The molecular regulation of the specific sets of endothelial receptors that mediate leukocyte diapedesis is not fully understood. Using elegant adoptive transfer experiments with cells from genetically deficient mouse models, in this issue of Blood Woodfin and colleagues show that the junctional molecules ICAM-2, JAM-A, and PECAM-1 mediate neutrophil transmigration in a stimulus-dependent manner and define how this ability is regulated by the activated endothelium.
Leukocyte transmigration across vascular endothelium is a key event in the inflammatory response. The extravasation process is orchestrated by the concerted action of cellular adhesion receptors and chemotactic factors, and requires cell shape changes in both leukocytes and endothelial cells. For both cell types, it is an active process and controls the rapid recruitment of leukocytes to inflammatory foci without altering vasculature integrity.1,2 Transendothelial migration is the last and least-understood step in the transfer of a leukocyte from the bloodstream to the inflamed tissue, and the mechanisms and signals by which leukocytes penetrate endothelial cell to cell junctions and traverse the gap are not well known.
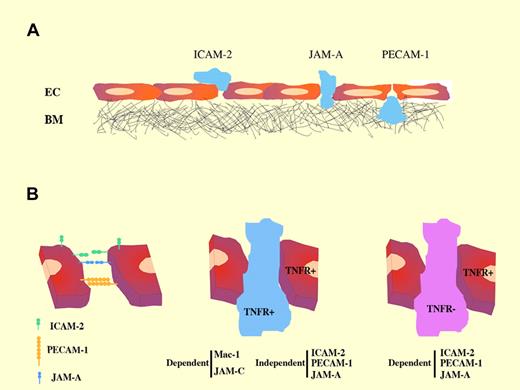
(A) Sequential roles of junctional molecules during neutrophil transmigration. (B) Stimulus-dependent requirement of endothelial junctional molecules in neutrophil transmigration.
(A) Sequential roles of junctional molecules during neutrophil transmigration. (B) Stimulus-dependent requirement of endothelial junctional molecules in neutrophil transmigration.
Many endothelial molecules that mediate leukocyte diapedesis are preferentially situated at endothelial cell to cell junctions; these include Junctional Adhesion Molecules A and C (JAM-A and -C), PECAM-1/CD31, and ICAM-2—addressed by Woodfin et al in this issue3 —and others such as ESAM-1 and CD99.2,4 Most of these molecular components of interendothelial junctions interact homophilically. JAM and CD31/PECAM-1 interact homophilically at endothelial junctions to regulate paracellular permeability and also interact with integrin counterreceptors on leukocytes to support transmigration.2,4 These molecular constituents are the basis of the so-called paracellular route of leukocyte transendothelial migration, which appears to be the main mode of leukocyte transfer. In addition, all these junctional molecules are organized in tetraspanin-based microdomains,5 which could facilitate their coordinated action.
Accumulating evidence underlines the role of endothelial junctional molecules in maintaining vascular monolayer integrity and controlling the passage of leukocytes.4 Previous studies found, by using blocking antibodies or deletion studies, that the adhesion molecules PECAM-1 and JAM-A are involved in different steps of leukocyte transfer.6 In the present study, Woodfin et al have carried out a detailed analysis of each molecule by confocal imaging and 3-dimensional fluorescence reconstruction from whole-mount preparations. This analysis clearly delineates the different positions at which neutrophils are arrested in vascular inflamed tissue from mice deficient in ICAM-2, PECAM-1, or JAM-A (panel A of the figure). These results, supported by functional assays, reinforce the conclusion that these 3 junctional molecules mediate distinct but sequential steps in the transmigration process.
An important issue addressed by Woodfin et al relates to the differential involvement of sets of endothelial junctional receptors (dependent on the proinflammatory stimulus; eg, IL-1 vs TNF-α) and the role of specific signaling/activation in leukocytes or endothelial target cells. Earlier evidence showed that ICAM-2, PECAM-1, and JAM-A EC adhesion molecules mediate neutrophil transmigration in a stimulus-dependent fashion,7,8 and that, in certain inflammatory models, this phenomenon seems to be dependent on the genetic background of the mouse strain used.7 However, the molecular mechanisms underlying such strain-related differences are not well understood. Woodfin et al use an experimental strategy based on adoptive transfer of fluorescently labeled donor leukocytes from wild-type or TNFR-deficient mice to wild-type recipients or recipients deficient in TNFR or one of ICAM-2, PECAM-1, or JAM-A. In this way, these authors provide evidence that these adhesion molecules mediate neutrophil transmigration when leukocytes lack TNF-α receptors (panel B of the figure). (Transmigration of TNFR+ leukocytes was dependent on interaction between Mac-1 and JAM-C.) Under these circumstances, high levels of leukocyte priming did not bypass the need for the endothelial junctional molecules. This is an important finding because neutrophil transmigration can be governed by the ability of the stimulus to activate leukocytes or tissue/EC receptors. It also opens a new avenue ofresearch toward understanding how different signaling cues (proinflammatory cytokines or chemokines) might stimulate distinct repertoires of endothelial receptors to proceed with the transmigration cascade.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal