | 28 | 80 | 28 | 80 | NS |
| Burkitt/Burkitt-like | 3 | 8.5 | 3 | 8.5 | NS |
| Peripheral T-cell | 4 | 11.5 | 4 | 11.5 | NS |
| IPI at diagnosis > 2 (except Burkitt) | 18 | 62 | 21 | 65.5 | NS |
| Hodgkin lymphoma | 18 | 34 | 18 | 34 | NS |
| Mixed cellularity | 8 | 61.5 | 3 | 17.5 | .01 |
| Nodular sclerosis | 3 | 23 | 11 | 65 | .03 |
| Ann Arbor stage at diagnosis > II | 11 | 61 | 11 | 61 | NS |
| Treatment before ASCT | |||||
| Median no. of treatment lines (range) | 2 (1-5) | 2 (1-4) | NS | ||
| Patients undergoing rituximab treatment | 18 | 35.6 | 22 | 41.5 | NS |
| Disease status at ASCT | |||||
| Complete remission | 25 | 47 | 25 | 47 | NS |
| First complete remission | 12 | 22.5 | 12 | 22.5 | |
| Partial remission/chemosensitive relapse | 10/13 | 19/24.5 | 13/10 | 24.5/19 | NS |
| Primary induction failure/chemoresistant relapse | 5 | 9.5 | 5 | 9.5 | NS |
| Conditioning regimen | |||||
| TBI-based regimens | 3 | 5.5 | 6 | 11.5 | .04 |
| BEAM/variants | 50 | 94.5 | 43 | 81 | NS |
| Other conditioning schemes | 0 | 0 | 4 | 7.5 | NS |
| Median CD34+ infused cells × 106/kg (range) | 5.1 (1.6-21) | 3.9 (0.9-17) | NS | ||
| Patients on G-CSF before engraftment | 47 | 89 | 31 | 58.5 | .001 |
| Post-ASCT antitumor preemptive treatment | 9 | 17 | 6 | 11 | NS |
| Local radiotherapy | 8 | 15 | 4 | 7.5 | NS |
| Rituximab | 1 | 2 | 2 | 3.5 | NS |
| Median months of follow-up from ASCT (range) | 30 (3-81) | 29.5 (3-98) | NS | ||
| Median months to ASCT from diagnosis (range) | 14.5 (4.5-106) | 17 (4-173) | NS | ||
| Patients with follow-up censored at second ASCT | 1 | 2 | 5 | 9.5 | NS |
HIV-Ly indicates cohort of AIDS-related lymphoma patients treated with autologous peripheral blood stem cell transplantation (ASCT); Control-Ly, matched cohort of HIV-negative lymphoma patients treated with ASCT; NS, not significant; TBI, total body irradiation; and BEAM, BCNU, etoposide, Ara-C, melphalan.
At the time of transplantation, 93% of HIV-Ly patients were on HAART, with a median CD4+ T-lymphocyte count of 163 cells/μL (range, 8-1159 cells/μL).
CI of neutrophil engraftment was 100% for HIV-Ly and control-Ly, after a median time of 11 days (range, 8-36 days) and 11 days (range, 7-19 days), respectively. CI of platelet engraftment was 92% (95% confidence interval [CInt], 84%-100%) for HIV-Ly and 98% (CInt, 94.5%-100%) for control-Ly (P = .03).
CI of relapse at a median follow-up of 30 months was 29% (CInt, 19.5%-49.5%) for HIV-Ly and 42% (CInt, 29.5%-59.5%) for control-Ly (P = not significant [NS]).
Causes of death for both cohorts are shown in Table 2. The CI of NRM at 1 year of follow-up was 8% (CInt, 3%-20%) for HIV-Ly and 2% (CInt, 0.5%-13%) for the control-Ly cohort (P = .2). The 4 nonrelapse deaths occurring in HIV-Ly patients within the first year after ASCT were related to bacterial infection in 3 cases (within 4 months of ASCT) and 1 nondocumented event at 3 months after ASCT.
Causes of death for HIV-Ly and Control-Ly cohorts
| . | HIV-Ly cohort . | Control-Ly cohort . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| Death events | 19 | 36 | 14 | 26.5 |
| Relapse/progression | 13 | 69 | 13 | 93 |
| Secondary malignancy | 1 | 5 | 0 | 0 |
| Infectious-related (< 4 mo after ASCT) | 3 | 16 | 0 | 0 |
| Non–ASCT-related late infection complication | 1 | 5 | 0 | 0 |
| Other | 0 | 0 | 1 | 7 |
| Unknown | 1 | 5 | 0 | 0 |
| . | HIV-Ly cohort . | Control-Ly cohort . | ||
|---|---|---|---|---|
| n . | % . | n . | % . | |
| Death events | 19 | 36 | 14 | 26.5 |
| Relapse/progression | 13 | 69 | 13 | 93 |
| Secondary malignancy | 1 | 5 | 0 | 0 |
| Infectious-related (< 4 mo after ASCT) | 3 | 16 | 0 | 0 |
| Non–ASCT-related late infection complication | 1 | 5 | 0 | 0 |
| Other | 0 | 0 | 1 | 7 |
| Unknown | 1 | 5 | 0 | 0 |
Survival
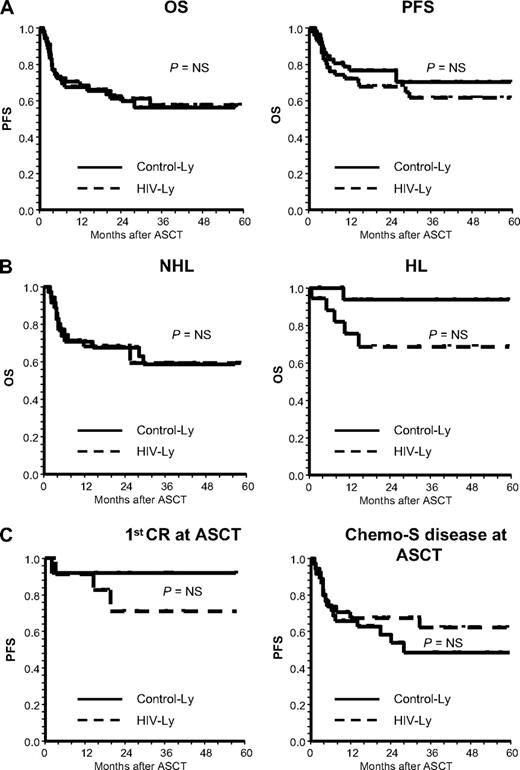
At a median follow-up of 30 months, the OS was 61.5% (CInt, 47%-76%) and 70% (CInt, 57%-84%) for HIV-Ly and control-Ly, respectively (P = NS), and the PFS was 61% (CInt, 47%-75%) and 56% (CInt, 41%-71%, P = NS; Figure 1A).
Survival according to HIV infection status: positive versus negative. (A) PFS and OS. HIV-Ly identifies the cohort of AIDS-related lymphoma patients treated with ASCT. Control-Ly identifies the cohort of matched HIV− lymphoma patients treated with ASCT. (B) OS for NHL and for HL. (C) PFS for patients in first CR and those with Chemo-S disease at the time of ASCT.
Survival according to HIV infection status: positive versus negative. (A) PFS and OS. HIV-Ly identifies the cohort of AIDS-related lymphoma patients treated with ASCT. Control-Ly identifies the cohort of matched HIV− lymphoma patients treated with ASCT. (B) OS for NHL and for HL. (C) PFS for patients in first CR and those with Chemo-S disease at the time of ASCT.
According to lymphoma histology, OS at a median follow-up of 30 months for HIV-Ly NHL was 58.5% (CInt, 40.5%-76.5%) and 59% (CInt, 41%-77%) for control-Ly NHL (P = NS). For HL, OS was 69% (CInt, 46%-91.5%) for HIV-Ly and 94% (CInt, 82%-100%) for control-Ly (P = NS) (Figure 1B). PFS for HIV-Ly NHL was 57.5% (CInt, 40.3%-74.6%) and 49.2% (CInt, 31.6%-66.8%) for control-Ly NHL (P = NS). PFS for HIV-Ly HL and control-Ly HL were 57% (CInt, 29%-85%) and 69% (CInt, 42%-95%), respectively (P = NS).
Patients in 1st CR at time of ASCT showed a PFS of 71% (CInt, 42%-99%) for HIV-Ly and 92% (CInt, 76%-100%) for control-Ly (P = NS; Figure 1C). For the remaining patients with Chemo-S disease at ASCT (CR > 1, PR or Chemo-S relapse), PFS was 67.5% (CInt, 51.5%-83%) for HIV-Ly and 48.5% (CInt, 29.5%-67.5%) for control-Ly (P = NS) (Figure 1C). Regarding patients with chemoresistant disease at ASCT, median time to progression was 3.4 months (2.3-20.8 months) in HIV-Ly and 2.5 months (1.5-2.9 months) in control-Ly (P = NS).
Discussion
Since the advent of HAART, ASCT has been successfully used in HIV-Ly patients, being particularly effective in Chemo-S patients.10-13 In a recent EBMT study,14 age more than 50 years was associated with a higher NRM. Relapse incidence was higher in patients requiring more than 2 pre-ASCT treatment lines and those not in CR at time of ASCT.
However, ASCT has not been considered as standard of care in HIV-Ly, probably because of the expectancy of an increased treatment-related toxicity. To address this issue, this is the first comparative study to evaluate ASCT outcomes between HIV-Ly and a matched control-Ly cohort.
Although neutrophil engraftment was similar in both groups, G-CSF was more frequently used within the HIV-Ly cohort. Nevertheless, we cannot conclude that G-CSF administration is strictly needed.10,13 Regarding platelet engraftment, a lower CI and a mild delay were observed within the HIV-Ly cohort, which might be related to different factors, such as posttransplant G-CSF use, HAART combination, or bone marrow damage resulting from chronic HIV infection.13
The difference in CI of relapse for both cohorts was found not statistically significant, although showing a favorable tendency for the HIV-Ly group. Whether this is related to the reported benefits of HAART use is unclear.6,7
OS and PFS were comparable in both cohorts (Figure 1A). Furthermore, similar results were obtained when both cohorts were compared according to histology (NHL vs HL; Figure 1B) and disease status at ASCT (1st CR, Chemo-S disease other than 1st CR [Figure 1C], and Chemo-R).
The main cause of death in both groups was disease relapse/progression. Although not statistically significant, a higher CI of 1 year of NRM was observed in the HIV-Ly group (8% vs 2%), mainly because of early bacterial infections. Nevertheless, the higher risk of NRM did not translate into survival differences between the 2 cohorts.
Our data suggest that, within the HAART era, HIV infection should not preclude lymphoma patients from undergoing ASCT according to the same eligibility criteria adopted for HIV− lymphoma patients.16-18 However, particular attention to infection prophylaxis and cautious immune recovery surveillance should take place early after ASCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Carmen Ruiz de Elvira, EBMT Registry, and all the Registry staff for their valuable help.
This study was supported in part by the Spanish Ministry of Health (grants BA05/90038, FIS 06/60230, and FIS 05/2505), Fundación MMA (grant 07/589), and the Program for Research Intensification in Nursing of the Agencia Pedro Laín Entralgo (Comunidad de Madrid, Spain) and Fundación para la Investigación Biomédica del Hospital Gregorio Marañón.
Authorship
Contribution: J.L.D.-M., P. Balsalobre, A. Re, D.S., and B.A. provided study concept and design; J.L.D.-M., P. Balsalobre, C.C., and A.S. analyzed data; J.L.D.-M., P. Balsalobre, and C.C. wrote the paper; and J.L.D.-M., P. Balsalobre, A. Re, M.M., J.M.R., E.C., A. Rosselet, I.G., R.V., B.A., K.C., P.G., I.E., P. Biron, N.S., A.E.H., A.F., G.G., M.H., M.J., P.F., D.S., and G.R. collected data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the European Group for Blood and Marrow Transplantation Lymphoma Working Party appears in the Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: José L. Díez-Martín, Hematology Department, Hospital General Universitario Gregorio Marañón, Pabellón Oncologico (Hematología), 1a pta, C/Dr Esquerdo, 46, 28007 Madrid, Spain; e-mail: jdiez.hgugm@salud.madrid.org.
References
Author notes
*J.L.D.-M. and P. Balsalobre contributed equally to this study.