Abstract
Leukocyte transmigration is mediated by endothelial cell (EC) junctional molecules, but the associated mechanisms remain unclear. Here we investigate how intercellular adhesion molecule-2 (ICAM-2), junctional adhesion molecule-A (JAM-A), and platelet endothelial cell adhesion molecule (PECAM-1) mediate neutrophil transmigration in a stimulus-dependent manner (eg, as induced by interleukin-1β [IL-1β] but not tumor necrosis factor-α [TNF-α]), and demonstrate their ability to act in sequence. Using a cell-transfer technique, transmigration responses of wild-type and TNF-α p55/p75 receptor-deficient leukocytes (TNFR−/−) through mouse cremasteric venules were quantified by fluorescence intravital microscopy. Whereas wild-type leukocytes showed a normal transmigration response to TNF-α in ICAM-2−/−, JAM-A−/−, and PECAM-1−/− recipient mice, TNFR−/− leukocytes exhibited a reduced transmigration response. Hence, when the ability of TNF-α to directly stimulate neutrophils is blocked, TNF-α–induced neutrophil transmigration is rendered dependent on ICAM-2, JAM-A, and PECAM-1, suggesting that the stimulus-dependent role of these molecules is governed by the target cell being activated. Furthermore, analysis of the site of arrest of neutrophils in inflamed tissues from ICAM-2−/−, JAM-A−/−, and PECAM-1−/− mice demonstrated that these molecules act sequentially to mediate transmigration. Collectively, the findings provide novel insights into the mechanisms of action of key molecules implicated in leukocyte transmigration.
Introduction
Leukocyte emigration into sites of inflammation occurs via a sequence of molecular and cellular responses, beginning with the tethering and rolling of leukocytes on the vascular lumen, leukocyte firm arrest, intravascular crawling, and finally penetration of the vascular wall.1 This final stage of leukocyte emigration involves the movement of leukocytes through the endothelium, its associated basement membrane (BM), and the pericyte coverage of venules, distinct stages in the inflammatory process that are relatively poorly understood.1 In penetrating the endothelial cell (EC) barrier, the predominant mechanism is via migration through junctions between adjacent cells where numerous adhesion complexes have now been identified and implicated in the transmigration process.1,2 These include platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31), intercellular adhesion molecule-2 (ICAM-2/CD102), CD99, and members of the junctional adhesion molecule (JAM) family (eg, JAM-A and JAM-C), although the precise mechanisms by which these proteins function and their potential additive/cooperative interactions remain largely unknown.
ICAM-2, JAM-A, and PECAM-1 are all members of the immunoglobulin superfamily of adhesion molecules and show many similarities in terms of their expression profiles and functions. Specifically, all 3 molecules are expressed on leukocytes, platelets, and on ECs where their expression is largely junctional.3,4 These molecules have been implicated in angiogenesis, immune functions, and most notably in the process of leukocyte transmigration.2 With respect to the latter, many in vivo studies have demonstrated the significant role of PECAM-1 in this process,5 and there is a growing body of data implicating JAM-A in leukocyte transmigration in different inflammatory models.4 Details of the mechanisms by which PECAM-1 and JAM-A mediate leukocyte migration through venular walls remain unclear, but there is now much evidence to suggest that a homophilic interaction between leukocyte PECAM-1 and EC PECAM-1 guides leukocytes through EC junctions in vitro.6 Furthermore, we have shown that homophilic PECAM-1 ligation at EC junctions can lead to enhanced expression of the laminin receptor, integrin α6β1, on the cell surface of neutrophils, supporting neutrophil migration through the venular BM.7,8 At endothelial junctions, JAM-A binds homophilically to JAM-A on adjacent cells,2 but the mechanism by which JAM-A mediates leukocyte transmigration is also complex and may depend on the relative contributions of leukocyte and EC JAM-A in different inflammatory scenarios.4
Several in vitro studies have suggested a role for ICAM-2 in leukocyte transendothelial cell migration,9-11 but only a limited number of investigations have addressed the role of ICAM-2 in leukocyte transmigration in vivo. Specifically, using a murine asthma model, ICAM-2–deficient mice exhibited delayed increase in eosinophil infiltration into airways,12 and an anti–ICAM-2–blocking monoclonal antibody (mAb) inhibited leukocyte infiltration in response to bacterial ocular infection in ICAM-1 KO mice.13 More recently, we have reported that ICAM-2 can mediate neutrophil transmigration in vivo in a stimulus-dependent manner, ie, support neutrophil transmigration as elicited by interleukin-1β (IL-1β) but not tumor necrosis factor-α (TNF-α) or thioglycollate.14 This pattern of “stimulus specificity” is similar to that observed for JAM-A and PECAM-1, both of which mediate neutrophil transmigration as induced by IL-1β but not TNF-α8,15 (Table 1). The reason for this “stimulus-specific” activation of ICAM-2–, JAM-A–, and PECAM-1–dependent pathways is currently unclear but may be governed at multiple levels, including the target cell being activated (leukocyte vs EC) and/or the signaling pathways linking stimulating receptors and adhesion pathways.
ICAM-2, JAM-A, and PECAM-1 mediate leukocyte transmigration in vivo in C57BL/6 mice in a stimulus-dependent manner
| Junctional protein . | Dependent . | Independent . |
|---|---|---|
| ICAM-2 | IL-1β,14 ischemia/reperfusion injury* | TNF-α,14 thioglycollate,14 LTB4* |
| JAM-A | IL-1β,8 ischemia/reperfusion injury8 | LTB4,8 PAF8 |
| PECAM-1 | IL-1β15 | TNF-α,15 thioglycollate22 |
| Junctional protein . | Dependent . | Independent . |
|---|---|---|
| ICAM-2 | IL-1β,14 ischemia/reperfusion injury* | TNF-α,14 thioglycollate,14 LTB4* |
| JAM-A | IL-1β,8 ischemia/reperfusion injury8 | LTB4,8 PAF8 |
| PECAM-1 | IL-1β15 | TNF-α,15 thioglycollate22 |
The table summarizes previous data from our group showing that the ability of the endothelial cell junctional molecules ICAM-2, JAM-A, and PECAM-1 to mediate leukocyte transmigration is dependent on the nature of the stimulus. All these studies were conducted in C57BL/6 mice.
Unpublished data.
With many aspects of the transmigration process remaining unclear, the present study aimed to extend our previous work on ICAM-2, JAM-A, and PECAM-1 by specifically investigating the mechanisms associated with the stimulus-dependent roles of these molecules in mediating neutrophil transmigration in vivo. The findings provide direct evidence to indicate that the functional role of these structures is regulated by the target cell being activated, ie, EC, and not neutrophil, activation leads to ICAM-2/JAM-A/PECAM-1–dependent transmigration. Furthermore, we show that ICAM-2, JAM-A, and PECAM-1 mediate distinct but sequential steps to support neutrophil migration through venular walls in vivo.
Methods
Animals
Male wild-type (WT), C57BL/6, FVB/n, and SJL mice were used, as well as male ICAM-2−/−,12 JAM-A−/−,16 PECAM-1−/−,17 and TNF-αR p55/p75 double knockout (TNFR−/−) mice. All genetically modified mice were on a C57BL/6 background (“Animals” in supplemental materials and methods, available on the Blood website; see the Supplemental Materials link at the top of the online article). All studies detailed in our manuscript involving mice had the approval of the Barts and The London School of Medicine & Dentistry Review Board.
Reagents
Reagents are detailed in “Reagents” in supplemental materials and methods.
Intravital microscopy
Intravital microscopy was used to directly observe leukocyte responses within mouse cremasteric venules as previously detailed.15 Briefly, IL-1β (50 ng in 400 μL), TNF-α (300 ng in 400 μL), or saline (control) was injected intrascrotally and 4 hours later the cremasters were surgically exteriorized and visualized with transmitted light on an upright microscope. These reactions are predominantly neutrophilic in nature (∼ 95% neutrophil infiltration).15 Leukocyte adhesion and transmigration responses were quantified in postcapillary venules as previously detailed.15 In some experimental groups, mice were pretreated with intravenous saline, an isotype control mAb, or blocking mAbs directed against ICAM-2 (3C4),14 (BD Biosciences, Oxford, United Kingdom), JAM-A (BV-11),18 PECAM-1 (Mec13.3; BD Biosciences), Mac-1 (56C),19 and JAM-C (H33),20 (all at 1-5 mg/kg, as indicated in appropriate legends, for maximal inhibitory effect), 15 minutes before the induction of inflammatory reactions.
Quantification of fluorescent leukocyte responses
To enable investigations into the contributions of leukocyte and EC-expressed proteins in leukocyte transmigration, a cell transfer technique was used as previously described.8 Bone marrow leukocytes were isolated from WT or TNFR−/− donor mice, fluorescently labeled with calcein AM, and injected intravenously into WT, ICAM-2−/−, JAM-A−/−, PECAM-1−/−, or TNF-R−/− recipient mice. The cremasters were then stimulated with TNF-α as detailed in “Intravital microscopy” and the number of fluorescent cells adherent or transmigrated quantified by the use of a fluorescent microscope. In most experiments, this quantification was performed at 4 hours after injection of the cytokine. In some experiments, the cremaster was exteriorized 2 hours after TNF-α injection, and the adhesion and transmigration responses of fluorescently labeled cells were quantified at 30-minute intervals for a further 2 hours. The results are shown as the number of adherent or transmigrated calcein-labeled cells/field of view. This is a tissue area of 38 mm2, equivalent to the area of the glass viewing window, across which the exteriorized cremaster is fixed.
Immunofluorescence labeling and analysis of cremaster muscle tissues by confocal microscopy
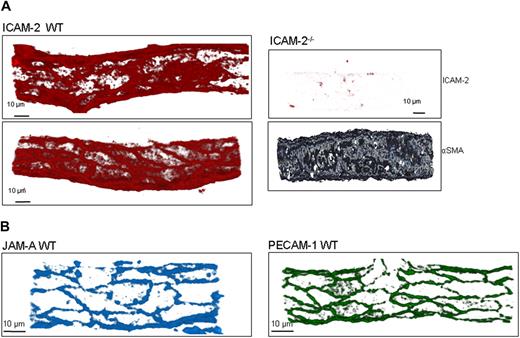
Whole-mounted tissues were examined by confocal microscopy to determine the location of leukocytes within inflamed tissues as previously described.8 Briefly, cremaster muscles of WT, ICAM-2−/−, JAM-A−/−, and PECAM-1−/− mice were stimulated with intrascrotal IL-1β for 4 hours and then immunolabeled with markers for neutrophils (MRP-14), the venular BM (collagen IV), and EC junctions (ICAM-2, JAM-A, or PECAM-1). Z-stack images of postcapillary venules were captured by confocal microscopy using a Zeiss LSM 5 Pascal confocal laser-scanning microscope equipped with argon and helium-neon lasers incorporating a 60× objective lens (0.9 aperture water immersion; Carl Zeiss, Welwyn Garden City, United Kingdom). Acquired Z-stack images were used for 3-dimensional reconstruction analysis of whole vessels using the LSM 5 Pascal software (version 3.2; Carl Zeiss). Images were postanalyzed using the image processing software IMARIS (Bitplane, Zurich Switzerland), which renders the optical sections into 3-dimensional images, enabling analysis of images from all angles, thus allowing the localization of leukocytes relative to the endothelium and the EC basement membrane to be determined in detail and accurately.
To observe the expression patterns of ICAM-2, JAM-A, and PECAM-1, WT tissues were immunostained for the relevant molecule using the mAbs listed. The labeling protocol was adapted from that described previously,8 and confocal images were captured and analyzed as detailed. Tissues obtained from mice genetically deficient in the target proteins were labeled and imaged using the same confocal microscopy settings to those used for samples from WT mice to assess the specific binding of the test mAbs. In these control studies, tissues were also labeled for α–smooth muscle actin (αSMA) to aid in localization of venules.
Statistics
Data analysis was performed using the statistical software GraphPad Prism 4 (GraphPad Software, San Diego, CA). All results are expressed as mean plus or minus SEM. Statistical significance was assessed by one-way analysis of variance with Student-Newman-Keuls multiple comparison test. Where 2 variables were analyzed, a rank-sum test was used. A P value less than .05 was considered significant.
Results
ICAM-2, JAM-A, and PECAM-1 exhibit a stimulus-dependent role in mediating neutrophil transmigration in multiple mouse strains
The EC adhesion molecules ICAM-2, JAM-A, and PECAM-1 mediate neutrophil transmigration in a stimulus-dependent manner (Table 1). For example, using the mouse cremaster muscle model, we have found that IL-1β– but not TNF-α–induced neutrophil responses are mediated by ICAM-2, JAM-A, and PECAM-1. However, because these studies have all been performed using the mouse strain C57BL/6, reported to be able to compensate for loss of PECAM-1 function in certain inflammatory models,21 we investigated whether this phenomenon accounts for the stimulus-specific roles of PECAM-1 in neutrophil transmigration through mouse cremasteric venules. For this purpose, the effect of an anti-PECAM-1 mAb (Mec13.3) was tested in this model using several mouse strains and the inflammatory stimuli IL-1β and TNF-α. Pretreatment of C57BL/6 mice with the anti–PECAM-1 mAb significantly suppressed neutrophil transmigration in response to IL-1β but not TNF-α (Table 2). This stimulus-dependent inhibitory effect of the anti–PECAM-1 mAb was also noted in 2 other mouse strains, FVB/n and SJL mice. Anti–ICAM-2 (3C4) and anti–JAM-A (BV-11) mAbs also exerted inhibitory effects on neutrophil transmigration through the vessel wall. Although all 3 blocking mAbs significantly suppressed IL-1β–induced neutrophil transmigration in the mouse strains investigated, the results suggested that mice on the C57BL/6 genetic background are less susceptible to PECAM-1 blockade (eg, 43% and 78% inhibitory effects of anti-PECAM-1 mAb on IL-1β–induced neutrophil transmigration noted in C57BL/6 and SJL mice, respectively). Of importance, in agreement with the findings of Schenkel et al,21 mice treated with the anti-PECAM-1 mAb exhibited reduced neutrophil transmigration in a thioglycollate peritonitis model in FVB/n and SJL mice but not in C57BL/6 mice (data not shown). Together, the findings demonstrate that ICAM-2, JAM-A, and PECAM-1 can mediate neutrophil transmigration in a “stimulus-specific” manner, although in some inflammatory models this phenomenon is dependent on the mouse strain used.
The stimulus-dependent role of endothelial cell junctional molecules in mediating cytokine-induced leukocyte transmigration is not governed by the mouse strain used
| Blocking antibody . | Percentage reduction in IL-1β–stimulated TEM . | Percentage reduction in TNF-α–stimulated TEM . | ||||
|---|---|---|---|---|---|---|
| C57BL/6 . | FVB/n . | SJL . | C57BL/6 . | FVB/n . | SJL . | |
| Anti–ICAM-2 | 48.4 ± 6.9* | 80.05 ± 7.1† | 49.95 ± 21.2 | −0.002 ± 6.8 | −1.04 ± 12.5 | −3.1 ± 20.2 |
| Anti–JAM-A | 62.2 ± 10.8‡ | 69.3 ± 5.6* | 66.7 ± 18.4* | −16.8 ± 17 | −5.98 ± 38.6 | −4.8 ± 29.9 |
| Anti–PECAM-1 | 43.1 ± 10.7† | 73.7 ± 34.1* | 78.3 ± 14.4† | −23.6 ± 5.2 | −15.59 ± 41.3 | 15.9 ± 20.3 |
| Blocking antibody . | Percentage reduction in IL-1β–stimulated TEM . | Percentage reduction in TNF-α–stimulated TEM . | ||||
|---|---|---|---|---|---|---|
| C57BL/6 . | FVB/n . | SJL . | C57BL/6 . | FVB/n . | SJL . | |
| Anti–ICAM-2 | 48.4 ± 6.9* | 80.05 ± 7.1† | 49.95 ± 21.2 | −0.002 ± 6.8 | −1.04 ± 12.5 | −3.1 ± 20.2 |
| Anti–JAM-A | 62.2 ± 10.8‡ | 69.3 ± 5.6* | 66.7 ± 18.4* | −16.8 ± 17 | −5.98 ± 38.6 | −4.8 ± 29.9 |
| Anti–PECAM-1 | 43.1 ± 10.7† | 73.7 ± 34.1* | 78.3 ± 14.4† | −23.6 ± 5.2 | −15.59 ± 41.3 | 15.9 ± 20.3 |
Three mouse strains, C57BL/6, FVB/n, and SJL, were used in studies of leukocyte transmigration through IL-1β (50 ng)– and TNF-α (300 ng)–stimulated cremasteric venules. Mice were pretreated with blocking mAbs directed against ICAM-2, JAM-A, PECAM-1, or appropriate control mAbs (all at 3 mg/kg intravenously) before local injection of cytokines. After 4 hours, leukocyte transmigration was quantified by intravital microscopy, as detailed in “Intravital microscopy.” Data show percentage reduction in number of transmigrated leukocytes in a 10−4 μm2 area of perivascular tissue in the different mouse strains and as treated with different blocking mAbs compared with control antibody-treated animals of the same strain (negative numbers indicate small and insignificant increases in responses). Results are mean ± SEM from n = 3 to 7 mice/group.
Significant reduction (P < .05).
Significant reduction (P < .01).
Significant reduction (P < .001).
Because leukocyte transmigration induced by TNF-α was found to be independent of ICAM-2, JAM-A, and PECAM-1, additional experiments were conducted to address the potential mechanisms through which this cytokine may be acting. In this context, TNF-α–induced leukocyte transmigration, but not adhesion, was found to be dependent on Mac-1 and JAM-C, as indicated by studies using blocking mAbs. Specifically, an anti–Mac-1 mAb (5C6) and an anti–JAM-C mAb (H33) were found to significantly and selectively suppress leukocyte transmigration elicited by TNF-α (anti–Mac-1: 48.4% ± 9.7% inhibition, n ≥ 4 mice/group, P < .05; anti–JAM-C: 56.4% ± 7.6% inhibition, n ≥ 4 mice/group, P < .001). Neither mAb had an effect on TNF-α–induced leukocyte adhesion (data not shown).
Stimulation of neutrophils can trigger transmigration independent of ICAM-2, JAM-A, and PECAM-1
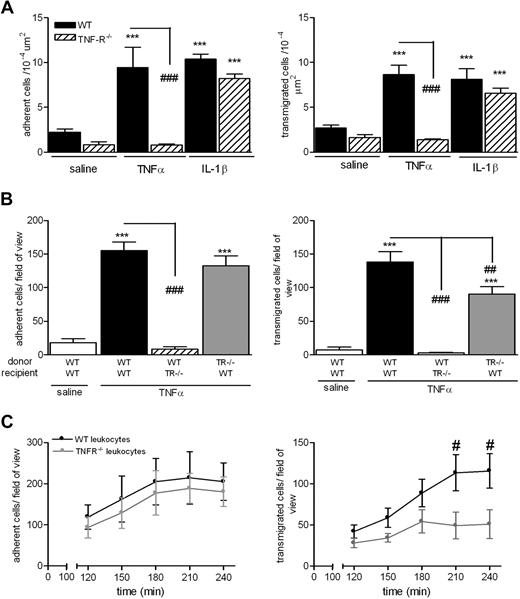
The findings in Tables 1 and 2 collectively indicate that stimuli that activate ECs (eg, IL-1β and I/R) induce neutrophil transmigration via recruitment of pathways involving ICAM-2, JAM-A, and PECAM-1, whereas mediators that stimulate neutrophils (eg, TNF-α and leukotriene B4 [LTB4]) elicit neutrophil transmigration via mechanisms that are independent of these adhesion molecules. The following studies aimed to obtain direct evidence for this possibility by investigating the roles of ICAM-2, JAM-A, and PECAM-1 under experimental conditions where TNF-α (capable of stimulating both ECs and neutrophils) is “forced” to activate either ECs or neutrophils alone. For this purpose, we used the TNF-α p55/p75 receptor double knockout mice (TNFR−/−) and a cell transfer technique previously developed in our laboratory.8 As expected, the TNFR−/− mice failed to respond to locally administered TNF-α but exhibited normal adhesion and transmigration responses to IL-1β, compared with WT mice (Figure 1A). To investigate the relative roles of leukocyte and EC/tissue TNF-α receptors in mediating neutrophil responses as elicited by TNF-α, bone marrow leukocytes were isolated from femurs of WT or TNFR−/− donor mice, labeled with calcein-AM, and injected intravenously into WT or TNFR−/− recipient mice before intrascrotal TNF-α (4-hour test period). WT leukocytes injected into WT recipients exhibited a significant leukocyte adhesion and transmigration response in tissues stimulated with TNF-α compared with saline-injected cremaster muscles (Figure 1B). In contrast, WT leukocytes injected into TNF-α–stimulated TNFR−/− recipients showed no adhesion or transmigration responses, indicating an absolute requirement for tissue TNF-α receptors for the induction of TNF-α–induced neutrophil firm adhesion. TNFR−/− leukocytes injected into WT recipients exhibited a normal adhesion response, but importantly a partial defect in transmigration was noted, suggesting a selective role for neutrophil TNF-α receptors in TNF-α–induced neutrophil transmigration. The latter findings were further investigated within a time-course protocol (Figure 1C). Briefly, WT or TNFR−/− leukocytes were fluorescently labeled and injected intravenously into WT mice that had been injected intrascrotally with TNF-α. After 2 hours, the mice were anesthetized and their cremaster muscles exteriorized for analysis of leukocyte/vessel wall interactions at 30-minute time intervals within a 2-hour period by fluorescence intravital microscopy. Using this protocol, WT and TNFR−/− cells showed the same time-dependent profile of adhesion in TNF-α–stimulated tissues. In contrast, whereas WT leukocytes showed a gradual increase in leukocyte transmigration within 4 hours, TNFR−/− cells exhibited a reduced transmigration response at all time points investigated, apparently reaching a plateau at 180 minutes after injection of TNF-α (Figure 1C). Collectively, these results indicate that, within the present 4-hour model, the observed reduction in TNF-α–induced transmigration of neutrophils under conditions of neutrophil TNF-α receptor deletion is not caused by a delay in the response.
Mechanism of TNF-α–induced leukocyte adhesion and transmigration in vivo. (A) WT or TNFR−/− mice were given intrascrotal injections of IL-1β (50 ng), TNF-α (300 ng), or saline; and 4 hours later, leukocyte responses of adhesion and transmigration were quantified in cremaster muscle by intravital microscopy as detailed in “Intravital microscopy.” (B) To investigate the contribution of TNF-α receptors on leukocytes or EC in responses to locally administered TNF-α, a cell-transfer technique was used. Bone marrow leukocytes were isolated from donor WT or TNFR−/− mice, labeled with calcein-AM, and injected intravenously into WT or TNFR−/− recipient mice. The mice were then injected intrascrotally with saline or TNF-α; and 4 hours later, the cremaster muscle was exteriorized and leukocyte responses of adhesion and transmigration of fluorescently labeled leukocytes were quantified. (C) As in panel B, WT or TNFR−/− cells were fluorescently labeled and injected intravenously into WT recipients that had received saline or TNF-α intrascrotally. The cremaster muscle was then exteriorized 120 minutes later, and adhesion and transmigration of fluorescent cells were quantified every 30 minutes for a further 120 minutes. Data are corrected for the small levels of responses detected in mice injected with intrascrotal saline and presented as mean ± SEM for n = 3 to 10 mice per group. Statistically significant differences between control and stimulated groups are indicated as follows: ***P < .001. Additional statistical comparisons are indicated as follows: #P < .05, ##P < .01, ###P < .001.
Mechanism of TNF-α–induced leukocyte adhesion and transmigration in vivo. (A) WT or TNFR−/− mice were given intrascrotal injections of IL-1β (50 ng), TNF-α (300 ng), or saline; and 4 hours later, leukocyte responses of adhesion and transmigration were quantified in cremaster muscle by intravital microscopy as detailed in “Intravital microscopy.” (B) To investigate the contribution of TNF-α receptors on leukocytes or EC in responses to locally administered TNF-α, a cell-transfer technique was used. Bone marrow leukocytes were isolated from donor WT or TNFR−/− mice, labeled with calcein-AM, and injected intravenously into WT or TNFR−/− recipient mice. The mice were then injected intrascrotally with saline or TNF-α; and 4 hours later, the cremaster muscle was exteriorized and leukocyte responses of adhesion and transmigration of fluorescently labeled leukocytes were quantified. (C) As in panel B, WT or TNFR−/− cells were fluorescently labeled and injected intravenously into WT recipients that had received saline or TNF-α intrascrotally. The cremaster muscle was then exteriorized 120 minutes later, and adhesion and transmigration of fluorescent cells were quantified every 30 minutes for a further 120 minutes. Data are corrected for the small levels of responses detected in mice injected with intrascrotal saline and presented as mean ± SEM for n = 3 to 10 mice per group. Statistically significant differences between control and stimulated groups are indicated as follows: ***P < .001. Additional statistical comparisons are indicated as follows: #P < .05, ##P < .01, ###P < .001.
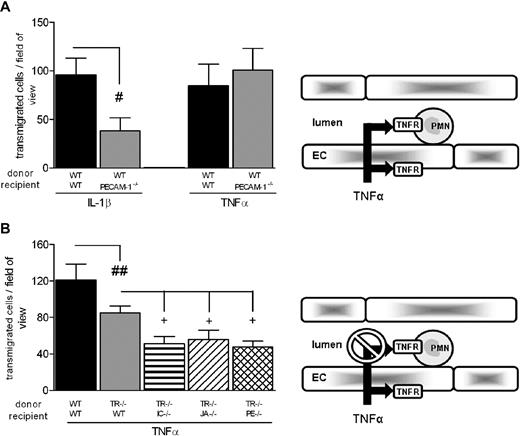
Having demonstrated a direct role for leukocyte TNFRs in neutrophil transmigration induced by TNF-α, these findings were exploited to plan a series of experiments aimed at investigating the functional roles of the EC molecules ICAM-2, JAM-A, and PECAM-1 in TNF-α–induced neutrophil transmigration under conditions where direct leukocyte activation could not occur. These studies were again performed using the cell-transfer technique. Initial experiments showed that WT leukocytes exhibited a significantly reduced transmigration response in PECAM-1−/− mice in response to IL-1β, but not TNF-α, compared with responses obtained from WT leukocytes injected into WT recipients (Figure 2A). Based on the data shown in Figure 1A, under these experimental conditions, TNF-α–induced responses were mediated through both activation of leukocyte and EC TNFRs. These studies were then extended to experimental conditions within which direct leukocyte activation was blocked. For this purpose, bone marrow leukocytes from TNFR−/− or WT mice were fluorescently labeled and injected intravenously into WT, ICAM-2−/−, JAM-A−/−, or PECAM-1−/− mice, and responses in TNF-α–stimulated cremasteric venules were quantified by fluorescent intravital microscopy (Figure 2B). In line with previous data, TNFR−/− leukocytes injected into WT mice exhibited a partial reduction in transmigration compared with responses detected from WT leukocytes in WT recipients. However, this response was further significantly reduced when TNFR−/− leukocytes were injected into ICAM-2−/−, JAM-A−/−, or PECAM-1−/− mice. These results demonstrate that, although in WT mice TNF-α–induced leukocyte transmigration occurs independently of the molecules ICAM-2, JAM-A, and PECAM-1 (Tables 1,2), under experimental conditions where the ability of TNF-α to stimulate leukocytes is blocked, that is, presumably TNF-α is “forced” to activate the endothelium alone (as occurs with IL-1β), the response induced by this cytokine becomes partially ICAM-2–, JAM-A–, and PECAM-1–dependent. Collectively, these findings provide direct evidence to support the hypothesis that the ability of ICAM-2, JAM-A, and PECAM-1 to mediate neutrophil transmigration is governed by the ability of the stimulus to activate the endothelium.
Roles of EC ICAM-2, JAM-A, and PECAM-1 in neutrophil transmigration induced by TNF-α. (A) The role of EC PECAM-1 in neutrophil transmigration as elicited by IL-1β and TNF-α was investigated. Fluorescently labeled WT leukocytes were injected into WT or PECAM−/− recipient mice treated with intrascrotal IL-1β or TNF-α. Four hours later, adhesion and transmigration of fluorescently labeled cells were quantified. (B) The role of junctional proteins in TNF-α–stimulated migration was investigated. WT or TNFR−/− fluorescently labeled bone marrow leukocytes were injected into WT, ICAM-2−/−, JAM-A−/−, or PECAM-1−/− recipient mice stimulated with intrascrotal TNF-α. Four hours later, the cremaster was exteriorized and leukocyte responses were quantified. The diagrams on the right illustrate the inflammatory conditions for which the experiments were planned, that is, stimulation of both leukocyte and EC TNFRs (A) or blockade of leukocyte TNFRs (B). Results are mean ± SEM from n = 4 to 14 mice/group. Statistically significant differences are indicated as follows: #P < .05, ##P < .01, +P < .05.
Roles of EC ICAM-2, JAM-A, and PECAM-1 in neutrophil transmigration induced by TNF-α. (A) The role of EC PECAM-1 in neutrophil transmigration as elicited by IL-1β and TNF-α was investigated. Fluorescently labeled WT leukocytes were injected into WT or PECAM−/− recipient mice treated with intrascrotal IL-1β or TNF-α. Four hours later, adhesion and transmigration of fluorescently labeled cells were quantified. (B) The role of junctional proteins in TNF-α–stimulated migration was investigated. WT or TNFR−/− fluorescently labeled bone marrow leukocytes were injected into WT, ICAM-2−/−, JAM-A−/−, or PECAM-1−/− recipient mice stimulated with intrascrotal TNF-α. Four hours later, the cremaster was exteriorized and leukocyte responses were quantified. The diagrams on the right illustrate the inflammatory conditions for which the experiments were planned, that is, stimulation of both leukocyte and EC TNFRs (A) or blockade of leukocyte TNFRs (B). Results are mean ± SEM from n = 4 to 14 mice/group. Statistically significant differences are indicated as follows: #P < .05, ##P < .01, +P < .05.
ICAM-2, JAM-A, and PECAM-1 do not compensate for one another and do not exhibit an additive role in mediating leukocyte transmigration
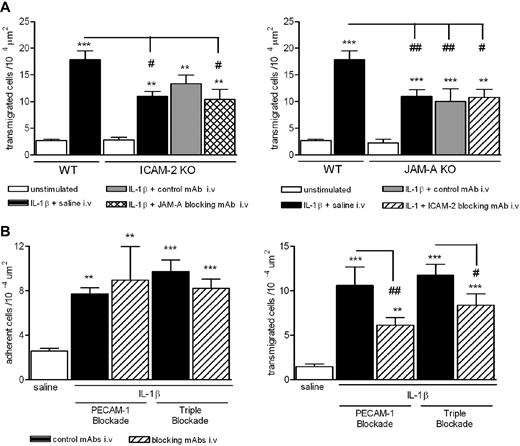
The similar structural, expression, and functional profiles of ICAM-2, JAM-A, and PECAM-1 have previously led us to investigate their potential additive and/or cooperative roles in vivo. In these studies, pharmacologic inhibition/deletion of JAM-A and PECAM-1 or ICAM-2 and PECAM-1 has shown no cumulative inhibitory effect on neutrophil transmigration through mouse cremasteric venules as induced by IL-1β.8,14 To extend these studies, we have now investigated the effects of loss of function of ICAM-2 and JAM-A and also the effect of triple blockade of all 3 molecules in the same model. Whereas both ICAM-2−/− and JAM-A−/− mice exhibited a reduced neutrophil transmigration response in IL-1β–stimulated cremasteric venules (Figure 3A), pretreatment of ICAM-2−/− and JAM-A−/− mice with blocking anti–JAM-A and anti-ICAM-2 mAbs, respectively, did not induce a greater inhibitory effect on neutrophil transmigration to that seen in control mAb-treated mice. Furthermore, pretreatment of WT mice with a cocktail of anti–ICAM-2, anti–JAM-A, and anti–PECAM-1 blocking mAbs exerted an inhibitory effect on IL-1β–induced neutrophil transmigration (but not adhesion) similar to the inhibitory effect seen with an anti–PECAM-1 mAb alone (Figure 3B). The lack of an additive effect of blockade/deletion of these molecules may suggest that ICAM-2, JAM-A, and PECAM-1 act to mediate different stages of the transmigration response elicited by IL-1β in that blockade of one step has the same effect as blockade of 2 or more of the molecules. This possibility was investigated next.
Effect of dual and triple blockade of ICAM-1–, JAM-A–, and PECAM-1–dependent pathways. (A) WT, ICAM-2−/−, or JAM-A−/− mice were given an intrascrotal injection of IL-1β (50 ng) or saline (control). Additional groups of mice were pretreated with an anti–JAM-A mAb (BV-11), anti–ICAM-2 mAb (3C4), or an appropriate control mAb (all at 1 mg/kg intravenously), as indicated. Four hours after the administration of IL-1β, cremaster muscles were exteriorized and leukocyte transmigration was quantified by intravital microscopy, as detailed in “Intravital microscopy.” (B) WT mice were pretreated with submaximal doses of an anti–PECAM-1 mAb (Mec 13.3), a cocktail of blocking mAbs directed against ICAM-2, JAM-A, and PECAM-1, or control mAbs as appropriate (all at 1 mg/kg intravenously). IL-1β (50 ng) or saline was injected intrascrotally; and after 4 hours, leukocyte responses of adhesion and transmigration were quantified. Results are mean ± SEM from n = 4 to 10 mice/group. Statistically significant differences between control and stimulated groups are indicated as follows: **P < .01, ***P < .001. Additional statistical comparisons are indicated as follows: #P < .05, ## P < .01.
Effect of dual and triple blockade of ICAM-1–, JAM-A–, and PECAM-1–dependent pathways. (A) WT, ICAM-2−/−, or JAM-A−/− mice were given an intrascrotal injection of IL-1β (50 ng) or saline (control). Additional groups of mice were pretreated with an anti–JAM-A mAb (BV-11), anti–ICAM-2 mAb (3C4), or an appropriate control mAb (all at 1 mg/kg intravenously), as indicated. Four hours after the administration of IL-1β, cremaster muscles were exteriorized and leukocyte transmigration was quantified by intravital microscopy, as detailed in “Intravital microscopy.” (B) WT mice were pretreated with submaximal doses of an anti–PECAM-1 mAb (Mec 13.3), a cocktail of blocking mAbs directed against ICAM-2, JAM-A, and PECAM-1, or control mAbs as appropriate (all at 1 mg/kg intravenously). IL-1β (50 ng) or saline was injected intrascrotally; and after 4 hours, leukocyte responses of adhesion and transmigration were quantified. Results are mean ± SEM from n = 4 to 10 mice/group. Statistically significant differences between control and stimulated groups are indicated as follows: **P < .01, ***P < .001. Additional statistical comparisons are indicated as follows: #P < .05, ## P < .01.
EC junctional molecules can act at distinct and sequential steps to mediate neutrophil transmigration
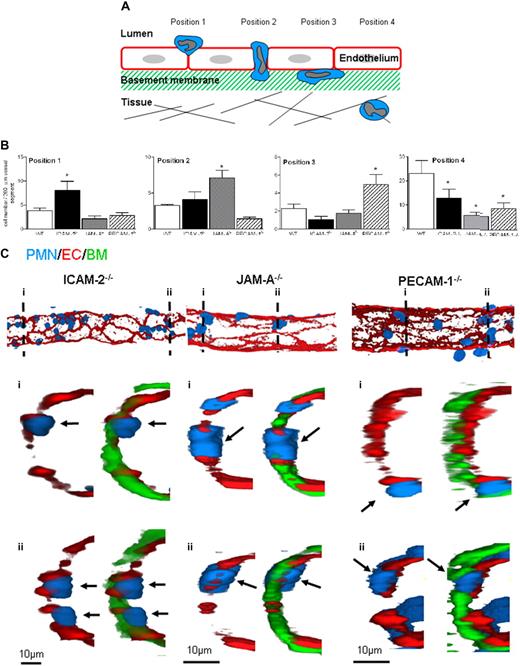
To investigate the precise step mediated by ICAM-2, JAM-A, and PECAM-1 in neutrophil transmigration in vivo, immunofluorescence and confocal microscopy were used to investigate the site of arrest of neutrophils in IL-1β–stimulated tissues in ICAM-2−/−, JAM-A−/−, and PECAM-1−/− mice compared with WT animals. Cremaster muscles were stimulated with IL-1β (4-hour reaction), and the mice were subjected to a vascular washout step just before the killing of animals and dissecting of the cremaster muscle. The tissues were then fixed, immunostained for markers of neutrophils (MRP14), EC junctional molecules (PECAM-1, ICAM-2, or JAM-A), and the vascular BM (collagen IV) to precisely delineate the position of neutrophils in the vessel wall. Venules were imaged and reconstructed in 3 dimensions by confocal microscopy and the image analysis software IMARIS (Bitplane, Zurich, Switzerland), respectively. Neutrophils were observed at multiple stages of their transmigration, and detailed analysis of the knockout mice indicated arrest of leukocytes at distinct and specific locations in venules of the different genotypes of animal. The reproducibility of these findings led us to create quantification criteria based on the position of the leukocytes (Figure 4A). Briefly, the sequential stages quantified represent the following steps: position 1, neutrophils identified as “investigating” a junction (ie, still largely within the vascular lumen but with the cell body beginning to protrude into the junction); position 2, neutrophils completely within an EC junction; position 3, neutrophils between ECs and the EC basement membrane; and finally, position 4, fully transmigrated neutrophils. Using this quantification criterion, the position of neutrophils in IL-1β–stimulated cremasters obtained from WT, ICAM-2−/−, JAM-A−/−, and PECAM-1−/− mice was measured (Figure 4B). Although all the genetically deficient strains exhibited reduced leukocyte migration into the extravascular tissue, compared with WT mice, the profile of arrest of neutrophils was very different in ICAM-2−/−, JAM-A−/−, and PECAM-1−/− mice. Specifically, samples from ICAM-2−/− mice showed a significantly greater number of neutrophils at an early stage of leukocyte transmigration, ie, beginning to penetrate the EC junction (position 1). Samples from JAM-A−/− mice indicated a significantly greater number of neutrophils fully embedded within the EC junctions (position 2), and PECAM-1−/− mice had significantly greater numbers of neutrophils between the endothelium and its associated BM (at position 3). Representative 3-dimensional images and a video of half venules, and selected associated cross sections, acquired by confocal microscopy from each knockout genotype, further illustrate the distinct positioning of neutrophils in the different mouse strains (Figure 4C; Video S1).
ICAM-2−/−, JAM-A−/−, and PECAM−/− mice exhibit distinct profiles in terms of site of arrest of transmigrating leukocytes in IL-1β–stimulated cremaster muscle tissues. (A) A schematic diagram illustrating the quantification criteria used to analyze the position of leukocytes within fixed and immunostained whole-mounted cremaster muscle tissues. (B) WT, ICAM-2−/−, JAM-A−/−, or PECAM−/− mice were given an intrascrotal injection of IL-1β (50 ng); and 4 hours later, the cremasters were dissected away from the mice, fixed, and immunostained to label neutrophils (MRP-14 with a 488-nm fluorochrome), EC (ICAM-2, JAM-A or PECAM-1 with a 555-nm fluorochrome), and venular basement membrane (collagen IV with a 633-nm fluorochrome), and prepared for analysis by confocal microscopy as detailed in “Immunofluorescence labeling and analysis of cremaster muscle tissues by confocal microscopy.” The localization of neutrophils in relation to the endothelium and its basement membrane according to the quantification criteria shown in panel A and detailed in text was determined and quantified by analyzing acquired 3-dimensional images of whole blood vessels as previously described.8 (C) The confocal images show representative longitudinal (top panels) and cross-sectional (bottom panels) images of IL-1β–stimulated venules from ICAM-2−/−, JAM-A−/−, and PECAM−/− mice. The top panels show 3-dimensional images of venules stained for EC junctions (red represents EC) and neutrophils (blue represents PMN) only. The images below were obtained by observing a cross-section (1 μm thick) of the associated venules along the indicated dotted lines (numbered) showing the staining of EC junctions, neutrophils, and the EC basement membrane (green represents BM). The arrows show the location of selected neutrophils. Results are mean ± SEM from n = 4 to 8 mice (4 venules analyzed in each sample). Statistically significant differences between WT and knockout groups are shown as follows: *P < .05.
ICAM-2−/−, JAM-A−/−, and PECAM−/− mice exhibit distinct profiles in terms of site of arrest of transmigrating leukocytes in IL-1β–stimulated cremaster muscle tissues. (A) A schematic diagram illustrating the quantification criteria used to analyze the position of leukocytes within fixed and immunostained whole-mounted cremaster muscle tissues. (B) WT, ICAM-2−/−, JAM-A−/−, or PECAM−/− mice were given an intrascrotal injection of IL-1β (50 ng); and 4 hours later, the cremasters were dissected away from the mice, fixed, and immunostained to label neutrophils (MRP-14 with a 488-nm fluorochrome), EC (ICAM-2, JAM-A or PECAM-1 with a 555-nm fluorochrome), and venular basement membrane (collagen IV with a 633-nm fluorochrome), and prepared for analysis by confocal microscopy as detailed in “Immunofluorescence labeling and analysis of cremaster muscle tissues by confocal microscopy.” The localization of neutrophils in relation to the endothelium and its basement membrane according to the quantification criteria shown in panel A and detailed in text was determined and quantified by analyzing acquired 3-dimensional images of whole blood vessels as previously described.8 (C) The confocal images show representative longitudinal (top panels) and cross-sectional (bottom panels) images of IL-1β–stimulated venules from ICAM-2−/−, JAM-A−/−, and PECAM−/− mice. The top panels show 3-dimensional images of venules stained for EC junctions (red represents EC) and neutrophils (blue represents PMN) only. The images below were obtained by observing a cross-section (1 μm thick) of the associated venules along the indicated dotted lines (numbered) showing the staining of EC junctions, neutrophils, and the EC basement membrane (green represents BM). The arrows show the location of selected neutrophils. Results are mean ± SEM from n = 4 to 8 mice (4 venules analyzed in each sample). Statistically significant differences between WT and knockout groups are shown as follows: *P < .05.
ICAM-2 exhibits both a luminal and EC junctional expression in venules
Previous studies have reported that the expression of ICAM-2 on cultured human ECs is junctional.23 However, as we found a role for ICAM-2 at an early stage of neutrophil transmigration, mostly at the interphase of the vascular lumen and EC junctions, we sought to investigate its expression profile within venules in more detail. Immunofluorescent staining and confocal microscopy analysis of unstimulated tissues from WT mice showed that ICAM-2 was expressed both within the vascular lumen and at EC junctions (Figure 5A). The luminal expression of ICAM-2 was not uniform and in many samples appeared to be more prevalent around the borders of cells as opposed to the central body of the endothelium. This expression profile, although at times variable, was specific as demonstrated by the lack of binding of the anti-ICAM-2 mAb in samples obtained from ICAM-2–deficient mice. In contrast to ICAM-2, the expression profiles of JAM-A and PECAM-1 were consistently junctional (Figure 5B). Collectively, these results show that the in vivo expression profile of ICAM-2 is different from that seen for JAM-A and PECAM-1, exhibiting both a junctional and luminal expression that appears in line with the indicated function of this molecule to mediate the transition from leukocyte/luminal wall interactions to junctional sites.
ICAM-2 exhibits both a luminal and EC junctional expression profile in venules in vivo. (A) Unstimulated cremaster muscle tissues from WT mice were immunostained for ICAM-2 and analyzed as detailed in “Immunofluorescence labeling and analysis of cremaster muscle tissues by confocal microscopy.” The specificity of binding of the anti-ICAM-2 mAb was confirmed in tissue samples obtained from ICAM-2–deficient mice in which the tissues were double stained for ICAM-2 and α–smooth muscle actin, the latter assisting in the identification of venules. (B) Unstimulated cremaster muscle tissues from WT mice were immunostained for JAM-A and PECAM-1 as detailed in “Immunofluorescence labeling and analysis of cremaster muscle tissues by confocal microscopy.” Samples were observed using a Zeiss LSM 5 PASCAL confocal laser-scanning microscope and analyzed using IMARIS software. Images are representative of 2 half-vessels per mouse, from 4 mice.
ICAM-2 exhibits both a luminal and EC junctional expression profile in venules in vivo. (A) Unstimulated cremaster muscle tissues from WT mice were immunostained for ICAM-2 and analyzed as detailed in “Immunofluorescence labeling and analysis of cremaster muscle tissues by confocal microscopy.” The specificity of binding of the anti-ICAM-2 mAb was confirmed in tissue samples obtained from ICAM-2–deficient mice in which the tissues were double stained for ICAM-2 and α–smooth muscle actin, the latter assisting in the identification of venules. (B) Unstimulated cremaster muscle tissues from WT mice were immunostained for JAM-A and PECAM-1 as detailed in “Immunofluorescence labeling and analysis of cremaster muscle tissues by confocal microscopy.” Samples were observed using a Zeiss LSM 5 PASCAL confocal laser-scanning microscope and analyzed using IMARIS software. Images are representative of 2 half-vessels per mouse, from 4 mice.
Discussion
Leukocyte transmigration is largely mediated by EC junctional molecules, but many aspects of this response are unknown. Here we demonstrate that the ability of ICAM-2, JAM-A, and PECAM-1 to mediate neutrophil transmigration is governed by the target cell being activated. Specifically, direct evidence is provided to show that neutrophil activation can lead to transmigration via mechanisms that are independent of ICAM-2, JAM-A, and PECAM-1. Furthermore, in reactions involving these molecules, considered to be triggered through activation of ECs, ICAM-2, JAM-A, and PECAM-1 act at distinct but sequential steps in supporting neutrophil migration through venular walls in vivo. Collectively, the findings indicate that the adhesion molecules ICAM-2, JAM-A, and PECAM-1 can support neutrophil emigration through venular walls in a highly organized and regulated manner after local EC activation, suggesting that they may play a critical role in mediating physiologic leukocyte infiltration as part of the host's immune response. On the other hand, strong activation of leukocytes, as might occur under pathologic scenarios where high levels of leukocyte priming/stimulatory molecules (eg, TNF-α, chemokines) may be generated, could possibly lead to an emigration response that bypasses the need for key EC junctional molecules.
There is mounting evidence demonstrating that the EC molecules ICAM-2, JAM-A, and PECAM-1 support neutrophil transmigration in vivo in a stimulus-dependent manner (Table 1). The field was rendered more complex when Schenkel et al reported that the functional role of PECAM-1 in leukocyte transmigration was dependent on the genetic background of the mouse strain used, specifically that the commonly used C57BL6 strain was abnormal in its lack of PECAM-1 dependency in certain models.21,24 As these findings have critical implications to understanding the role of PECAM-1 in leukocyte transmigration, we felt it important to address this phenomenon in the widely used cremaster muscle model where we have previously used the C57BL/6 mice and found a clear stimulus-dependent role for PECAM-1 in neutrophil transmigration. In this context, blockade of PECAM-1, ICAM-2, or JAM-A inhibited neutrophil transmigration as elicited by IL-1β, but not TNF-α, in several mouse strains. The results show that the ability of these molecules to mediate leukocyte transmigration by certain stimuli is not globally governed by the mouse strain used, although in some inflammatory models (eg, thioglycollate peritonitis model), the genetic background of the experimental animal may play a more decisive role.21 How this is achieved is currently unclear but may be related to local factors, such as profile of resident inflammatory cells, their portfolio of stored/generated mediators, and/or phenotype of ECs in different vascular beds of different mouse strains.
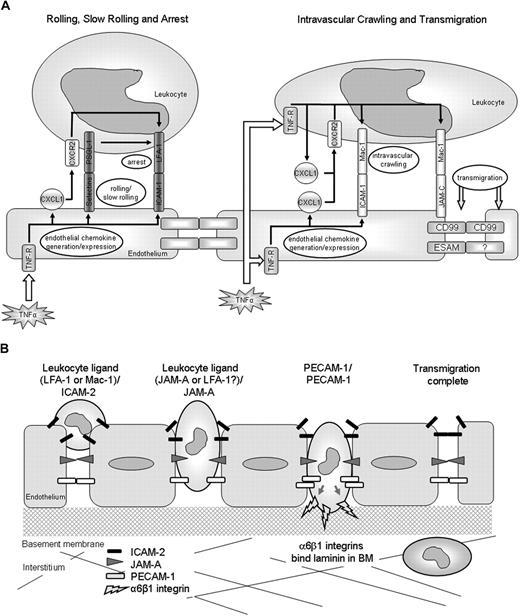
We next investigated the mechanism associated with the stimulus-dependent roles of ICAM-2, JAM-A, and PECAM-1. Based on the available data, the hypothesis under investigation was that stimuli that activate ECs (eg, IL-1β) recruit adhesion pathways involving ICAM-2, JAM-A, and PECAM-1, whereas stimuli that can directly activate neutrophils (eg, TNF-α, FMLP, and LTB4) appear to bypass the need for such molecules.8,14,15 To address this, we took advantage of the ability of TNF-α to directly stimulate both ECs and neutrophils and, using TNFR-deficient mice and a cell transfer technique, investigated the functional roles of ICAM-2, JAM-A, and PECAM-1 under experimental conditions where leukocytes lacked TNF-α receptors. In initial studies, we investigated the relative roles of leukocyte and tissue (considered to be largely accounted for by the endothelium) TNFRs in TNF-α–induced leukocyte responses. The results indicated an absolute requirement for tissue TNFRs in TNF-α–induced neutrophil firm adhesion and a selective role for neutrophil TNF-α receptors in neutrophil transmigration. The former is in line with comprehensive works showing that EC TNF-α receptors play a critical role in mediating TNF-α–induced enhanced expression of EC adhesion/stimulatory molecules (eg, E-selectin, ICAM-1, and chemokines) on cultured ECs, supporting leukocyte adhesion in vitro,25,26 and normal leukocyte adhesion to cremasteric venules in vivo.27 TNF-α can also stimulate/prime neutrophils to exhibit L-selectin shedding, enhanced expression of CD11b, rapid adhesion to EC- and protein-coated surfaces, and generation of proinflammatory molecules, including chemokines from adherent cells.15,28-31 Zarbock et al have recently addressed the roles of leukocyte and EC TNF-α receptors in the leukocyte adhesion cascade, providing data to suggest that stimulation of EC TNF-α receptors can support neutrophil slow rolling via E-selectin/PSGL-1, a response that can be driven to neutrophil arrest via LFA-1 activation as induced by TNF-α–stimulated generation of neutrophil-derived chemokines (CXCL1).32 Less is known, however, about the selective mechanisms associated with TNF-α–induced leukocyte transmigration. It is potentially possible that, although activation of EC TNF-α receptors primarily supports early intravascular responses such as rolling and arrest, the subsequent stimulation of adherent leukocytes via their TNF-α receptors may lead to generation of proinflammatory mediators, such as chemokines, which can in turn stimulate steps subsequent to the firm adhesion phase, leading to higher levels of transmigration. The lack of response of WT leukocytes in TNFR−/− mice may be a consequence of the lack of up-regulation of endothelial adhesion molecules on the TNFR−/− tissue, which prevents circulating WT leukocytes from forming interactions with the endothelium where they may then be exposed to the locally injected TNF-α. Based on this model, a potential sequence of events involved in TNF-α–induced neutrophil interactions with the venular endothelium, as mediated via both neutrophil and EC TNFRs, is illustrated in Figure 6A. In support of this potential cascade of events, neutralizing antibodies directed against the chemokines MIP-2 and KC (CXCL1) have been shown to selectively inhibit the transmigration phase of leukocyte emigration in TNF-α–stimulated mouse cremaster muscles as viewed by intravital microscopy.33 The precise mechanism by which chemokines may trigger the transmigration phase remains unanswered but may involve activation of leukocyte Mac-1 by an autocrine/paracrine mechanism, triggering intravascular crawling.34 Mac-1 may also play a direct role in mediating leukocyte transmigration through interactions with other EC ligands, such as JAM-C, with EC-derived chemokines providing directional cues to the emigrating cells.26,35,36 Such a cascade of events is supported by data presented here demonstrating that blockers of both Mac-1 and JAM-C can suppress leukocyte transmigration elicited by TNF-α, some in agreement with previous studies.20 Of importance, as the precise mechanism of action of JAM-C in mediating leukocyte transmigration remains unclear,20,37 eg, it is unclear to what extent JAM-C–mediated leukocyte transmigration in vivo is mediated through its interactions with Mac-1 and/or JAM-B,37,38 details of the components and cascade of molecular events that mediate leukocyte transmigration as elicited by TNF-α require further investigations. Collectively, the present results do, however, provide direct in vivo evidence to indicate that activation of EC and leukocyte TNF-α receptors may act in sequence to mediate leukocyte firm adhesion and transmigration, respectively (Figure 6A).
Schematic diagrams illustrating potential pathways involved in leukocyte/vessel wall interactions. (A) The potential roles of leukocyte and EC TNF-α receptors in mediating leukocyte/vessel wall interactions. The diagrams are based on our findings and published works. (B) The EC molecules ICAM-2, JAM-A, and PECAM-1 act sequentially to mediate leukocyte transmigration through venular walls in vivo. ICAM-2 acts at an early stage where leukocytes are thought to be “investigating” an EC junction, JAM-A mediates migration through EC junctions, and PECAM-1 supports migration through the vascular BM.
Schematic diagrams illustrating potential pathways involved in leukocyte/vessel wall interactions. (A) The potential roles of leukocyte and EC TNF-α receptors in mediating leukocyte/vessel wall interactions. The diagrams are based on our findings and published works. (B) The EC molecules ICAM-2, JAM-A, and PECAM-1 act sequentially to mediate leukocyte transmigration through venular walls in vivo. ICAM-2 acts at an early stage where leukocytes are thought to be “investigating” an EC junction, JAM-A mediates migration through EC junctions, and PECAM-1 supports migration through the vascular BM.
The aforementioned findings were extended to studies aimed at directly investigating the possibility that the ability of TNF-α to induce neutrophil transmigration independently of ICAM-2, JAM-A, and PECAM-1 is accounted for by the ability of this cytokine to stimulate neutrophil TNF-α receptors. For this purpose, the responses of fluorescently labeled TNFR−/− leukocytes in TNF-α–stimulated cremaster muscles of WT, ICAM-2−/−, JAM-A−/−, and PECAM-1−/− mice were quantified using a cell transfer technique. Under these experimental conditions where TNF-α was unable to stimulate leukocytes, the transmigration response to this cytokine was rendered dependent on ICAM-2, JAM-A, and PECAM-1. The reduced TNF-α–elicited transmigration of TNFR−/− leukocytes observed in the EC molecule-deleted recipients was partial, an effect in line with the partial inhibition of IL-1β–induced responses previously noted in these mice.8,14,15 These findings provide direct evidence to suggest that the ability of the adhesion molecules ICAM-2, JAM-A, and PECAM-1 to mediate neutrophil transmigration in a stimulus-dependent manner is governed by the ability of the stimuli to activate leukocytes or tissue (largely EC) receptors. At present, it is unclear how leukocyte or EC stimulation can lead to pathways independent or dependent of ICAM-2/JAM-A/PECAM-1, respectively, or what the nature of the independent pathways is. It is potentially possible that stimulation of neutrophils directly or indirectly (eg, via release of chemokines and other neutrophil stimulatory molecules) activates β2/ICAM-1-mediated transmigration and/or pathways involving other EC junctional molecules, such as endothelial cell-selective adhesion molecule, CD99, or JAM-C as discussed,35,36,39-41 as well as possibly transmigration via the transcellular route.1 Addressing these possibilities will form the subject of future investigations.
As we have previously found that blockade/deletion of PECAM-1/JAM-A or PECAM-1/ICAM-2 does not result in an additive inhibitory effect on neutrophil transmigration through mouse cremasteric venules as induced by IL-1β,8,14 in the present study we extended these findings to conditions of ICAM-2/JAM-A functional blockade and also, importantly, to blockade of all 3 molecules. The dual and triple functional blockade studies provided no evidence to suggest that ICAM-2, JAM-A, and PECAM-1 act in an additive manner, suggesting that they may act in sequence to mediate neutrophil emigration through venular walls. Although complete inhibition was not seen, it is thought that these data do represent a true inhibition rather than a delay in transmigration based on previous studies,8,42 which observed dynamic changes in the number of transmigrated leukocytes over time and saw no evidence for numbers “catching up” in the absence of functional JAM-A. Our previous studies have shown that JAM-A and PECAM-1 act at distinct steps in the transmigration process, with JAM-A mediating neutrophil migration at the level of the endothelium and PECAM-1 supporting leukocyte transmigration at the level of the vascular BM.8,15,22,43 To investigate this in more detail, and importantly to investigate the precise role of ICAM-2 in the transmigration response, IL-1β–stimulated cremaster muscles from WT, ICAM-2−/−, JAM-A−/−, and PECAM-1−/− mice were analyzed by confocal microscopy for localization of neutrophils. Although both ICAM-2 and JAM-A were noted to mediate leukocyte transmigration at the level of the endothelium, clear differences were observed in the site of arrest of neutrophils in the relevant knockout mice. Specifically, ICAM-2–deficient mice exhibited a large number of neutrophils arrested at the interphase between the vascular lumen and EC junctions; ie, the neutrophils appeared to have stopped at a very early stage in the transmigration response, just before penetrating the EC junctions. In contrast, in JAM-A–deficient animals, a significant number of neutrophils were found well within the EC junctions. The labeling protocol used to show the location of endothelial junctions and the single time point at which cells were observed made it impossible to determine whether transcellular migration was occurring in these tissues. Furthermore, in agreement with previous findings,15,22 PECAM-1−/− mice exhibited a defect in neutrophil emigration through the vascular BM. Some previous studies have observed a role for PECAM-1 in migration through the endothelium as opposed to the BM,44,45 but this was only seen when antibodies against specific domains of PECAM-1 were used, as opposed to full genetic deletion of the protein as with this work. Collectively, the present findings provide the first evidence to demonstrate that the adhesion molecules ICAM-2, JAM-A, and PECAM-1 can act in sequence to mediate distinct but sequential steps in supporting neutrophil emigration from the vascular lumen to the extravascular tissue (Figure 6B). PECAM-1–mediated neutrophil migration through the vascular BM appears to be regulated at least partly via PECAM-1–mediated up-regulation of the integrin α6β1,22 but the mechanisms associated with ICAM-2– and JAM-A–mediated events are at present unclear. However, because ICAM-2 is expressed both on the vascular lumen and at EC junctions, this expression profile appears in line with its role in mediating an early phase of neutrophil transmigration, possibly acting to guide leukocytes to EC junctions. The role of JAM-A in neutrophil migration through EC junctions is also unclear, but its strong junctional expression, regulated by pro-inflammatory cytokines, suggests that endothelial JAM-A may direct the movement of leukocytes through cell-cell junctions via binding with its leukocyte ligand.4 Leukocyte JAM-A has also been shown to mediate directional leukocyte migration, a response that may facilitate leukocyte migration through EC junctions and beyond in certain inflammatory scenarios.4,42
In conclusion, results presented here demonstrate the ability of the EC adhesion molecules ICAM-2, JAM-A, and PECAM-1 to mediate neutrophil transmigration in a stimulus-dependent manner in multiple mouse strains and provide direct evidence to show that this phenomenon can be governed by the target cell being activated. Furthermore, the findings show that ICAM-2, JAM-A, and PECAM-1 mediate distinct but sequential steps in the transmigration process to support emigration of neutrophils through venular walls in vivo.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Urban Deutsch for his help in generating SPF ICAM-2−/− mice on the C57BL/6 genetic background.
This work was supported by funds from the Wellcome Trust (081172/Z/06/Z) (S.N.) and the European Community (NoE MAIN 502935).
Wellcome Trust
National Institutes of Health
Authorship
Contribution: A.W. designed and performed experiments, analyzed data, and contributed to the writing of the manuscript; M.-B.V. designed and performed experiments and contributed to data analysis and interpretation; B.E., E.D., and B.A.I. provided key reagents and contributed to data analysis; S.N. provided project supervision and contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sussan Nourshargh, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, United Kingdom; e-mail: s.nourshargh@qmul.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal