Abstract
Various studies analyzed the inhibitory effect exerted by mesenchymal stem cells (MSCs) on cells of the innate or acquired immunity. Myeloid dendritic cells (DCs) are also susceptible to such inhibition. In this study, we show that MSCs strongly inhibit DC generation from peripheral blood monocytes. In the presence of MSCs, monocytes supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) did not acquire the surface phenotype typical of immature (CD14−, CD1a+) or mature (CD80+, CD86+, CD83+) DCs, failed to produce IL-12, and did not induce T-cell activation or proliferation. Analysis of the molecular mechanism(s) responsible for the inhibitory effect revealed a major role of prostaglandin E2 (PGE2). Thus, addition of the PGE2 inhibitor NS-398 restored DC differentiation and function. Moreover, PGE2 directly added to cultures of monocytes blocked their differentiation toward DCs in a manner similar to MSCs. Although IL-6 has been proposed to play a role in MSC-mediated inhibition of DC differentiation, our data indicate that PGE2 and not IL-6 represents the key inhibitory mediator. Indeed, NS-398 inhibited PGE2 production and restored DC differentiation with no effect on IL-6 production. These data emphasize the role of MSCs in inhibiting early DC maturation and identifying the molecular mechanisms responsible for the inhibitory effect.
Introduction
Human mesenchymal stem cells (MSCs) represent a relatively rare stromal cell population that resides primarily in the bone marrow1 but can be isolated also from other adult and fetal tissues, including adipose tissue,2 umbilical cord blood,3 amniotic fluid,4 and fetal lung.5 MSCs do not express specific markers but can be phenotypically identified on the basis of the absence of hemopoietic cell markers, namely, CD45, CD34, CD3, CD14, and by the expression of markers, such as CD29, CD90, CD73, CD105, and CD106.6 MSCs represent multipotent cells capable of differentiating into various lineages, including adipose, osteogenic, and chondrogenic tissues.7 They secrete several cytokines, growth factors, and extracellular matrix molecules that play an important role in the proliferation and maturation of hematopoietic stem cells (HSCs), thus revealing their potential usefulness for promoting engraftment of HSC transplantation.8,9 Recently, MSCs have gained attraction not only in regenerative medicine but also in the prevention and treatment of graft-versus-host disease (GVHD)10-14 thought to reflect their capability (documented in humans, rodents, and primates) of suppressing allogeneic T-cell responses.15-18 Subsequent studies further investigated other possible cellular targets of the MSC-mediated immunosuppression. MSCs were shown to exert a strong inhibitory effect on other cells belonging to either innate or adaptive immunity, including natural killer (NK) cells,19,20 dendritic cells (DCs),21-23 B cells,24 and unconventional T cells, including NKT and γδ+ cells.25 Notably, the modulation exerted by MSCs on DC maturation may have important implications for a more precise understanding of their inhibitory effect on GVHD. Indeed, DCs play a critical role in initiating and regulating immune responses by promoting antigen (Ag)–specific T-cell activation.26,27 In addition, as revealed by recent studies, they can efficiently stimulate cells of the innate immune system.28-30 They are the most effective antigen-presenting cells and prime naive T cells to initiate adaptive immune responses, including those against allogeneic cells, that can be measured in vitro in mixed lymphocyte reactions (MLRs). Some DCs reside in an immature state in peripheral tissues and are highly specialized in Ag uptake. They display low levels of surface major histocompatibility complex and costimulatory molecules (CD80, CD86), which become up-regulated on DC maturation. Immature DCs (iDCs) can be rapidly recruited at the site of inflammation where Ag capture and processing is required. After Ag cleavage into peptides, peptide loading on major histocompatibility complex molecules and migration to T-cell areas of the draining lymph nodes, DCs undergo complete maturation. Mature DCs (mDCs) down-regulate their capability of taking up Ags and acquire the ability to stimulate T cells and to induce polarized Th1, Th2 (or indifferentiated Th0) responses as well as tolerogenic T cells.
Several recent studies have focused on the influence of MSCs on DC function. MSCs were shown to inhibit T-cell activation also indirectly by interfering with the differentiation of monocytes21,22,31 or CD34+ hemopoietic precursors23,32 into mDCs. The mechanisms by which MSCs exert their inhibitory effect on DC maturation is still poorly defined. Thus, although MSCs could prevent the interleukin-4 (IL-4)– and granulocyte-macrophage colony-stimulating factor (GM-CSF)–induced monocyte differentiation to fully mDCs, the stage(s) at which MSCs actually act remains to be understood. In addition, no clear information is available on the molecular mechanism(s) (cytokines?) involved in such inhibition. Previous studies suggested that MSC-derived IL-6 and macrophage colony-stimulating factor (M-CSF) could be responsible for the observed inhibitory effect.22,23 However, only phenotypic data, limited to the analysis of surface expression of CD14 and CD1a on monocyte-derived cells, were provided and no DC-mediated functions were investigated. In addition, in these studies, cytokine-specific neutralizing monoclonal antibodies (mAbs) substantially failed to restore the acquisition of mDC phenotype in monocyte-MSC cocultures. Regarding the MSC-mediated inhibition of T and NK cell proliferation and functional maturation, a major inhibitory role was ascribed, in previous studies, to prostaglandin E2 (PGE2) and indoleamine 2,3-dioxygenase (IDO).20,33,34
In the present study, we show that MSCs exert their inhibitory effect on DC maturation by acting early, during the progression from monocytes to iDCs induced by GM-CSF and IL-4, although they do not interfere with lipopolysaccharide (LPS)–induced iDC differentiation into mDCs. Moreover, we show that PGE2 and not IL-6 plays a major role in MSC-mediated inhibition of DC maturation.
Methods
Samples were obtained after ethics committee approval from the Institutional Review Board of the Giannina Gaslini Institute, Genova, Italy, and informed consent was obtained from patients' legal guardians in accordance with the Declaration of Helsinki.
MSC isolation and culture
MSCs were derived from discarded bone fragments of pediatric patients undergoing orthopedic surgery to correct major scoliosis. Bone marrow cells were plated in 25-cm2 tissue culture flasks at a concentration of 106 cells/mL in 5 mL of Mesencult basal medium supplemented with MSC stimulatory supplements (both from StemCell Technologies, Vancouver, BC). After 48-hour incubation at 37°C in a 5% CO2 humidified atmosphere, nonadherent cells were removed and the adherent fraction was cultured in fresh medium. For further expansion passages of confluent cells, MSCs were detached by treatment with trypsin/ethylenediaminetetraacetic acid solution (Lonza Verviers SPRL, Verviers, Belgium) at 37°C for 7 minutes and replated after washing in 75-cm2 tissue culture flasks. Half the medium was replaced twice a week. MSCs were assessed by cytofluorimetric analysis for the expression of the typical markers CD105, CD106, CD166, and CD29, and the absence of the hematopoietic markers CD45, CD34, and CD14. MSCs were used in the experiments only after 2 to 4 expansion passages, to ensure depletion of monocytes/macrophages.
Generation of monocyte-derived DCs
To culture sufficient number of cells, monocytes were separated from buffy coats that were obtained from the Gaslini Institute's transfusion center. Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-Hypaque density gradient centrifugation (specific gravity, 1.077 g/mL; Lympholyte, Cedarlane, CA). CD14+ cells were positively selected from PBMCs using the MACS CD14 MicroBeads (Monocyte Isolation Kit, Miltenyi Biotec, Auburn, CA), according to the manufacturer's instructions. Purity of separated monocytes was assessed by flow cytometry. Cells were then cultured in 6-well plates in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 1% penicillin/streptomycin, 1% L-glutamine (all from Lonza Verviers SPRL), and recombinant GM-CSF (50 ng/mL) and IL-4 (20 ng/mL; both from PeproTech, Rocky Hill, NJ), either in the absence or in the presence of MSCs. After 5 days, cultured cells were harvested by gentle aspiration and analyzed by flow cytometry to assess iDC phenotype. In some experiments, DC maturation was induced at day 5 with 40 ng/mL LPS stimulation (Sigma-Aldrich, St Louis, MO) for 2 additional days, after which cytofluorimetric analysis was performed to evaluate DC maturation.
DC-MSC cocultures
Cocultures were initiated by plating MSCs in 6-well plates overnight at a concentration of 2 × 105cells/well in RPMI 1640 medium supplemented with 10% FCS, 1% penicillin/streptomycin, and 1% L-glutamine (all from Lonza Verviers SPRL). MSCs were not irradiated before the cocultures. A total of 106, freshly separated monocytes (mean purity, 93% of CD14+ cells) were plated with the appropriate cytokine concentrations (as described in “Generation of monocyte-derived DCs”) either in the absence or in the presence of previously plated MSCs at a 5:1 monocyte/MSC ratio. A titration of MSC to monocyte cell ratio of 1:1 up to 1:100 was also performed using different numbers of MSCs. In some experiments, iDCs, differentiated under standard conditions (ie, cultured alone with GM-CSF and IL-4), were induced to mature at day 5 by LPS stimulation for 2 additional days in the presence or in the absence of MSCs. For transwell culture experiments, cultures were performed in 24-well plates. A total of 2 × 105 monocytes were seeded with a previously plated MSC layer (4 × 104 MSCs/well) in cell contact or separately in a 6.5-mm diameter, 0.4-μm pore size transwell chamber.
To investigate whether MSCs exerted their inhibitory effect on DC differentiation by PGE2 production, the inhibitor of PGE2 synthesis, NS-398 (5 μM, Cayman Chemical, Ann Arbor, MI), was added to the initial cocultures. In other experiments, monocytes were cultured alone (without MSCs) with GM-CSF and IL-4 and with the addition of 1 μM PGE2 (Sigma-Aldrich). The dose of 1 μM PGE2 was selected on the basis of preliminary titration experiments as the minimal concentration capable of mediating optimal inhibition of DC differentiation. To evaluate also a possible role of IL-6 and M-CSF in the MSC-mediated inhibition of DC differentiation, neutralizing anti–IL-6 and anti–M-CSF monoclonal antibodies (both from R&D Systems Europe, Abingdon, United Kingdom) were used in monocyte-MSC cocultures at a concentration of 10 μg/mL.
Cytofluorimetric analysis
To analyze DC phenotype, the anti–CD14-PC7 mAb was purchased from Beckman Coulter (Fullerton, CA), the anti–CD1a-FITC, anti–CD14-phycoerythrin (PE), anti–CD80-PE, anti–CD83-PE, anti–CD86-PE, anti–HLA-A,B,C-PE, and anti–HLA-DR-PE were from BD Biosciences PharMingen (San Diego, CA). Cytofluorimetric analysis of DCs was performed by triple-color staining. Briefly, cells were first incubated for 10 minutes at 4°C with saturating concentrations (1 mg/mL) of human IgGs to prevent unspecific binding of mAbs to Fcγ receptors. Then, DCs were stained with the fluorochrome-conjugated mAbs and incubated for 30 minutes at 4°C. Finally, unbound reagents were removed by washing, and cells were resuspended in RPMI 1640 5% FCS and analyzed on a FACSCalibur equipped with CellQuest software (BD Biosciences, San Jose, CA). To compare the surface densities of DC-specific markers in DCs cultured in different conditions, we calculated the mean ratio fluorescence intensity (MRFI), which is the ratio between the mean fluorescence intensity of cells stained with the selected mAb and that of unstained cells (negative control).
For phenotypic analysis of MSCs, the anti-CD29, anti–CD105-PE, anti-CD106, and anti-CD166 mAbs were from Ancell (Bayport, MN), and the anti-CD14, anti-CD34, anti-CD45, and anti-CD73 mAbs were purchased from BD Biosciences PharMingen. MSC phenotype was analyzed by indirect single fluorescence analysis. MSCs were first incubated with primary marker-specific mAbs for 30 minutes at 4°C. Then, cells were washed and incubated with PE-conjugated AffiniPure F(ab′)2 goat anti–mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). After 30 minutes, cells were washed and resuspended in RPMI 1640 5% FCS before the analysis.
T-cell proliferation assays
Monocyte-derived cells were used as stimulators in T-cell proliferation assays after 5 days of culture with GM-CSF and IL-4 and 2 additional days of stimulation with LPS. Cells were irradiated (50 Gy) and plated in triplicate in U-bottom 96-well plates at 5 × 102 up to 20 × 104 cells per well in serial dilution. Allogeneic peripheral blood lymphocytes (PBLs) were used as responder cells. Cells were obtained from the nonadherent fraction of PBMCs of healthy donors after 1 hour and 30 minutes of adherence at 37°C in a 5% CO2 humidified atmosphere. PBLs were plated at 105 cells/well. After 5-day culture, cells were pulsed for 16 additional hours with 3H-thymidine at 1 μCi (0.037 MBq)/well (GE Healthcare, Little Chalfont, United Kingdom) and then harvested. 3H-thymidine incorporation of PBL was measured using the Plate Chameleon Multilabel Counter (Hidex, Turku, Finland).
ELISA
Quantification of PGE2 was performed on supernatants collected at 24, 48, and 72 hours of culture. IL-6 production was evaluated in supernatant (SN) after 5 days of culture. To measure IL-12p70 production by mDCs, culture SNs were collected at day 7 (5 days + 48 hours after LPS stimulation).
PGE2 levels were measured by competitive enzyme-linked immunosorbent assay (ELISA) technique using a commercially available ELISA kit (R&D Systems Europe). IL-6 and IL-12p70 were quantified using a sandwich type assay (Beckman Coulter for IL-6; BioSource Europe, Nivelles, Belgium for IL-12p70), according to the manufacturer's instructions. Concentrations were calculated by comparison with known standards with a lowest detection limit of 39 pg/mL, 3 pg/mL, and 0.5 pg/mL for PGE2, IL-6, and IL-12p70, respectively. All determinations were made in duplicates.
Statistical analysis
Descriptive and analytical statistics were performed using the SPSS (version 15; Chicago, IL) software package. Descriptive data were presented as mean plus or minus SD. Comparison between different conditions was performed using the paired sample t test, except in the case of IL-12p70 production, for which the nonparametric Wilcoxon test was used as the result of the marked variability of IL-12 secretion. Correlation between PGE2 levels and CD14 or CD1a expression was performed by Pearson correlation coefficient (r value). A P value of less than .05, less than .01, or less than .001 was considered statistically significant.
Results
MSCs inhibit monocyte-derived DC differentiation induced by GM-CSF and IL-4
CD14+ cells purified from peripheral blood of healthy donors were cultured in medium with GM-CSF and IL-4 to induce DC differentiation. Cultures were set either in the absence or in the presence of MSCs at different monocyte/MSC ratios (ranging from 100:1 to 1:1). After 5 days, DC differentiation was assessed by analyzing the expression of CD1a, CD14, and of the costimulatory molecules CD80 and CD86. CD14+ cells cultured alone underwent differentiation into classic iDCs (the mean percentage of CD14- and CD1a-positive cells was < 0.5% and 81.3%, respectively, whereas the mean MRFI of CD80 and CD86 was 5.5 and 3.8, respectively). The same cells, cultured in the presence of MSCs, displayed a surface phenotype that revealed a poor degree of differentiation. For example, at 5:1 monocyte/MSC ratio, the mean percentage of CD14+ and CD1a+ cells in 20 independent experiments was 66% and 18%, respectively, whereas the mean MRFI of CD80 and CD86 was 3.9 and 4.4, respectively (not shown). Thus, only a small fraction of monocytes had lost CD14 and expressed CD1a. Figure 1 shows a representative experiment. Notably, maximal inhibition could be observed from 1:1 up to 10:1 monocyte/MSC ratios. The inhibitory effect decreased at 20:1 and was virtually absent at 100:1 ratio (not shown). Moreover, it is of note that total cell recovery did not differ between cells cultured under standard conditions (ie, with GM-CSF and IL-4) and cells cultured in the presence of MSCs (not shown), thus suggesting that MSCs affect DC differentiation but not viability.
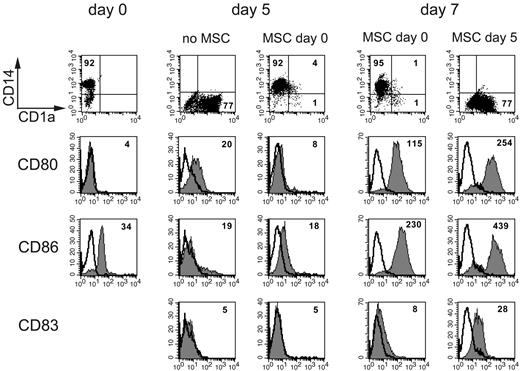
MSC inhibit monocyte progression to immature DCs. A representative experiment. Expression of CD14, CD1a, CD80, CD86, and CD83 on monocytes (at day 0) and after induction of DC differentiation to immature DCs (day 5) or full maturation (day 7). Phenotypic analysis of monocytes was performed on PBMCs by gating on the leukocyte subset according to the forward scatter and side scatter scatter, whereas iDC surface phenotype was analyzed in CD14+ cells cultured for 5 days with GM-CSF and IL-4 either in the absence or in the presence of MSCs. Mature DC phenotype was analyzed after LPS-induced maturation of cells cultured alone or with MSCs either starting at day 0 or at day 5. Numbers indicate percentages of positive cells for CD14 and CD1a expression, whereas they represent mean fluorescence intensity for the surface density of CD80, CD86, and CD83 markers.
MSC inhibit monocyte progression to immature DCs. A representative experiment. Expression of CD14, CD1a, CD80, CD86, and CD83 on monocytes (at day 0) and after induction of DC differentiation to immature DCs (day 5) or full maturation (day 7). Phenotypic analysis of monocytes was performed on PBMCs by gating on the leukocyte subset according to the forward scatter and side scatter scatter, whereas iDC surface phenotype was analyzed in CD14+ cells cultured for 5 days with GM-CSF and IL-4 either in the absence or in the presence of MSCs. Mature DC phenotype was analyzed after LPS-induced maturation of cells cultured alone or with MSCs either starting at day 0 or at day 5. Numbers indicate percentages of positive cells for CD14 and CD1a expression, whereas they represent mean fluorescence intensity for the surface density of CD80, CD86, and CD83 markers.
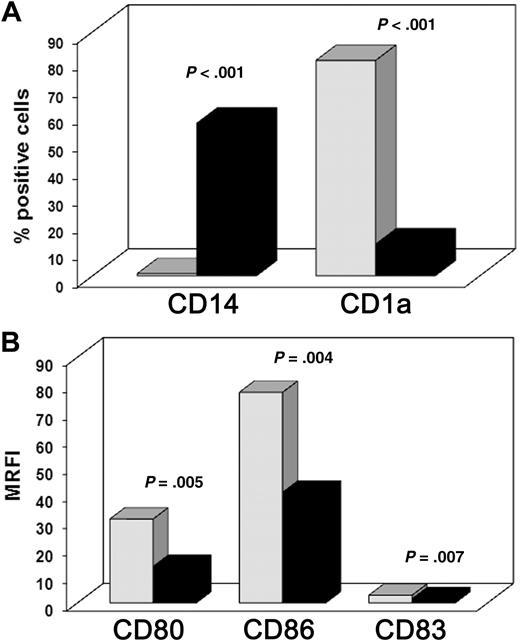
To induce further DC maturation, LPS was added to cells that had been cultured for 5 days either in the absence or in the presence of MSCs. Surface marker analysis was performed after 2 additional days in culture. In agreement with previous data,22,23 the surface density of the costimulatory molecules CD80 and CD86 and of the maturation marker CD83 was significantly lower in cells cultured in the presence of MSCs. Data obtained from 12 independent experiments are shown in Figure 2: the mean percentages plus or minus SD of CD14+ and CD1a+ cells cultured in the absence of MSCs were 0.06% plus or minus 0.01% and 79.5% plus or minus 18.1%, respectively. In contrast, in the presence of MSCs, they were 56.2% plus or minus 19.5% and 11.7% plus or minus 9.1%, respectively (Figure 2A; P < .001 for both). Figure 2B shows the MRFI for CD80, CD86, and CD83 of cells cultured in the absence (30.7 ± 18.1, 77.1 ± 41.8, and 2.8 ± 1.2, respectively) or in the presence of MSCs (13.4 ± 5.3, 40.5 ± 19.6, and 1.5 ± 0.6; P < .01 for all). In another series of experiments, we analyzed whether the addition of MSCs to monocyte-derived iDCs could prevent the progression toward fully differentiated mDCs. To this aim, iDCs were cultured with LPS either in absence or in the presence of MSCs (added at day 5). As shown in Figure 1, MSCs did not interfere with LPS-induced maturation of iDCs because the surface density of CD80, CD86, and CD83 was comparable with that of mDCs obtained in the absence of MSCs. These findings indicate that MSCs exert a strong inhibitory effect on the differentiation process from monocytes to iDCs but not on the LPS-induced maturation of iDCs to mDCs.
MSC-induced inhibition of monocyte differentiation into mDCs. Freshly purified CD14+ cells were cultured with GM-CSF and IL-4 for 5 days, LPS was added for 2 additional days to induce DC maturation. Cultures were set either in the presence (■) or in the absence ( ) of MSCs. (A) Mean of percentages of CD14 and CD1a positive cells. (B) Mean of the surface density of CD80, CD86, and CD83 evaluated as MRFI (“Cytofluorimetric analysis”). Results were obtained from 12 independent experiments.
) of MSCs. (A) Mean of percentages of CD14 and CD1a positive cells. (B) Mean of the surface density of CD80, CD86, and CD83 evaluated as MRFI (“Cytofluorimetric analysis”). Results were obtained from 12 independent experiments.
MSC-induced inhibition of monocyte differentiation into mDCs. Freshly purified CD14+ cells were cultured with GM-CSF and IL-4 for 5 days, LPS was added for 2 additional days to induce DC maturation. Cultures were set either in the presence (■) or in the absence ( ) of MSCs. (A) Mean of percentages of CD14 and CD1a positive cells. (B) Mean of the surface density of CD80, CD86, and CD83 evaluated as MRFI (“Cytofluorimetric analysis”). Results were obtained from 12 independent experiments.
) of MSCs. (A) Mean of percentages of CD14 and CD1a positive cells. (B) Mean of the surface density of CD80, CD86, and CD83 evaluated as MRFI (“Cytofluorimetric analysis”). Results were obtained from 12 independent experiments.
Monocyte-derived cells cultured in the presence of MSCs do not produce IL-12 and fail to induce T-cell responses in MLR
Monocyte-derived cells cultured either in the presence or in the absence of MSCs were also tested for IL-12p70 production by the collection of cell-free culture SN 48 hours after LPS stimulation. The amount of IL-12p70 secreted was analyzed by ELISA. As shown in Figure 3A, cells cocultured with MSCs produced low amounts of IL-12 (8.8 ± 11 pg/mL), whereas much higher amounts (334 ± 376 pg/mL) were released by cells cultured alone (displaying a typical mDC phenotype; P < .05). It is of note that, as observed for the DC surface phenotype, also the IL-12 production was not impaired in DCs induced to mature in the presence of MSCs added at day 5 of culture (notably, the mean IL-12 levels were even higher: 487 ± 512 pg/mL).
MSCs inhibit the production of IL-12 and the capability of stimulating T-cell response in MLR of monocyte-derived cells. (A) Il-12p70 production was measured in culture SN after 48-hour stimulation with LPS of monocyte-derived cells cultured for 5 days with GM-CSF and IL-4 under different culture conditions. Standard (ctr), with the addition of MSCs or of MSCs and the PGE2 inhibitor NS-398 or of PGE2 (1 μM) added at day 0, with MSCs added at day 5. Results are expressed as pg/mL. (B) In MLR experiments, monocyte-derived cells were irradiated and used in graded doses to stimulate allogeneic T cells (105 responder cells/well). After culture for 5 days, T-cell proliferation was evaluated by incubating cells with 3H-thymidine for additional 16 hours. Cells were then harvested and 3H-thymidine uptake was measured. Because of the variable degree of proliferation of different T-cell populations used as responder cells, data are not expressed as cpm values but as percentages. We considered 100% the maximal proliferation of T cells stimulated with DCs (5 × 102 cells/well) obtained under standard conditions, and relative percentage the proliferation of T cells stimulated by cells obtained under the other culture conditions. Bars represent mean ± SD from 5 independent experiments. *P < .05; **P < .01.
MSCs inhibit the production of IL-12 and the capability of stimulating T-cell response in MLR of monocyte-derived cells. (A) Il-12p70 production was measured in culture SN after 48-hour stimulation with LPS of monocyte-derived cells cultured for 5 days with GM-CSF and IL-4 under different culture conditions. Standard (ctr), with the addition of MSCs or of MSCs and the PGE2 inhibitor NS-398 or of PGE2 (1 μM) added at day 0, with MSCs added at day 5. Results are expressed as pg/mL. (B) In MLR experiments, monocyte-derived cells were irradiated and used in graded doses to stimulate allogeneic T cells (105 responder cells/well). After culture for 5 days, T-cell proliferation was evaluated by incubating cells with 3H-thymidine for additional 16 hours. Cells were then harvested and 3H-thymidine uptake was measured. Because of the variable degree of proliferation of different T-cell populations used as responder cells, data are not expressed as cpm values but as percentages. We considered 100% the maximal proliferation of T cells stimulated with DCs (5 × 102 cells/well) obtained under standard conditions, and relative percentage the proliferation of T cells stimulated by cells obtained under the other culture conditions. Bars represent mean ± SD from 5 independent experiments. *P < .05; **P < .01.
The inhibitory activity exerted by MSCs on DC maturation was further analyzed in experiments in which monocyte-derived cells, cultured in the presence or in the absence of MSCs, were used as a source of simulating cells in MLR. As shown in Figure 3B, monocyte-derived cells cultured in the presence of MSCs displayed a severely impaired capability of stimulating T-cell responses compared with control cultures in the absence of MSCs (ie, undergone differentiation toward mDCs; P < .01). Notably, addition of MSCs at day 5 to monocyte-derived iDCs did not affect the generation of cells capable of T-cell stimulation in MLR. Indeed, these cells were even more efficient stimulator cells than DCs obtained under standard conditions (P < .01).
Altogether, these results confirm that MSCs exert a profound inhibition on DC differentiation and functional maturation from monocytes.
PGE2 plays a key role in the MSC-mediated inhibition of DC differentiation
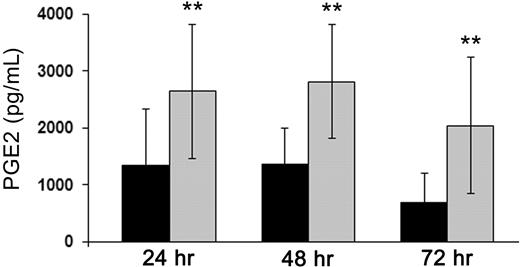
To investigate the mechanism(s) involved in the MSC-mediated inhibition of DC differentiation, we analyzed whether the inhibitory effect required cell-to-cell contact. To this aim, we performed experiments in which monocytes and MSCs were cocultured using the transwell chamber system. Under this culture condition, a significant (although not maximal) inhibition of DC differentiation was observed at day 5 (not shown), thus suggesting the involvement of a soluble factor. We previously showed that MSCs may exert their suppressive effect on NK cell proliferation and functional maturation as a result of the production of PGE2.20 To evaluate its possible involvement also in the MSC-mediated inhibition of DC differentiation, PGE2 produced by MSCs cultured alone or with monocytes in the presence of GM-CSF and IL-4 was first measured in preliminary experiments. Supernatants were collected from culture combinations of MSCs and monocytes derived from different donors at 24, 48, and 72 hours of culture and analyzed for PGE2 production. As shown in Figure 4, MSCs produced PGE2 constitutively; moreover, this production was significantly increased on coculture with monocytes (P < .01). Notably, monocytes cultured alone did not produce any detectable PGE2 (not shown). A statistically significant correlation was found between the levels of PGE2 detected in culture SN and the degree of inhibition of DC differentiation evaluated by the expression of CD1a and CD14 (not shown). This suggests that MSCs, derived from different donors, can produce different amounts of PGE2. As a consequence, it is conceivable that different MSC populations may exert variable inhibitory effects on DC differentiation.
PGE2 production by MSCs is up-regulated in monocyte-MSC cocultures. PGE2 levels were measured in culture SN of MSCs cultured alone (■) or with monocytes ( ) induced to differentiate into DCs. PGE2 was evaluated by ELISA assay performed on SN collected at 24, 48, and 72 hours of culture. Results are expressed as mean of PGE2 levels measured in 7 independent experiments performed using different MSC populations. Data are expressed in pg/mL. **P < .01.
) induced to differentiate into DCs. PGE2 was evaluated by ELISA assay performed on SN collected at 24, 48, and 72 hours of culture. Results are expressed as mean of PGE2 levels measured in 7 independent experiments performed using different MSC populations. Data are expressed in pg/mL. **P < .01.
PGE2 production by MSCs is up-regulated in monocyte-MSC cocultures. PGE2 levels were measured in culture SN of MSCs cultured alone (■) or with monocytes ( ) induced to differentiate into DCs. PGE2 was evaluated by ELISA assay performed on SN collected at 24, 48, and 72 hours of culture. Results are expressed as mean of PGE2 levels measured in 7 independent experiments performed using different MSC populations. Data are expressed in pg/mL. **P < .01.
) induced to differentiate into DCs. PGE2 was evaluated by ELISA assay performed on SN collected at 24, 48, and 72 hours of culture. Results are expressed as mean of PGE2 levels measured in 7 independent experiments performed using different MSC populations. Data are expressed in pg/mL. **P < .01.
To confirm that PGE2 may indeed play a major role in the MSC-mediated inhibition of DC maturation, we added the PGE2-specific synthesis inhibitor NS-398 to the monocyte-MSC cocultures. PGE2 secretion was assessed in culture SN by quantitative ELISA assays. In the presence of the inhibitor, no PGE2 could be detected. Moreover, the phenotypic analysis of cells from day 5 cocultures with MSCs in the presence of NS-398 revealed virtually complete restoration of DC differentiation (Figure 5A). As shown in Figure 5B, cells displayed high CD1a (65% ± 23%) and low CD14 expression (9.4% ± 9%) compared with cells cultured with MSCs in the absence of the inhibitor (17.2% ± 11.4% for CD1a and 64.1% ± 26.4% for CD14; P < .001 for both). Notably, analysis of DC function revealed that, in the presence of NS-398, there was a partial restoration of IL-12 production (P < .05) and of the ability of cells to stimulate T-cell response in MLR (P < .01; Figure 3). Taken together, these data strongly suggest that PGE2 plays a major role in the inhibition of DC differentiation and function.
Involvement of MSC-derived PGE2 in the inhibition of DC differentiation: analysis of CD1a and CD14 surface expression. Purified CD14+ cells were cultured with GM-CSF and IL-4 to induce differentiation into DCs. Cultures were performed either in the absence (■) or in the presence (▧) of MSCs. In addition, the PGE2 inhibitor NS-398 (5 μM) was added to monocyte-MSC cocultures ( ) or 1 μM PGE2 was added to monocytes cultured alone (
) or 1 μM PGE2 was added to monocytes cultured alone ( ). After 5 days, phenotypic analysis was performed to check DC differentiation. (A) A representative experiment. Numbers represent percentages of positive cells. (B) Expression of CD14 and CD1a in cells cultured under the described culture conditions. Results are expressed as mean ± SD of the percentages of marker-positive cells obtained from the analysis of 11 independent experiments performed. ***P < .001.
). After 5 days, phenotypic analysis was performed to check DC differentiation. (A) A representative experiment. Numbers represent percentages of positive cells. (B) Expression of CD14 and CD1a in cells cultured under the described culture conditions. Results are expressed as mean ± SD of the percentages of marker-positive cells obtained from the analysis of 11 independent experiments performed. ***P < .001.
Involvement of MSC-derived PGE2 in the inhibition of DC differentiation: analysis of CD1a and CD14 surface expression. Purified CD14+ cells were cultured with GM-CSF and IL-4 to induce differentiation into DCs. Cultures were performed either in the absence (■) or in the presence (▧) of MSCs. In addition, the PGE2 inhibitor NS-398 (5 μM) was added to monocyte-MSC cocultures ( ) or 1 μM PGE2 was added to monocytes cultured alone (
) or 1 μM PGE2 was added to monocytes cultured alone ( ). After 5 days, phenotypic analysis was performed to check DC differentiation. (A) A representative experiment. Numbers represent percentages of positive cells. (B) Expression of CD14 and CD1a in cells cultured under the described culture conditions. Results are expressed as mean ± SD of the percentages of marker-positive cells obtained from the analysis of 11 independent experiments performed. ***P < .001.
). After 5 days, phenotypic analysis was performed to check DC differentiation. (A) A representative experiment. Numbers represent percentages of positive cells. (B) Expression of CD14 and CD1a in cells cultured under the described culture conditions. Results are expressed as mean ± SD of the percentages of marker-positive cells obtained from the analysis of 11 independent experiments performed. ***P < .001.
To further support the notion that indeed PGE2 has a main responsibility in the MSC-mediated inhibition of DC differentiation from monocytes, experiments were performed in which exogenous PGE2 (at the concentration of 1 μM) was added at day 0 to purified CD14+ cells cultured with GM-CSF and IL-4. Cytofluorimetric analysis performed at day 5 of culture revealed a surface phenotype similar to that of cells cocultured with MSCs, ie, very low CD1a and persistence of CD14 surface expression (Figure 5). Moreover, addition of PGE2 inhibited the induction of DC functions. As shown in Figure 3, cells cultured with GM-CSF and IL-4 in the presence of PGE2 and then stimulated with LPS for additional 2 days, did not produce IL-12 (Figure 3A, P < .05) and were unable to elicit proliferative T-cell responses in MLR (Figure 3B, P < .01).
MSC-derived PGE2 acts independently of IL-6
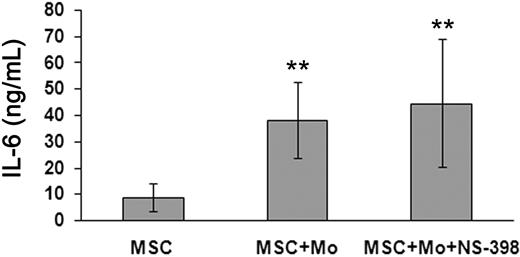
Previous studies reported that IL-6 (together with M-CSF), produced by MSCs, would play a major role in the MSC-mediated inhibition of DC differentiation.22,23 Because our present study strongly suggests a major involvement of PGE2, we evaluated whether PGE2 could exert its inhibitory effect indirectly, ie, by promoting IL-6 production either by MSCs or monocytes. IL-6 was measured in SN of different culture combinations by ELISA. As shown in Figure 6, MSCs produced constitutively a significant amount of IL-6 (mean ± SD of 6 independent experiments was 8.8 ± 5.4 ng/mL), which was highly increased when MSCs were cocultured with monocytes (38.1 ± 14.3 ng/mL; P < .01). Although not shown, monocytes alone did not produce significant levels of IL-6, also on exposure to PGE2 (< 0.01 ng/mL). We also assessed whether blocking of PGE2 production by NS-398 could affect IL-6 secretion. In these experiments, the inhibitor was added to cultures containing different cell combinations. The amount of IL-6 in SN of cocultures of monocytes and MSCs either in the presence or in the absence of NS-398 was similar (44.5 ± 24.2 ng/mL vs 38.1 ± 14.3 ng/mL), thus indicating that, in cultures containing the PGE2 inhibitor, DC maturation occurred despite the presence of high amounts of IL-6. We also assessed whether blocking of IL-6 and M-CSF with specific neutralizing mAbs could interfere with the MSC-mediated inhibition of DC differentiation. Only marginal effect on DC maturation, evaluated on the basis of CD1a expression and CD14 decrease, could be detected (not shown).
IL-6 production by MSCs under different culture conditions. IL-6 production was evaluated in MSCs cultured alone or in combination with monocytes (either in the absence or in the presence of the PGE2 inhibitor NS-398) with GM-CSF and IL-4. After 5 days, culture SNs were collected and IL-6 was measured by ELISA assay. Data are represented as mean ± SD of IL-6 levels (expressed as ng/mL) evaluated in 6 independent experiments performed. **P < .01.
IL-6 production by MSCs under different culture conditions. IL-6 production was evaluated in MSCs cultured alone or in combination with monocytes (either in the absence or in the presence of the PGE2 inhibitor NS-398) with GM-CSF and IL-4. After 5 days, culture SNs were collected and IL-6 was measured by ELISA assay. Data are represented as mean ± SD of IL-6 levels (expressed as ng/mL) evaluated in 6 independent experiments performed. **P < .01.
Altogether, these data strongly support the notion that PGE2 plays a fundamental role in the MSC-mediated inhibition of DC differentiation by acting independently of IL-6.
Discussion
In the present study, we analyzed the effect of MSCs on monocyte-derived DC maturation. We confirm that MSCs mediate a potent inhibition on DC differentiation. More importantly, we show that the effect is restricted to the early stages of this event, ie, to the cytokine-induced progression from monocytes to iDCs. The inhibition affected both the expression of informative DC surface markers and the acquisition of DC functions, such as IL-12 production and the capability of stimulating T-cell responses in MLR. Finally, we show that the inhibitory effect is primarily mediated by PGE2.
MSCs have been shown to exert a potent suppression on both innate and adaptive immunity by acting on NK19,20 and T15-17 and B lymphocytes.24 In addition, previous reports documented a potent inhibitory effect of MSCs on myeloid DC maturation. In these experiments, MSCs were added to cultures of monocytes containing GM-CSF and IL-4 for 5 days and further supplemented with LPS to induce full DC maturation.22,23 However, no information was provided on the actual stage(s) at which the inhibition had occurred. Here we show that MSCs exert their inhibitory effect only when present at the early stages of the differentiation process. Indeed, addition of even low proportions of MSCs (1:10) could prevent monocyte differentiation toward iDCs, as revealed by the persistence of CD14 (a monocyte marker) and by the lack of expression of CD1a (an iDC marker). In contrast, addition of MSCs at later stages did not interfere with the progression of iDCs toward mDCs. DC maturation was evaluated not only on the basis of the surface expression of CD80, CD86, and CD83, but also according to the acquisition of typical DC functions, such as the production of the polarizing cytokine IL-12 and the ability to trigger T-cell responses in MLR. Therefore, MSCs can exert a profound inhibitory effect on DC maturation and function, provided that their interaction occurs early in the process of DC maturation. Because DCs play a central role in the induction of T cell–mediated transplantation rejection or GVHD,35 our present data suggest that the infusion of MSCs (in protocols of adoptive immunotherapy) may be more efficacious when administered before or immediately after transplantation to achieve optimal immunosuppressive effect.
Different soluble factors have been reported to mediate the inhibitory effect exerted by MSCs on different cells of the innate or adaptive immune system. Thus, both IDO and PGE2 would play an important role in the inhibition of NK- and T-cell proliferation and function. IDO would also inhibit B-cell proliferation in the presence of interferon-γ.36 On the other hand, IL-6 and M-CSF have been reported to interfere with DC maturation.23,23,37 It should be noted, however, that the addition of anti–IL-6 and anti–M-CSF neutralizing mAbs to monocyte-MSC cocultures had a marginal effect on restoration of DC maturation because only a partial decrease of CD14 was observed and no expression of CD1a occurred. In addition, no information was provided on whether such neutralizing mAbs could restore DC function. Because PGE2 appears to play a central role in the MSC-mediated immunosuppressive effect against other immune cell types, we reevaluated its possible involvement in the MSC-DC inhibitory interactions. Our study provides convincing evidence of the pivotal role of PGE2 in the inhibitory effect. First, MSCs produce PGE2 constitutively, and this production is increased when MSCs are cocultured with monocytes. Second, the addition of the PGE2 inhibitor NS-398 to monocyte-MSC cocultures almost completely restored DC maturation and function. Third, direct addition of exogenous PGE2 inhibited the generation of iDCs from monocytes, whereas it had no effect when added at later stages, thus paralleling the effect of MSCs. Notably, in culture conditions in which PGE2 synthesis was inhibited by NS-398, but that contained high doses of IL-6, no inhibition of DC differentiation was detected, thus reinforcing the concept that PGE2 and not IL-6 plays a major role in the MSC-mediated inhibition of DC maturation. The mechanism responsible for the increased production of PGE2 in monocyte-MSC cocultures is still unclear. Recently, it has been shown that LPS can induce PGE2 release from murine MSCs via interaction with Toll-like receptor 4.38 However, we can exclude that this mechanism is involved in our experimental system. Indeed, PGE2 increases start early, at 24 hours of coculture, whereas LPS is added only at day 5 to induce further DC maturation.
MSCs were originally considered a valuable tool in regenerative medicine because of their ability to differentiate toward different cells of the mesodermal lineage, primarily osteocytes and chondrocytes.7 More recent studies have also highlighted their potent modulatory effect on both innate and adaptive immunity.39 A pioneering clinical study by Le Blanc et al13 revealed that infusion of MSCs could successfully treat severe acute GVHD. This was confirmed in several subsequent studies both in adult and in pediatric patients.12,14,40 The effect of MSCs on GVHD probably reflects the inhibition of DC maturation and function, as well as of T-cell proliferation and effector function. Antigen presentation by immature DCs has been associated with tolerance induction. Thus, preventing iDC generation may have a negative effect on inflammation control. However, different data obtained in both in vitro and in vivo studies suggest that the MSC-mediated impairment of DC differentiation would primarily result in inhibition of T-cell responses as well as in generation of regulatory T cells.34 Remarkably, although early MSC infusion (ie, before or immediately after bone marrow transplantation) may prevent the onset of GVHD, also a later administration of MSCs to treat severe GVHD can block further generation of both DCs and alloreactive effector T cells, as suggested by the successful clinical outcome reported in different studies.10,12,14,40 One may ask how MSCs, after intravenous infusion, can encounter maturing DCs in patients receiving HSC transplantation. Because the early stages of DC maturation (ie, from monocytes to iDCs) occur primarily in inflamed tissues, MSCs should express appropriate adhesion molecules and chemokine receptors allowing their migration to these sites. Indeed, in vitro cultured MSCs can extravasate from the blood vessels as a result of the expression of surface adhesion molecules. MSCs show coordinated rolling and adhesion behavior on endothelial cells in a P-selectin– and V-CAM–dependent manner.41 In addition, they express receptors for chemokines, including the proinflammatory chemokines CCR1, CXCL16, and CX3CL1 that allow their migration to inflamed tissues.42 Remarkably, such chemokines can be released by monocytes/macrophages and DCs on activation by microbial products and/or cytokine-mediated signaling. This further facilitates the interaction between MSCs and DC precursors in inflamed tissues.
In conclusion, our present study provides novel information on the molecular mechanisms and on the timing of MSC-mediated inhibition of DC maturation and function. These data are relevant not only for a better understanding of the biology of MSCs, DCs, and their functional interactions but also for a better insight into the in vivo mechanisms leading to MSC-mediated immunosuppression. As a corollary, this would allow an improved exploitation of MSCs in HSC transplantation and in other diseases requiring immunosuppression.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro, Fondazione CARIPLO (agreement no. 20060034085), Fondazione Carige (prat. 2006.1051-53), Ministero dell'Istruzione, dell'Università e della Ricerca (Ministero Istruzione Universita Ricerca [MIUR]–Programma di Ricerca di Rilevante Interesse Nazionale [PRIN] 2006/project 2006061378_003 and 2007/project 2007473C72_002), MIUR-Fondo Investimenti Ricerca di Base (FIRB; 2003 project RBLA039LSF-001), and Ministero della Salute (Ricerca Finalizzata 2005/agreement no. 57 and Ricerca Oncologica-Project of Integrated Program 2006-08, agreement no. RO strategico 3/07; L.M.).
Authorship
Contribution: G.M.S. designed and performed research, analyzed and interpreted data, and wrote the manuscript; H.A. designed and performed research and performed statistical analysis; F.B. provided selected samples; and L.M. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lorenzo Moretta, Istituto Giannina Gaslini, Largo Gerolamo Gaslini 5, 16147 Genova, Italy; e-mail: lorenzomoretta@ospedale-gaslini.ge.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal