In this issue, Li and colleagues describe a new mechanism promoting thrombus dissolution involving thrombin-induced secretion and cleavage of an “orphan” metalloproteinase ADAMTS-18.
Platelet thrombus formation is a dynamic process that is essential for the cessation of bleeding at sites of vascular injury. Dysregulation of this process can lead to excessive thrombus formation, particularly at sites of atherosclerotic plaque rupture, precipitating the acute coronary syndromes or ischemic stroke. The hemostatic response is regulated by competing prothrombotic and antithrombotic forces. A key player in this regard is the serine protease, thrombin.1 In its free form, thrombin is procoagulant, activating several components of the coagulation cascade as well as converting fibrinogen to fibrin. It is also one of the most potent platelet agonists known. Conversely, thrombin bound to thrombomodulin on vascular endothelial cells can activate protein C, a potent physiologic anticoagulant.
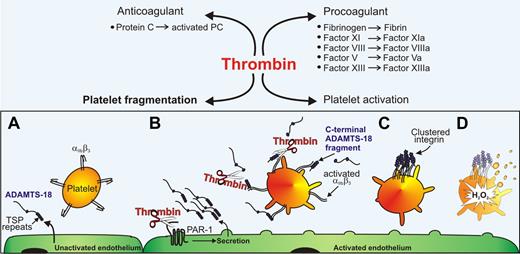
ADAMTS-18 Binds and Clusters β3 integrins and Destroys platelet aggregates. (A) Under resting conditions, the endothelium constitutively releases a small amount of ADAMTS-18. (B) Endothelial activation by thrombin enhances the secretion of ADAMTS-18, which binds to platelet αIIbβ3. Thrombin cleaves ADAMTS-18 to produce an active 45-kDa C-terminal fragment, which (C) clusters β3 integrins. (D) Clustering activates12-lipoxygenase and NADPH oxidase to regulate thrombus size by oxidative fragmentation. (TSP, thrombospondin; PAR-1, protease activated receptor-1; H2O2, hydrogen peroxide.)
ADAMTS-18 Binds and Clusters β3 integrins and Destroys platelet aggregates. (A) Under resting conditions, the endothelium constitutively releases a small amount of ADAMTS-18. (B) Endothelial activation by thrombin enhances the secretion of ADAMTS-18, which binds to platelet αIIbβ3. Thrombin cleaves ADAMTS-18 to produce an active 45-kDa C-terminal fragment, which (C) clusters β3 integrins. (D) Clustering activates12-lipoxygenase and NADPH oxidase to regulate thrombus size by oxidative fragmentation. (TSP, thrombospondin; PAR-1, protease activated receptor-1; H2O2, hydrogen peroxide.)
In this issue of Blood, Li et al describe a surprising new role for thrombin: promoting the dissolution of platelet thrombi.2 They demonstrate that thrombin, generated at the site of vessel injury, is able to cleave a protease of previously unknown function, ADAMTS-18, which, following secretion from activated endothelium, binds to the β3 subunit of the platelet adhesion receptor integrin αIIbβ3. The thrombin-generated 45-kDa C-terminal cleavage product of ADAMTS-18 clusters the β3 integrins and induces oxidative fragmentation of the platelet, leading to thrombus dissolution. This process is dependent on the sequential activation of platelet 12-lipoxygenase and NADPH oxidase, resulting in the intracellular production of reactive oxygen species (ROS) including hydrogen peroxide (H2O2).
Like many breakthroughs, the path leading to this discovery has been an unexpected one. It began with the seemingly unrelated observation that patients with HIV-1 infection develop autoimmune thrombocytopenia due to the presence of an antiplatelet antibody directed against the epitope GPIIIa49-66 of αIIbβ3 (GPIIb-IIIa).3 In general, the higher the titer of antibody, the lower the platelet count. It was determined that this antibody induced thrombocytopenia by inducing platelet fragmentation via a process dependent on the generation of H2O2 and other ROS. The search for a physiologic ligand that could induce oxidative platelet fragmentation was undertaken using the platelet GPIIIa49-66 peptide as bait in a phage surface display library. From 20 clones isolated, 1 had 70% identity with a C-terminal region of ADAMTS-18. Li et al demonstrate that an active C-terminal fragment of ADAMTS-18 can completely dissolve platelet aggregates formed in vitro. Moreover, this fragment lyses thrombi formed in the carotid artery of mice and reduces infarction and neurologic impairment in a cerebral stroke model. Importantly, this protection was afforded whether the fragment was infused 2 hours before or 2 hours after the 90-minute ischemia and reperfusion, with no adverse bleeding events.
These provocative findings shed new light on the physiologic processes regulating thrombus dissolution in vivo. It has long been recognized that platelet thrombus formation is a dynamic process, dependent on the equilibrium between platelet deposition and dispersal. As a consequence, impairment of thrombin/ADAMTS-18–dependent platelet dissolution could conceivably produce a prothrombotic phenotype. It is also possible that thrombin/ADAMTS-18 could act as a safeguard in situations where the anticoagulant function of thrombin is compromised, as occurs in proinflammatory states.4
As with any good study, this work poses more questions than it answers. Foremost is whether thrombin-induced cleavage of ADAMTS-18 represents the dominant physiologic mechanism controlling thrombus dissolution in vivo. In this regard, the generation of ADAMTS-18 null mice or mice expressing noncleavable ADAMTS-18 will be informative. This issue aside, the authors clearly demonstrate that the C-terminal fragment of ADAMTS-18 is highly effective at promoting platelet thrombus dissolution in vivo. This may have direct clinical relevance, as platelet-rich thrombi are notoriously difficult to lyse with fibrinolytic therapies alone. Hence, therapeutic approaches that induce platelet fragmentation may represent an innovative approach to enhance fibrinolysis. At a basic level, the study by Li et al raises interesting issues with respect to the physiologic mechanisms regulating H2O2 and ROS production during thrombus development. These molecules are known to be generated following platelet activation,5 which begs the question as to what stage in the thrombotic process these molecules are generated and how their platelet-stimulating functions are coordinated with their ability to induce platelet fragmentation. Additionally, whether thrombin is the only (or major) proteolytic enzyme with the capacity to regulate the activity of ADAMTS-18 remains to be determined. Nonetheless, despite being one of the most intensely investigated proteases, new and unanticipated functions for thrombin continue to emerge, reinforcing its role as a master of thromboregulation.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal