Abstract
The immunoregulatory enzyme indoleamine 2,3-dioxygenase (IDO) is expressed by a subset of murine plasmacytoid DCs (pDCs) in tumor-draining lymph nodes (TDLNs), where it can potently activate Foxp3+ regulatory T cells (Tregs). We now show that IDO functions as a molecular switch in TDLNs, maintaining Tregs in their normal suppressive phenotype when IDO was active, but allowing inflammation-induced conversion of Tregs to a polyfunctional T-helper phenotype similar to proinflammatory T-helper-17 (TH17) cells when IDO was blocked. In vitro, conversion of Tregs to the TH17-like phenotype was driven by antigen-activated effector T cells and required interleukin-6 (IL-6) produced by activated pDCs. IDO regulated this conversion by dominantly suppressing production of IL-6 in pDCs, in a GCN2-kinase dependent fashion. In vivo, using a model of established B16 melanoma, the combination of an IDO-inhibitor drug plus antitumor vaccine caused up-regulation of IL-6 in pDCs and in situ conversion of a majority of Tregs to the TH17 phenotype, with marked enhancement of CD8+ T-cell activation and antitumor efficacy. Thus, Tregs in TDLNs can be actively reprogrammed in situ into T-helper cells, without the need for physical depletion, and IDO serves as a key regulator of this critical conversion.
Introduction
Regulatory T cells (Tregs) represent a critical barrier to immunotherapy of tumors. Established tumors suppress immune responses against their own antigens, and Tregs are emerging as a key mechanism contributing to this state of functional unresponsiveness.1 In murine models, host Tregs become activated within days of tumor implantation.2 Once activated, Tregs are difficult to eliminate and serve to potently and dominantly inhibit otherwise effective immune responses against the tumor.3
We have shown that Foxp3+ Tregs in the draining lymph nodes of mouse tumors become highly activated by exposure to the immunoregulatory enzyme indoleamine 2,3-dioxygenase (IDO).4,5 In tumor-draining lymph nodes (TDLNs), IDO is expressed by a specific subset of IDO-competent plasmacytoid dendritic cells (DCs).6 The combination of these IDO-expressing pDCs and IDO-activated Tregs renders the local milieu in the TDLNs profoundly inhibitory for T-cell activation.7
Tregs can be suppressive, but this is not a fixed and immutable attribute. Resting Tregs are not spontaneously suppressive, and require an activation step before they become functionally inhibitory.8 Conversely, the suppressive phenotype of Tregs is plastic. When Foxp3 is artificially ablated in mature Tregs, the suppressor phenotype is converted to a proinflammatory, T helper–like phenotype that can participate in autoimmunity.9 Likewise, Tregs exposed to certain inflammatory signals (eg, from activated DCs or TLR ligands) can lose their suppressor activity10 and may alter their phenotype (be “reprogrammed”) to resemble proinflammatory effector cells.11-13 Thus, at least in these experimental models, Tregs show a significant degree of phenotypic plasticity and are susceptible to both activation and deactivation (reprogramming) by signals from their local microenvironment.
However, it is not known whether this apparent plasticity of Tregs is of biologic relevance for tumor immunology. In the current study, we test the hypothesis that, under conditions of antigen-driven T-cell response to tumors, IDO functions as a critical molecular “switch” in TDLNs, regulating the phenotype and functional activity of Tregs. We show that, when IDO is active, Tregs are maintained in their normal potently suppressive state; but when IDO is blocked, Tregs undergo an inflammation-induced, interleukin-6 (IL-6)–dependent conversion into a nonsuppressive, proinflammatory phenotype similar to T-helper-17 (TH17) cells. These findings position IDO as a previously unsuspected key molecular regulator of Treg phenotype and function in TDLNs.
Methods
Reagents, cell lines, and mouse strains
A complete list of reagents, 1-methyl-D-tryptophan (1MT) preparation, tumor cell lines, and all transgenic and knockout mouse strains is given in supplemental materials (available on the Blood website; see the Supplemental Materials link at the top of the online article). Animal studies were approved by the Institutional Animal Care and Use Committee of the Medical College of Georgia. Details are included in the supplemental data.
Tumors
Tumor implantation and harvesting of TDLNs are described in detail in Sharma et al.4 A large inoculum of B16F10 and B16-OVA tumor cells was used (106) to ensure that established tumors rapidly drove Treg activation and suppression in the TDLNs. Tumor area was measured at necropsy on day 11 as the product of orthogonal diameters; or was measured serially 3 times per week.
Vaccines
Details of the vaccine preparations are given in supplemental materials.
Adoptive transfer
For OT-I adoptive transfer, mice received 2 × 106 sorted CD8+ OT-I spleen cells intravenously.4 For Treg adoptive transfer, Tregs were isolated from spleens of TCR-tg OT-II mice bred onto the Foxp3GFP (Thy1.1-congenic) background,14 and fluorescence-activated cell sorter (FACS)-sorted as CD4+GFP+ cells. OT-IIFoxp3-GFP Thy1.1 Tregs (106) were mixed with OT-I cells for coadoptive transfer.
Treg activation cocultures and readout assays
The Treg culture system has been described in detail,4 and complete methods are given in the supplemental data. Activation cocultures contained 2 × 103 pDCs (CD11c+B220+ cells from TDLNs); 105 CD8+ OT-I cells; SIINFEKL peptide; and 5 × 103 Tregs (either CD4+CD25+ cells or CD4+GFP+ Tregs from Foxp3GFP mice).
FACS staining
For intracellular cytokine staining, cells were harvested from cocultures, or isolated from mechanically disaggregated TDLNs ex vivo, and incubated for 4 hours with 5 ng/mL phorbol myristate acetate plus 2 μM ionomycin15 in the presence of brefeldin A (GolgiPlug; BD Biosciences, San Jose, CA), then fixed in Cytoperm/Cytofix (BD Biosciences) on ice, and stained in BD Permwash solution per the manufacturer's instructions. For tumor-disaggregation studies, tumors were treated for 1 hour with 1 mg/mL collagenase (C5138; Sigma-Aldrich, St Louis, MO), 0.1 mg/mL DNAse (D5025; Sigma-Aldrich), and 0.1 mg/mL hyaluronidase (H3884; Sigma-Aldrich) in RPMI 1640 medium.
IDO-transfected TREX cells and Western blot
Detailed methods are given in the supplemental materials.
Statistical analysis
Multiple treatment groups were compared by analysis of variance with Tukey honestly significant difference (HSD) test.
Results
IDO plus effector T cells activate Foxp3+ Tregs for suppression
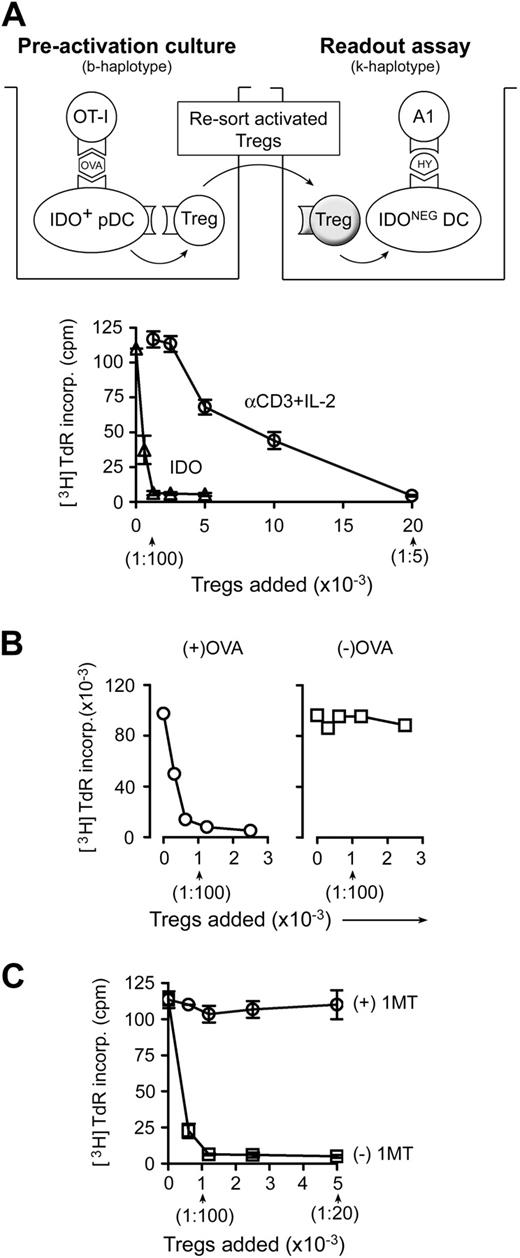
In vitro studies were performed using the coculture system shown in Figure 1A (described in “Treg activation cocultures”).4 Resting Tregs were sorted from spleens of B6 mice without tumors. IDO-expressing pDCs were enriched from the TDLNs of mice with B16 melanoma tumors. As a source of activated effector T cells, OVA-specific OT-I T cells (sorted CD8+) were added to cocultures with cognate OVA peptide antigen. After 2 days, Tregs were recovered from cocultures by FACS sorting and tested for suppressor activity in a readout assay comprising allogeneic A1 T cells (TCR-tg, recognizing a peptide of HY) plus congenic CBA spleen DCs.
Activation of Treg suppressor activity by IDO and effector T cells. (A) Resting splenic Tregs (FACS-sorted CD4+CD25+) were cocultured with pDCs from TDLNs (CD11c+B220+) plus OT-I T cells, OVA peptide antigen, and feeder layer (all on the B6 background). After 2 days, the Tregs were harvested, resorted, and added to readout assays to measure suppressor activity (A1 T cells + congenic spleen DCs, CBA background). Graph represents proliferation in the readout assay by tritiated-thymidine incorporation, using either IDO-activated Tregs or the same Tregs activated with αCD3 cross-linking plus recombinant IL-2 (with IDO blocked using 1MT). Bars represent SD of replicate wells. (B) Tregs were activated with IDO+ pDCs as in panel A, with or without the cognate OVA peptide antigen for OT-I. (C) Tregs in panel A were activated in the presence of OT-I and OVA, with (○) or without (□) D-1MT to block IDO. Experiments in each panel were repeated 3 to 10 times with similar results.
Activation of Treg suppressor activity by IDO and effector T cells. (A) Resting splenic Tregs (FACS-sorted CD4+CD25+) were cocultured with pDCs from TDLNs (CD11c+B220+) plus OT-I T cells, OVA peptide antigen, and feeder layer (all on the B6 background). After 2 days, the Tregs were harvested, resorted, and added to readout assays to measure suppressor activity (A1 T cells + congenic spleen DCs, CBA background). Graph represents proliferation in the readout assay by tritiated-thymidine incorporation, using either IDO-activated Tregs or the same Tregs activated with αCD3 cross-linking plus recombinant IL-2 (with IDO blocked using 1MT). Bars represent SD of replicate wells. (B) Tregs were activated with IDO+ pDCs as in panel A, with or without the cognate OVA peptide antigen for OT-I. (C) Tregs in panel A were activated in the presence of OT-I and OVA, with (○) or without (□) D-1MT to block IDO. Experiments in each panel were repeated 3 to 10 times with similar results.
Figure 1A shows that IDO-activated Tregs acquired efficient suppressor activity, comparable with the most potent suppression reported in the literature,16,17 and an order of magnitude more efficient on a per-cell basis than the same Tregs activated using anti-CD3 antibodies plus recombinant IL-2 (Figure 1A). (For CD3-induced activation, IDO was blocked by adding the IDO-inhibitor 1MT.) We have previously shown that similar IDO-induced Treg activation also occurs in vivo in TDLNs.4
For Tregs to become activated by IDO, it was also necessary for antigen-activated OT-I cells to be present. If the cognate OVA antigen for OT-I was omitted, then Tregs failed to acquire suppressor activity (Figure 1B). Blocking IDO with 1MT also prevented Tregs from acquiring suppressor activity (Figure 1C). Thus, 2 conditions were required for Tregs to become activated for suppression: functional IDO and activated OT-I.
In the absence of IDO, Tregs undergo conversion to a TH17-like phenotype
A key point not elucidated by the preceding experiments was the fate of those Tregs exposed to activated OT-I cells but without the signal from IDO. It is known that, under certain proinflammatory conditions, Tregs can lose their suppressor phenotype. TH17 cells bear a reciprocal developmental relationship to inducible Tregs,15 and some Tregs that lose their suppressive phenotype may up-regulate IL-17.9,11,13,18 Therefore, we asked whether Tregs exposed to activated OT-I in the absence of IDO might convert to a phenotype resembling TH17 cells.
Tregs were FACS-sorted from mice bearing a Foxp3-GFP fusion protein in place of one normal Foxp3 gene.14 Sorted CD4+GFP+ cells from these mice were thus unambiguously known to be Foxp3+ Tregs at the start of the assay. Preactivation cocultures were performed as in Figure 1, with or without OVA and 1MT. After 2 days, cocultures were harvested and stained for intracellular IL-17; Figure 2A shows the gated Treg population (CD4+Foxp3-GFP+). Tregs exposed to activated OT-I cells when IDO was active (no 1MT) showed no IL-17 expression; but Tregs exposed to activated OT-I when IDO was blocked by 1MT contained a substantial proportion of CD4+GFP+ cells that had up-regulated IL-17. IL-17 up-regulation required OT-I activation because IL-17 was not induced in the absence of OVA antigen (Figure 2A last panel).
In the absence of IDO, activated T cells drive conversion of Tregs to a TH17-like phenotype. (A) Sorted CD4+GFP+ Tregs from Foxp3GFP knockin mice were activated in cocultures with pDCs and OT-I as in Figure 1, with or without OVA and 1MT as indicated. After 2 days, cocultures were stained for intracellular IL-17 after a 4-hour stimulation with phorbol myristate acetate/ionomycin plus brefeldin A. The top dot plot represents an example of a representative gate for the Foxp3GFP-positive CD4+ Tregs. The bottom plots represent the gated Treg population in each treatment group. (B) Tregs (CD4+CD25+) were sorted from homozygous-null Rorγt gfp/gfp mice lacking functional RORγt, or from WT controls, and activated in cocultures with and without 1MT as shown. After 2 days, cultures were stained for CD4 to identify Tregs versus IL-17. (The only CD4+ cells in cocultures were the original sorted Tregs.) (C) Tregs from Rorγt-null mice, or control wild-type Tregs, were activated in cocultures for 2 days, with (□) or without (◊) 1MT. Tregs were sorted and functional suppressor activity measured against A1 T-cell readout as in Figure 1. (D) Foxp3GFP Tregs were sorted and activated in cocultures with 1MT and OVA. Plots represent 4-color staining from a representative sample, gated on GFP+CD4+. (E) Foxp3GFP Tregs were activated as in panel A and stained for IL-17 versus the cytokines shown. Plots represent the gated CD4+GFP+ cells. Experiments were repeated 3 to 12 times with similar results.
In the absence of IDO, activated T cells drive conversion of Tregs to a TH17-like phenotype. (A) Sorted CD4+GFP+ Tregs from Foxp3GFP knockin mice were activated in cocultures with pDCs and OT-I as in Figure 1, with or without OVA and 1MT as indicated. After 2 days, cocultures were stained for intracellular IL-17 after a 4-hour stimulation with phorbol myristate acetate/ionomycin plus brefeldin A. The top dot plot represents an example of a representative gate for the Foxp3GFP-positive CD4+ Tregs. The bottom plots represent the gated Treg population in each treatment group. (B) Tregs (CD4+CD25+) were sorted from homozygous-null Rorγt gfp/gfp mice lacking functional RORγt, or from WT controls, and activated in cocultures with and without 1MT as shown. After 2 days, cultures were stained for CD4 to identify Tregs versus IL-17. (The only CD4+ cells in cocultures were the original sorted Tregs.) (C) Tregs from Rorγt-null mice, or control wild-type Tregs, were activated in cocultures for 2 days, with (□) or without (◊) 1MT. Tregs were sorted and functional suppressor activity measured against A1 T-cell readout as in Figure 1. (D) Foxp3GFP Tregs were sorted and activated in cocultures with 1MT and OVA. Plots represent 4-color staining from a representative sample, gated on GFP+CD4+. (E) Foxp3GFP Tregs were activated as in panel A and stained for IL-17 versus the cytokines shown. Plots represent the gated CD4+GFP+ cells. Experiments were repeated 3 to 12 times with similar results.
Of note, the IL-17–expressing cells in cocultures were known to arise specifically from conversion of preexisting Foxp3+ Tregs (not from differentiation of naive CD4+ cells) because the only CD4+ cells present in cocultures were the original Foxp3GFP-positive Tregs. Furthermore, the newly converted IL-17+ cells uniformly continued to express residual Foxp3GFP fluorescence, confirming their origin from the original Foxp3+ Tregs. (Similar coexpression of Foxp3 and IL-17 during Treg reprogramming has been observed in other systems as well.13,19 )
The transcription factor RORγt is required for normal differentiation of naive CD4+ T cells along the TH17 lineage.20 We asked whether reprogramming of Foxp3+ Tregs to TH17-like cells also required RORγt. Rorγt gfp/gfp have a knockin of an enhanced green fluorescent protein sequence that disrupts the normal RORγt locus21 ; homozygous-null mice are unable to up-regulate IL-17 during TH17 differentiation.20 Tregs were isolated from Rorγt gfp/gfp mice by sorting for CD4+CD25+ cells. Figure 2B shows that Rorγt-null Tregs were unable to convert to IL-17 expression in our system. We next asked whether RORγt was required for the loss of functional Treg suppressor activity observed when IDO was blocked by 1MT (Figure 1C). Figure 2C shows that wild-type Tregs activated in the presence of 1MT lost all functional suppressor activity, as expected, whereas RORγt-deficient Tregs activated under identical conditions retained significant suppressor activity, even in the presence of 1MT. Thus, the loss of suppressor activity seen when IDO was blocked by 1MT represented an active, RORγt-dependent conversion of Tregs to the TH17 phenotype, not simply a passive failure of Treg activation.
IL-17 is expressed early during TH17 differentiation, whereas IL-22 is expressed later and is thus is a marker of an established TH17 phenotype.22 Figure 2D shows 4-color staining of Foxp3+ Tregs activated as in Figure 2A, under (+)1MT(+)OVA conditions. IL-22 was coexpressed by essentially all the Tregs that had up-regulated IL-17. Thus, based on IL-17 expression, RORγt dependence, and coexpression of IL-22, the Tregs in our system converted to a phenotype similar to authentic TH17 cells.
Under strong proinflammatory conditions, CD4+ T-helper cells may coexpress multiple cytokines (so-called polyfunctional T-helper cells), including IL-17, IL-2, and others.23 TH17 cells can coexpress IL-2 in vivo,24 and TH17 cells differentiated in vitro can produce IL-2 and tumor necrosis factor-α (TNF-α).25 Figure 2E shows that, in our system, most of the reprogrammed Tregs coexpressed IL-2 and TNF-α, in addition to IL-17 and IL-22. Only a small number of reprogrammed cells expressed interferon-γ or IL-10. Thus, reprogrammed Tregs appeared highly activated and a source of multiple proinflammatory cytokines.
Up-regulation of IL-17 in Tregs is driven by IL-6
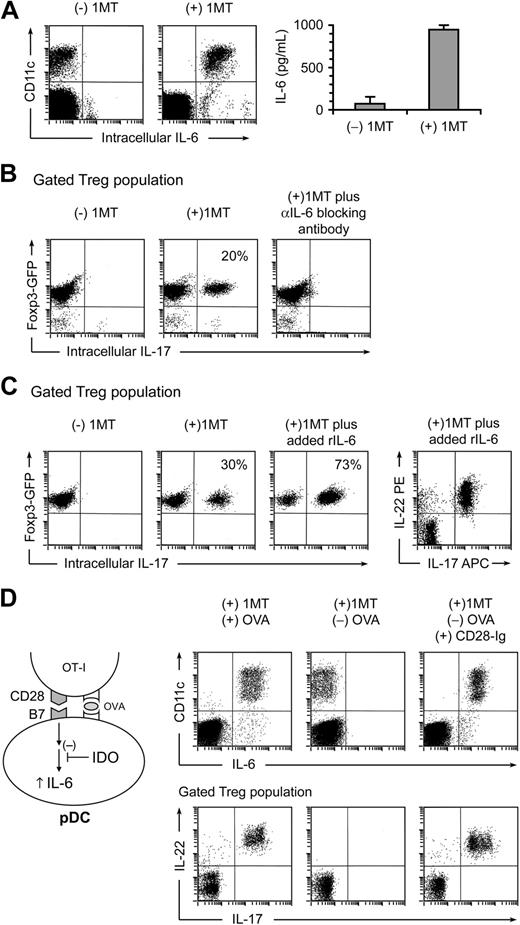
IL-6 is a proinflammatory cytokine that, in conjunction with transforming growth factor β (TGFβ), can drive the differentiation of naive CD4+ T cells toward the TH17 lineage.15 Under certain conditions, IL-6 can be produced by activated pDCs, so we asked whether pDCs from TDLNs produced IL-6 in our cocultures (Figure 3A). For these studies, the feeder layers in cocultures were depleted of macrophages (a potential contaminating source of IL-6). IL-17 up-regulation in Tregs was unaffected by macrophage depletion, and essentially all of the IL-6–producing cells under these conditions were the pDCs (identified as CD11c+ in the FACS plots). IL-6 was expressed only when IDO was blocked with 1MT; if IDO was enzymatically active, then IL-6 was suppressed. The suppressive effect of IDO was further confirmed by measuring IL-6 in coculture supernatants by enzyme-linked immunosorbent assay (Figure 3A right panel).
Up-regulation of IL-17 is driven by IL-6. (A) Cocultures were performed as in Figure 2, with and without 1MT. After 2 days, cultures were stained for IL-6 versus CD11c (to mark the sorted pDCs). The right graph represents IL-6 measurement by enzyme-linked immunosorbent assay on supernatants of cocultures (error bars show SD of quadruplicate wells). (B) Tregs were sorted from Foxp3GFP mice and activated in cocultures, with or without 1MT plus neutralizing polyclonal antibody against IL-6. After 2 days, cocultures were stained for CD4 versus IL-17. Plots represent the gated CD4+ (Treg) population. (C) Sorted Foxp3GFP Tregs were activated in cocultures with or without 1MT. Recombinant IL-6 (100 ng/mL) was added as shown. Plots represent the gated CD4+ (Treg) population. Plot at right indicates a representative example of coexpression of IL-22 and IL-17 on gated Tregs in IL-6–treated cocultures. (D) IL-6 up-regulation in pDCs requires OVA antigen or CD28→B7 engagement. Cocultures were performed in the presence of 1MT, with and without OVA or recombinant CD28-Ig fusion protein (20 μg/mL), as indicated. After 2 days, cultures were stained for IL-6 versus CD11c (top plots), and for CD4 versus IL-17 versus IL-22 (bottom plots, gated on the CD4+ Treg population). All experiments were repeated 3 to 10 times with similar results.
Up-regulation of IL-17 is driven by IL-6. (A) Cocultures were performed as in Figure 2, with and without 1MT. After 2 days, cultures were stained for IL-6 versus CD11c (to mark the sorted pDCs). The right graph represents IL-6 measurement by enzyme-linked immunosorbent assay on supernatants of cocultures (error bars show SD of quadruplicate wells). (B) Tregs were sorted from Foxp3GFP mice and activated in cocultures, with or without 1MT plus neutralizing polyclonal antibody against IL-6. After 2 days, cocultures were stained for CD4 versus IL-17. Plots represent the gated CD4+ (Treg) population. (C) Sorted Foxp3GFP Tregs were activated in cocultures with or without 1MT. Recombinant IL-6 (100 ng/mL) was added as shown. Plots represent the gated CD4+ (Treg) population. Plot at right indicates a representative example of coexpression of IL-22 and IL-17 on gated Tregs in IL-6–treated cocultures. (D) IL-6 up-regulation in pDCs requires OVA antigen or CD28→B7 engagement. Cocultures were performed in the presence of 1MT, with and without OVA or recombinant CD28-Ig fusion protein (20 μg/mL), as indicated. After 2 days, cultures were stained for IL-6 versus CD11c (top plots), and for CD4 versus IL-17 versus IL-22 (bottom plots, gated on the CD4+ Treg population). All experiments were repeated 3 to 10 times with similar results.
To test whether IL-6 was mechanistically required for up-regulation of IL-17, we used neutralizing anti-IL-6 antibody. Figure 3B shows that blocking IL-6 completely abrogated up-regulation of IL-17 in Tregs in cocultures. Consistent with a mechanistic role for IL-6, addition of exogenous recombinant IL-6 to cocultures drove even more conversion of Tregs, such that the large majority now became converted to the TH17-like phenotype (Figure 3C).
IL-6 expression in pDCs is triggered by activated OT-I cells
IL-6 induction in pDCs also required a signal from activated OT-I cells, in addition to IDO blockade. Thus, if OVA antigen was omitted from cocultures, IL-6 was not induced even in the presence of 1MT (Figure 3D middle panel). This suggested that antigen presentation by pDCs to OT-I might be necessary to trigger IL-6 induction. During the process of antigen presentation, it is known that CD28 and its cognate B7 counterligands actively cluster in the immunologic synapse26 ; and in other systems, CD28-mediated engagement of B7 can generate an intracellular “reverse” signal in DCs that triggers IL-6 production.27 Therefore, we asked whether the requirement OVA antigen in our system could be replaced by artificially cross-linking B7 molecules, using a recombinant CD28-Ig fusion protein, as described by others.27 Figure 3D (right panel) shows that CD28-Ig was able to fully substitute for the OVA signal, both for IL-6 induction in the pDCs, and for driving IL-17 and IL-22 up-regulation in Tregs. These findings were thus consistent with a model (diagrammed in Figure 3D) in which antigen-activated OT-I delivered an IL-6–inducing signal to pDCs via CD28-mediated engagement of B7 molecules.
IDO suppresses expression of IL-6 in pDCs
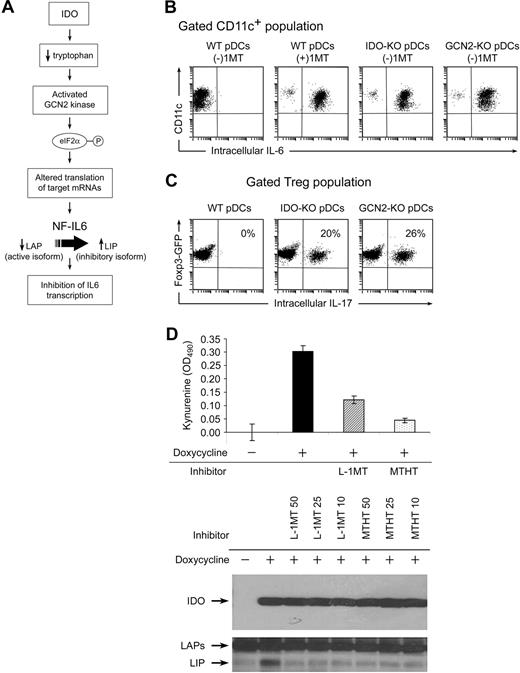
Figure 3A had shown that IL-6 was produced by pDCs only when IDO was blocked by 1MT. This suggested that the IDO in pDCs might actively suppress their own production of IL-6. (We have previously demonstrated such an autocrine/paracrine effect of IDO on type I interferon production by pDCs.28 ) IDO depletes the amino acid tryptophan, which can activate the amino acid-sensitive GCN2-kinase pathway, as diagrammed in Figure 4A. Activated GCN2 phosphorylates ribosomal eIF2α, which alters translation of target mRNAs. One mRNA species known to be sensitive to amino acid-induced regulation is the transcription factor NF-IL-6 (C/EBPβ), which is a key regulator of IL6 gene transcription.29 Therefore, we asked whether IDO blocked IL-6 production by activating the GCN2 pathway in pDCs.
Evidence that IDO acts via the GCN2-kinase pathway in pDCs to block IL-6 up-regulation. (A) Hypothesized pathway for IDO-induced translational regulation of NF-IL-6 transcription factor. (B) pDCs were isolated from TDLNs of tumors grown in genetically defined hosts (IDO1-KO, GCN2-KO, or WT). pDCs were then used in activation cocultures with OT-I and Tregs, as Figure 2. After 2 days, cocultures were stained for CD11c versus IL-6. (C) Foxp3GFP Tregs were cocultured as in Figure 2A, using pDCs from TDLNs of WT, IDO1-KO, or GCN2-KO hosts. All cultures were without 1MT. After 2 days, cultures were harvested and stained for intracellular IL-17 versus CD4. (D) Analysis of the inhibitory LIP isoform of NF-IL-6 in T-REX cells stably transfected with inducible IDO cDNA. IDO was either uninduced or induced by treatment with doxycycline (20 ng/mL) as indicated. Induced cells were treated with 50, 25, and 10 μM of the IDO inhibitors L-1MT or methyl-thiohydantoin-tryptophan (MTHT), as indicated. Graph documents production of kynurenine by functional IDO (error bars show SD of triplicate wells). The top Western blot represents expression of IDO after induction; the bottom Western blot represents induction of the 21-kDa LIP isoform of NF-IL-6, and the higher molecular weight LAP isoforms. All experiments were repeated 3 to 4 times with similar results.
Evidence that IDO acts via the GCN2-kinase pathway in pDCs to block IL-6 up-regulation. (A) Hypothesized pathway for IDO-induced translational regulation of NF-IL-6 transcription factor. (B) pDCs were isolated from TDLNs of tumors grown in genetically defined hosts (IDO1-KO, GCN2-KO, or WT). pDCs were then used in activation cocultures with OT-I and Tregs, as Figure 2. After 2 days, cocultures were stained for CD11c versus IL-6. (C) Foxp3GFP Tregs were cocultured as in Figure 2A, using pDCs from TDLNs of WT, IDO1-KO, or GCN2-KO hosts. All cultures were without 1MT. After 2 days, cultures were harvested and stained for intracellular IL-17 versus CD4. (D) Analysis of the inhibitory LIP isoform of NF-IL-6 in T-REX cells stably transfected with inducible IDO cDNA. IDO was either uninduced or induced by treatment with doxycycline (20 ng/mL) as indicated. Induced cells were treated with 50, 25, and 10 μM of the IDO inhibitors L-1MT or methyl-thiohydantoin-tryptophan (MTHT), as indicated. Graph documents production of kynurenine by functional IDO (error bars show SD of triplicate wells). The top Western blot represents expression of IDO after induction; the bottom Western blot represents induction of the 21-kDa LIP isoform of NF-IL-6, and the higher molecular weight LAP isoforms. All experiments were repeated 3 to 4 times with similar results.
B16 tumors were grown in WT B6 mice or in mice lacking IDO1 (IDO1-KO) or GCN2 (GCN2-KO). pDCs from TDLNs were used as antigen-presenting cells in activation cocultures (the Tregs and other cells in coculture were all from WT mice). Figure 4B shows that pDCs lacking IDO1 were unable to suppress their own IL-6 production in cocultures (ie, even without 1MT, IL-6 was still expressed when pDCs lacked IDO1). Similarly, GCN2-KO pDCs were unable to suppress their own IL-6 production. Both IDO1-KO and GCN2-KO pDCs spontaneously drove conversion of Tregs to TH17-like cells in cocultures, without the requirement for added 1MT (Figure 4C).
The NF-IL-6 (C/EBPβ) transcription factor exists in 2 forms: transcriptionally active liver-enriched transcriptional activator protein (LAP) isoforms that promote IL-6 transcription, and the dominant-negative liver inhibitory protein (LIP), which inhibits LAP. Both LIP and LAP are generated from the same mRNA via alternate ribosomal start sites.29 GCN2 kinase is known to alter ribosomal initiation of many mRNA species.30 Therefore, we asked whether the IDO→GCN2 pathway might up-regulate the dominantly inhibitory LIP isoforms of NF-IL-6. These mechanistic studies could not be performed on the tiny number of primary pDCs from TDLNs, so we used a model of T-REX cells transfected with a doxycycline-inducible IDO cDNA construct. Figure 4D shows that induction of the IDO transgene triggered up-regulation of the inhibitory LIP isoform of NF-IL-6, and that this was blocked by 2 different functional inhibitors of IDO enzymatic activity. Thus, taken together, our data are consistent with the hypothesis that IDO directly suppresses IL-6 induction, via GCN2-mediated regulation of NF-IL-6.
Replacement of Foxp3+ Tregs by TH17-like cells in vivo
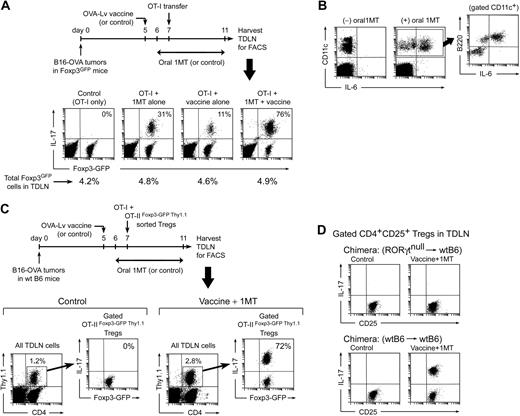
Our in vitro model showed that 3 cell types (ie, Tregs, pDCs, and activated OT-I cells) needed to come together under conditions in which IDO was blocked to convert Tregs to TH17-like cells. To test whether this interaction could occur in vivo, we used B16 tumor cells transfected with an ovalbumin transgene (B16-OVA)31 implanted in Foxp3GFP mice. On day 7 of tumor growth, resting OT-I cells were adoptively transferred intravenously, as shown in the schematic in Figure 5A. Before adoptive transfer, mice were treated with or without oral 1MT in drinking water to block IDO. To further drive activation of OT-I cells, some mice were immunized with a vaccine containing the OVA DNA sequence delivered in a lentiviral vector (OVA-Lv vaccine).
Generation of TH17-like cells in TDLNs in vivo. (A) Foxp3GFP mice with B16-OVA tumors were treated with OVA-Lv vaccine, oral D-1MT, and adoptive transfer–sorted OT-I cells, as shown. On day 11, TDLNs were harvested and stained for IL-17. Percentages in the right-upper quadrants of each plot give the fraction of the Foxp3GFP-positive cells that coexpressed IL-17. Percentages below give total Foxp3GFP-positive cells in each LN. (B) Mice were treated as in panel A and TDLN cells stained for CD11c versus B220 versus intracellular IL-6. Plots represent total LN cells; inset represents gated CD11c+ population from the (+)1MT group. (C) Wild-type B6 mice with B16-OVA tumors were treated with control (vehicle only) or oral D-1MT plus OVA-Lv vaccine. All mice received coadoptive transfer of 106 CD8+ OT-I cells mixed with 106 sorted Foxp3+ Tregs (CD4+GFP+Thy1.1+) from OT-IIFoxp3-GFP Thy1.1 mice. On day 11, TDLNs were harvested and stained for CD4/Thy1.1/IL-17 versus Foxp3GFP by 4-color FACS. Each left plot represents the population of transferred Tregs (CD4+Thy1.1+) as a percentage of total TDLN cells; right plots represent GFP versus IL-17 expression in the gated GFP+ Tregs (the percentage gives the fraction of Foxp3GFP-positive cells that coexpress IL-17). (D) Bone marrow chimeras (RORγt-null marrow into wt B6 hosts, or control wtB6→wtB6) received B16-OVA tumors, and mice were treated as in panel A with either control (vehicle only) or oral D-1MT plus OVA-Lv vaccine. All mice received OT-I adoptive transfer on day 7. Plots indicate representative IL-17 up-regulation in gated CD4+CD25+ population in TDLNs from each treatment group on day 11. Experiments were repeated 3 to 8 times with similar results.
Generation of TH17-like cells in TDLNs in vivo. (A) Foxp3GFP mice with B16-OVA tumors were treated with OVA-Lv vaccine, oral D-1MT, and adoptive transfer–sorted OT-I cells, as shown. On day 11, TDLNs were harvested and stained for IL-17. Percentages in the right-upper quadrants of each plot give the fraction of the Foxp3GFP-positive cells that coexpressed IL-17. Percentages below give total Foxp3GFP-positive cells in each LN. (B) Mice were treated as in panel A and TDLN cells stained for CD11c versus B220 versus intracellular IL-6. Plots represent total LN cells; inset represents gated CD11c+ population from the (+)1MT group. (C) Wild-type B6 mice with B16-OVA tumors were treated with control (vehicle only) or oral D-1MT plus OVA-Lv vaccine. All mice received coadoptive transfer of 106 CD8+ OT-I cells mixed with 106 sorted Foxp3+ Tregs (CD4+GFP+Thy1.1+) from OT-IIFoxp3-GFP Thy1.1 mice. On day 11, TDLNs were harvested and stained for CD4/Thy1.1/IL-17 versus Foxp3GFP by 4-color FACS. Each left plot represents the population of transferred Tregs (CD4+Thy1.1+) as a percentage of total TDLN cells; right plots represent GFP versus IL-17 expression in the gated GFP+ Tregs (the percentage gives the fraction of Foxp3GFP-positive cells that coexpress IL-17). (D) Bone marrow chimeras (RORγt-null marrow into wt B6 hosts, or control wtB6→wtB6) received B16-OVA tumors, and mice were treated as in panel A with either control (vehicle only) or oral D-1MT plus OVA-Lv vaccine. All mice received OT-I adoptive transfer on day 7. Plots indicate representative IL-17 up-regulation in gated CD4+CD25+ population in TDLNs from each treatment group on day 11. Experiments were repeated 3 to 8 times with similar results.
Figure 5A shows that mice receiving only OT-I cells (control group) had no IL-17 expression by the endogenous Foxp3GFP Tregs in TDLNs. Mice receiving OT-I plus concomitant 1MT administration showed a minority of Foxp3GFP cells converting to IL-17 expression (typically 25%–30%). Mice receiving OT-I plus vaccine (without 1MT) showed little IL-17 expression. However, the combination of vaccine plus 1MT resulted in conversion of the majority of Tregs to IL-17 expression (up to 75% or greater coexpression of Foxp3GFP and IL-17; Figure 5A fourth panel). In all groups, the total percentage of Foxp3GFP-expressing cells in the TDLNs remained constant (shown as the percentages below each dot plot in Figure 5A), with the change occurring in the relative fraction of cells coexpressing IL-17.
Furthermore, consistent with the predictions of our in vitro model, many of the pDCs in TDLNs up-regulated IL-6 when challenged with OT-I cells in the presence of 1MT (Figure 5B). Typically, 2% to 3% of total TDLN cells were found to be DCs (defined as CD11c+); within these, the expression of IL-6 was confined to the CD11c+B220+ (plasmacytoid DC) fraction, as shown in the gated population in Figure 5B. (In these studies, the LN disaggregation protocol was optimized for recovery of pDCs, so recovery of myeloid DCs may not have been quantitative; but qualitatively the expression of IL-6 was confined to the pDCs.)
Direct conversion of mature Foxp3+ Tregs to TH17-like cells in vivo
In Figure 5A, the presence of residual Foxp3GFP fluorescence in essentially all of the IL-17–expressing cells suggested that the IL-17+ cells might arise from conversion of preexisting Foxp3+ Tregs (which we had shown to occur in our in vitro model). To test this, wild-type B6 mice with B16-OVA tumors were immunized in the presence of 1MT, and a defined population of mature, Foxp3+ Tregs were adoptively transferred at the time of OT-I injection (Figure 5C). The transferred Tregs were isolated from TCR-tg OT-II mice (CD4+, specific for a peptide of ovalbumin) that had been crossed with Foxp3GFP mice and bred on a Thy1.1 congenic background (described in “Mouse strains” in supplemental materials). OT-IIFoxp3-GFP Thy1.1 Tregs were sorted as CD4+GFP+ cells and thus were known to be uniformly Foxp3+ at the time of transfer. Figure 5C shows that, in control recipients (without vaccination or 1MT), none of the transferred OT-IIFoxp3-GFP Thy1.1 Tregs in TDLNs converted to IL-17 expression. However, in mice treated with OVA-Lv vaccine and 1MT, the majority of transferred Tregs in TDLNs up-regulated IL-17. These IL-17–expressing cells were unambiguously identified as the transferred Tregs by the Thy1.1 congenic marker and retained residual Foxp3GFP fluorescence (just as in our in vitro model). Thus, these studies formally demonstrated that mature preexisting Foxp3+ Tregs could be directly converted to the IL-17–expressing phenotype in vivo. For the studies shown, we chose OT-II Tregs with a TCR recognizing a tumor antigen, as used by others,32 but we obtained similar results using polyclonal natural Tregs from Foxp3GFP donors (data not shown), so the observed in vivo reprogramming was not restricted to OT-II cells.
Figure 5D shows that up-regulation of IL-17 by Tregs in TDLNs also required an intact RORγt transcription factor in the Tregs (consistent with the in vitro model shown in Figure 2B). For these studies, the tumor-bearing hosts were bone marrow chimeras of RORγtnull marrow transplanted into wt B6 hosts because the RORγt-deficient mice themselves are defective in peripheral LN development.21
Enhanced antitumor response to vaccine plus 1MT
We next asked whether replacement of Tregs by TH17-like cells in TDLNs was associated with enhanced functional antitumor immune response. We first addressed this question in the nominal B16-OVA system, where the CD8+ effector cells were known. B16-OVA tumors grow aggressively in immunocompetent hosts, despite the potent xenoantigen31 ; and once established, tumors induce unresponsiveness in naive OT-I cells4,33 and convert naive CD4+ OT-II cells into adaptive Tregs.32 Thus, B16-OVA is informative because the artificial antigen is highly immunogenic, yet the antitumor immune response is suppressed.
Mice with B16-OVA tumors received various combinations of OVA-Lv vaccine, 1MT in drinking water, and OT-I adoptive transfer as indicated in Figure 6A (delivered via the same protocol as in Figure 5A). On day 11, tumors were measured in situ at necropsy. (Day 11 was chosen because even partial responses were evident at this time point; whereas at later time points, minor differences became obscured as tumors grew out.) The maximum reduction in tumor size on day 11 was obtained by adding 1MT to the regimen of vaccination plus OT-I, corresponding to the conditions that produced maximum conversion of Tregs to TH17-like cells (compare Figure 5A). When followed for a longer period, tumors treated with 1MT plus OT-I and vaccine showed sustained growth delay (Figure 6B).
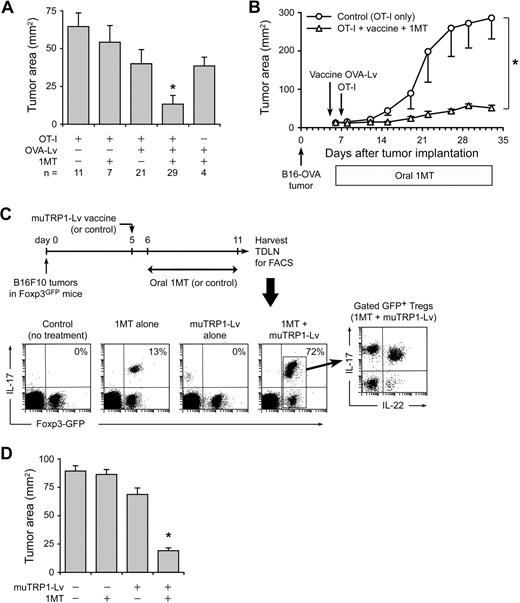
Replacement of Tregs by TH17-like cells is associated with enhanced antitumor efficacy. (A) Foxp3GFP mice with established B16-OVA tumors were treated using the protocol shown in Figure 5, with or without resting OT-I cells, OVA-Lv vaccine, and oral D-1MT, as indicated below the axis. On day 11, tumors were dissected and the tumor area measured as the product of orthogonal diameters. Values reflect the mean of pooled data from 7 independent experiments (error bars show SD); the total number of tumors analyzed in each treatment (n) is shown. *P < .01 by analysis of variance versus all other groups; bars represent SD. (B) B6 mice with established B16-OVA tumors were treated with adoptive transfer of resting OT-I cells (control) or OT-I cells plus OVA-Lv vaccine plus oral D-1MT, as in the previous panel. Data points represent average of 5 mice; bars represent SD. One of 2 experiments. (C) Foxp3GFP mice were injected with 106 B16F10 tumor cells. On day 5, mice received muTRP1-Lv vaccine (or control). D-1MT in drinking water (or control) was started on day 6. On day 11, TDLNs were harvested and stained for intracellular IL-17 as in Figure 5. Percentages give the fraction of Foxp3GFP-positive cells coexpressing IL-17. Representative of4 independent experiments. (D) Foxp3GFP mice with established B16F10 tumors were treated with or without muTRP1-Lv vaccine and D-1MT in drinking water, as indicated. Tumor size was measured at necropsy on day 11. Each data point is a mean of 6 tumors from 3 independent experiments (error bars show SD). *P < .01 by analysis of variance.
Replacement of Tregs by TH17-like cells is associated with enhanced antitumor efficacy. (A) Foxp3GFP mice with established B16-OVA tumors were treated using the protocol shown in Figure 5, with or without resting OT-I cells, OVA-Lv vaccine, and oral D-1MT, as indicated below the axis. On day 11, tumors were dissected and the tumor area measured as the product of orthogonal diameters. Values reflect the mean of pooled data from 7 independent experiments (error bars show SD); the total number of tumors analyzed in each treatment (n) is shown. *P < .01 by analysis of variance versus all other groups; bars represent SD. (B) B6 mice with established B16-OVA tumors were treated with adoptive transfer of resting OT-I cells (control) or OT-I cells plus OVA-Lv vaccine plus oral D-1MT, as in the previous panel. Data points represent average of 5 mice; bars represent SD. One of 2 experiments. (C) Foxp3GFP mice were injected with 106 B16F10 tumor cells. On day 5, mice received muTRP1-Lv vaccine (or control). D-1MT in drinking water (or control) was started on day 6. On day 11, TDLNs were harvested and stained for intracellular IL-17 as in Figure 5. Percentages give the fraction of Foxp3GFP-positive cells coexpressing IL-17. Representative of4 independent experiments. (D) Foxp3GFP mice with established B16F10 tumors were treated with or without muTRP1-Lv vaccine and D-1MT in drinking water, as indicated. Tumor size was measured at necropsy on day 11. Each data point is a mean of 6 tumors from 3 independent experiments (error bars show SD). *P < .01 by analysis of variance.
Treg conversion can be driven by endogenous T cells against a shared self/tumor antigen
The OVA system was informative for mechanistic studies, but a more realistic clinical scenario is vaccination against a shared self/tumor antigen to which the host is already tolerant. Under these conditions, it was not clear whether there would be adequate endogenous CD8+ T-cell response to drive conversion of Tregs to TH17-like cells. To test this, we used an altered peptide ligand sequence developed against the melanoma-associated antigen TRP1, optimized to break tolerance to native TRP1 in tumor-bearing hosts.34,35 The muTRP1-Lv vaccine was delivered via the same recombinant lentivirus vector used in Figure 6C to deliver OVA.36 B16F10 tumors were grown in Foxp3GFP knockin mice, and mice were immunized with muTRP1-Lv vaccine, with or without oral 1MT, as shown in Figure 6C. Mice receiving 1MT alone showed few GFP+ Tregs converting to IL-17 expression, and mice receiving vaccine alone showed minimal conversion. However, mice receiving the combination of muTRP1-Lv vaccine and 1MT showed conversion of a large majority of Tregs in TDLNs into TH17-like cells. Thus, vaccination against an endogenous shared self/tumor antigen was able to drive extensive reprogramming of Tregs when combined with 1MT.
Similar to the nominal OVA system, reprogramming of Tregs was associated with enhanced functional antitumor responses to muTRP1-Lv vaccine, measured by tumor size on day 11 (Figure 6D). As in the B16-OVA experiments, a large inoculum of B16F10 tumor cells (106) and an early time point were used; under these stringent conditions, vaccine and 1MT were each minimally effective as single agents, but the combination of vaccine + 1MT showed significant synergistic antitumor effect.
1MT enhances response to CpG-based vaccine
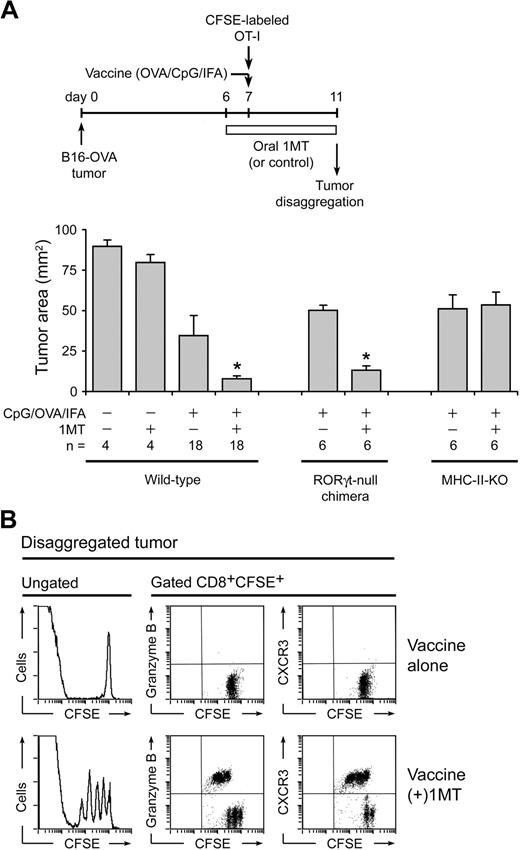
To confirm that the effect of 1MT was not restricted only to lentivector vaccines, we tested 1MT with a vaccine comprising OVA protein emulsified in incomplete Freund's adjuvant plus CpG oligodeoxynucleotide 1826, a TLR9 ligand.37 Figure 7A shows that this vaccine, by itself, had only modest effect against established (day 7) B16-OVA tumors, but that the addition of 1MT showed significant synergy with vaccine. When similar studies were performed in RORγt-null bone marrow chimeric mice (RORγt-null marrow→B6 hosts, as in Figure 5D), the synergistic effect of 1MT was preserved, indicating that the RORγt/IL-17 pathway itself was not indispensible for antitumor effect of 1MT (Figure 7A). However, we noted that the RORγt pathway is selective for IL-17, and RORγt-null Tregs could still up-regulate IL-22 and undergo other proinflammatory changes. Therefore, we asked whether mice lacking all CD4+ T helper cells (not just RORγt/IL-17) were still able to respond to vaccine plus 1MT. For these studies, we used major histocompatibility complex (MHC) class II–deficient mice (IAb-KO mice), which lack all detectable CD4+ T cells (both Tregs and T-helper cells).38 In these mice, the synergistic effect of 1MT was completely abrogated (Figure 7A). Thus, the helper activity of CD4+ cells appeared required for the synergistic effect of 1MT.
Enhancement of CpG-based vaccine by 1MT. (A) Wild-type B6 mice with established B16-OVA tumors were treated with adoptive transfer of OT-I cells, with or without 1MT, and with or without vaccine (OVA protein in incomplete Freund adjuvant plus CpG-1826). In parallel experiments, wild-type hosts were compared with RORγt-null bone marrow chimeric hosts (as in Figure 5D) or with MHC class II-deficient (IAb-KO) hosts. Mean data are indicated, pooled from a total of 8 experiments; bars represent SD. *P < .05 by analysis of variance. (B) B6 mice with B16-OVA tumors received CFSE-labeled OT-I cells plus OVA/CpG/IFA vaccine, with or without 1MT as shown. After 4 days, tumors were disaggregated and stained for CD8 versus granzyme B or CXCR3.
Enhancement of CpG-based vaccine by 1MT. (A) Wild-type B6 mice with established B16-OVA tumors were treated with adoptive transfer of OT-I cells, with or without 1MT, and with or without vaccine (OVA protein in incomplete Freund adjuvant plus CpG-1826). In parallel experiments, wild-type hosts were compared with RORγt-null bone marrow chimeric hosts (as in Figure 5D) or with MHC class II-deficient (IAb-KO) hosts. Mean data are indicated, pooled from a total of 8 experiments; bars represent SD. *P < .05 by analysis of variance. (B) B6 mice with B16-OVA tumors received CFSE-labeled OT-I cells plus OVA/CpG/IFA vaccine, with or without 1MT as shown. After 4 days, tumors were disaggregated and stained for CD8 versus granzyme B or CXCR3.
In the tumors themselves, CFSE-labeled OT-I cells showed better ability to divide and up-regulate differentiation markers (granzyme B and CXCR3) in mice treated with 1MT plus vaccine, compared with vaccine alone (Figure 7B). Indeed, proliferation of OT-I in these large established tumors was poor in the absence of 1MT, reminiscent of the reported suppression of OT-I by other established tumors.39 In these studies, as with the lentivector experiments in Figure 6, stringent conditions (large established tumors) were chosen to favor suppression.
Discussion
The current study demonstrates that Tregs in TDLNs retain a remarkable degree of phenotypic plasticity. Under the right conditions, a large majority of Tregs in TDLNs could be reprogrammed in situ into a polyfunctional T-helper phenotype resembling proinflammatory TH17 cells. Using in vitro and in vivo models, we show that this conversion requires a signal from activated effector T cells, combined with inhibition of the immunosuppressive IDO pathway.
The CD4+ T-cell lineage is emerging as more plastic than previously thought.40 In the case of Tregs, it is known that certain forms of inflammation or activated DCs can block Treg-suppressive activity11,41-43 via a mechanism dependent, at least in part, on IL-6.10 Tregs that have been “deactivated” by such signals may down-regulate Foxp3 and up-regulate helper/effector cytokines, such as IL-2 and IL-17.9,11,13,44,45 However, it has not been known whether this Treg plasticity was biologically relevant to tumor immunology or whether it was amenable to therapeutic manipulation. We now show that widespread reprogramming of Tregs can occur physiologically in TDLNs, that IDO is a key molecular regulator of this critically important checkpoint, and that this checkpoint can be pharmacologically targeted by an orally bioavailable small-molecule inhibitor of IDO.
The phenotype of reprogrammed Tregs was similar to activated TH17 cells or to “polyfunctional” T-helper cells because they coexpressed both IL-17 and IL-22 (associated with the TH17 lineage), and also IL-2 and TNF-α. Some TH17 cells are known to coexpress other cytokines, such as IL-2.24,25 In our system, we refer to the reprogrammed Tregs as “TH17-like” because of their RORγt-dependent induction of IL-17 expression, but whether they are considered TH17 cells or polyfunctional T-helper cells is largely a matter of semantics. The important mechanistic finding is that they appear to be a potent source of helper cytokines. Our studies with CD4-deficient mice (MHC-II-KO) suggest that CD4+ T-helper cells play an indispensable role in the synergistic antitumor effects of 1MT. These helper effects are more than just the proinflammatory effects of IL-17, as shown by the studies with RORγt-null mice. We speculate that the helper cytokines from reprogrammed Tregs may be an important mechanism of CD4+ help in vivo in the setting of vaccination plus IDO blockade. Further studies will be required to test the hypothesis that reprogrammed Tregs directly supply helper cytokines to antitumor CD8+ cells and to determine whether this occurs in lymph nodes, tumors, or both.
A key finding from our study is that signals from activated effector T cells were strictly required to drive conversion of Tregs to TH17-like cells when IDO was blocked. In vitro, this was shown by the obligate requirement for antigen-activated OT-I to up-regulate IL-6 in pDCs and to drive conversion of Tregs. The signal supplied by antigen could be replaced by artificial ligation of B7 molecules using CD28-Ig fusion protein, suggesting that the role of activated OT-I was to provide a CD28→B7-mediated intracellular signal to the pDCs. This is consistent with the previously described role of CD28→B7 signaling as an inducer of IL-6 in other models.27,46 In vivo, OVA-activated OT-I cells could drive conversion of Tregs in TDLNs of OVA-expressing tumors. Importantly, however, conversion of Tregs could also be driven by a vaccine against TRP1 (a shared self/tumor antigen) when combined with 1MT. For these studies, we used an immunogenic mutated TRP1 peptide capable of breaking tolerance to the native TRP1 protein,34 delivered in a lentivirus vaccine vector that stimulates robust CD8+ T-cell responses.35,36 The efficacy of this vaccine in driving Treg conversion when combined with 1MT is an important finding because it means that Treg conversion could be driven by the natural frequency of T cells against an endogenous self/tumor antigen, as long as IDO was blocked.
In our system, conversion of Tregs to TH17-like cells required IL-6. IL-6 has been implicated in abrogation of Treg suppression in a variety of experimental models.10,11,19 Although other cytokines may also serve to bias cells toward the TH17 phenotype,13,47 neutralizing-antibody studies showed that IL-6 was strictly required in our system. In turn, IL-6 expression was regulated by IDO, such that when IDO was active, production of IL-6 was suppressed. Thus, we hypothesize that a key molecular mechanism by which IDO maintains Tregs in the suppressive phenotype is by blocking the induction of IL-6 in activated pDCs. This hypothesis would be consistent with previous reports showing that IDO and its downstream metabolites are able to suppress local inflammation, including inflammation-induced production of IL-17.48,49 Those studies used bulk populations of T cells and thus could not address our finding that IDO regulates the direct reprogramming of Foxp3+ Tregs to TH17-like cells, but our results are consistent with these earlier reports.
Taken together, our findings suggest a model in which IDO functions as a molecular “switch” during certain forms of inflammation, acting to control the phenotype of local Tregs. Mice deficient in functional IDO do not show a global defect in Tregs, but they do show a profound defect in acquired peripheral tolerance, including acquired tolerance to transplanted tissues, fetal antigens, and antigens presented at mucosal surfaces.50 Because tumors represent a dramatic example of acquired tolerance to their own antigens, the regulatory role of IDO may be highly relevant in this context.
Clinically, our findings suggest that, instead of attempting to physically deplete Tregs, it may be possible to reprogram Tregs in situ into proinflammatory T-helper/TH17-like cells. If this proves generalizable to human tumors, then the combination of antitumor vaccination plus an IDO inhibitor drug could be an effective strategy to deactivate and reprogram Tregs. The IDO-inhibitor drug D-1MT is currently in phase 1 clinical trials, so it may soon become possible to test this hypothesis in a clinical setting.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jose Guevara-Patino for the generous gift of mutant-TRP1 DNA; David Ron and his laboratory for generously sharing GCN2-KO mice; Joyce Wilson, Judy Gregory, and Doris McCool for expert technical assistance; Jeanene Pihkala for cell sorting; and Yibing Peng for expert preparation of viral vectors
This work was supported by the National Institutes of Health (grants CA103320, CA096651, and CA112431, D.H.M.; HD41187 and AI063402, A.L.M.; and CA116444, Y.H.).
National Institutes of Health
Authorship
Contribution: M.D.S. and D.-Y.H. performed experiments; Y.L. and Y.H. prepared lentivectors and contributed to vaccination studies; P.A.K. provided specialized mice and advice; R.M. designed and performed experiments on NF-IL-6; P.C. performed bone marrow transplantations; A.L.M. and Y.H. assisted in planning and interpretation of experiments; and D.H.M. and M.D.S. planned experiments and wrote the paper.
Conflict-of-interest disclosure: D.H.M. and A.L.M. have intellectual property interests in the therapeutic use of IDO and IDO inhibitors, and receive consulting income and research support from NewLink Genetics, Inc. R.M. is currently an employee of NewLink Genetics, Inc. The remaining authors declare no competing financial interests.
Correspondence: David H. Munn, Medical College of Georgia, Cancer Immunotherapy Program, 1120 15th St, Rm CN-4141, Augusta, GA 30912; e-mail: dmunn@mail.mcg.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal