Abstract
Emerging evidence suggests that all hematopoietic and endothelial cells originate from Flk-1+ mesoderm in the mouse. However, this concept has not been completely proven, especially for the origin of blood cells. Using either Flk1+/Cre;Rosa26R-EYFP or Flk1+/Cre;Rosa26R-LacZ mice, we permanently marked Flk-1+ cells and their progenies to determine the relationship between hematopoietic tissues and cells that express Flk-1. In embryos, all blood cells within the yolk sac and aorta were of Flk-1+ origin. In addition, nearly all CD45+ cells in bone marrow and circulating blood in adults were of Flk-1+ origin. These results provide clear evidence that all blood cells, primitive and definitive, in mice are derived from Flk-1+ mesodermal cells.
Introduction
Flk-1 is a receptor tyrosine kinase that binds vascular endothelial growth factor (VEGF). In primitive streak–stage embryos, Flk1 expression is first detectable in cells within and exiting the primitive streak as well as the extraembryonic mesoderm.1,2 Subsequently, it can be detected in the endothelial cells lining the blood islands of the extraembryonic yolk sac, the first site of embryonic hematopoiesis by embryonic day (E) 7.5.
Genetic studies indicate that Flk1 is required for blood and vessel formation because Flk1−/− mice fail to develop blood or vessels and die by E9.5.3 By making chimeric mice with Flk1−/− embryonic stem (ES) cells, it was revealed that Flk1 is also required for adult blood, as no Flk1−/− cells contributed to adult hematopoietic tissues.4 Flk-1+ cells within the primitive streak are capable of giving rise to hematopoietic cells, whereas Flk-1− cells of the primitive streak do not possess hematopoietic potential.5 Using the in vitro ES differentiation system to generate Flk-1+ cells, it is clear that hematopoietic and endothelial progenitors are contained within Flk-1+ cells.6-8 In addition, Flk-1+ but not Flk-1− cells derived from ES cells could generate T lymphocytes when cocultured with lymphocyte-depleted thymic lobes,9 and Flk-1+ cells differentiated from ES cells in vitro were able to reconstitute the hematopoietic systems of severe combined immunodeficiency (SCID) mice upon transplantation.10
Though these studies show a functional requirement for Flk1 in hematopoietic development, they do not explain the origin of embryonic or adult blood. The complicated issue in hematopoietic ontogeny is that Flk1 is down-regulated within cells of the hematopoietic system such that any given hematopoietic cell is Flk-1−.11 To circumvent this issue, it is necessary to use a lineage-tracing system in which cells that express Flk1, or are the progeny of Flk-1+ cells, will be permanently marked. An ideal means to perform this tracing is by using a Flk1+/Cre mouse, which was generated by knocking in Cre recombinase into the Flk1 locus by homologous recombination.12 By comparing the endogenous Flk-1 and LacZ expression in Flk1+/LacZ and Cre expression in Flk1+/Cre mice, it was established that Cre expression patterns recapitulate endogenous Flk-1 protein and mRNA. Previous studies using this strategy to permanently mark cells expressing Flk1 suggest blood cells within the yolk sac blood islands originate from Flk-1+ mesoderm.12 Conversely, lineage tracing using chimeric mice suggested that not all primitive blood cells are derived from Flk-1+ mesoderm.13 In this report, we examine the origin of primitive and definitive blood cells by lineage tracing and demonstrate that all blood cells are the progeny of Flk-1+ mesoderm.
Methods
Flk1+/Cre mice12 were crossed with Rosa26R-LacZ14,15 or Rosa26R-EYFP16 mice to generate Flk1+/Cre; Rosa26R-LacZ and Flk1+/Cre; Rosa26R-EYFP reporter mice, respectively. The use of mouse models in these experiments received Institutional Animal Care and Use Committee (IACUC) approval (approval no. 20070074) from all participating institutions.
Fluorescence-activated cell sorter (FACS) analyses were performed as previously described.7,8 E9.5 yolk sacs were dissected. Embryos were subjected to genotyping, and yolk sacs were incubated for 90 minutes at 37°C in 0.1% collagenase (Sigma-Aldrich, St Louis, MO) with 20% fetal bovine serum in phosphate-buffered saline (PBS). After incubation, the yolk sacs were separated into single-cell suspension by passing through 20-gauge syringes. The cells were stained with Ter119 and Mac1 antibodies (eBioscience, San Diego, CA), and analyzed by FACS. Bone marrow (BM) cells were obtained by flushing the femur, and peripheral blood (PB) samples were taken retro-orbitally. Both BM and PB were treated with red blood cell lysis buffer. After centrifugation, cells were stained with CD45, CD4, CD8, B220, Gr1, and Mac1 antibodies (eBioscience) and analyzed by FACS.
Whole-mount LacZ staining was performed as previously described.4 After staining, embryos were cryosectioned at 5 to 6 μm.
Results and discussion
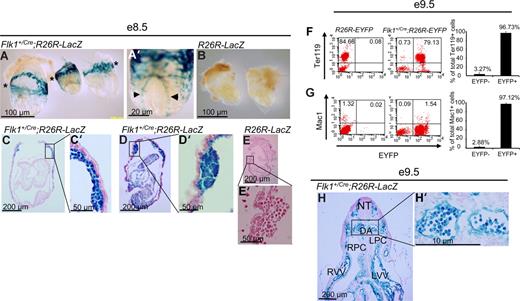
To trace the lineage of Flk-1+ cells, Flk1+/Cre mice,12 in which Cre recombinase is knocked into the Flk1 locus, were crossed to flox-STOP-flox-LacZ (Rosa26R-LacZ)14,15 or flox-STOP-flox-EYFP (Rosa26R-EYFP)16 Rosa reporter mice so that cells that express Flk1 will express Cre recombinase and delete the floxed-STOP sequence. Due to the constitutively active nature of the Rosa26 locus, the cells and their progeny will permanently express LacZ or EYFP. We first examined embryonic hematopoiesis in the yolk sac blood islands of E8.5 Flk1+/Cre;Rosa26R-LacZ mice. As shown in Figure 1A, all blood island endothelial and blood cells are LacZ+. Whole-mount examination revealed the obvious presence of LacZ+ cells in the extraembryonic yolk sac (asterisks in Figure 1A) and in the dorsal aorta (arrowheads in Figure 1A′). In control Rosa26R-LacZ littermates, no LacZ+ cells were found throughout the embryos or yolk sacs (Figure 1B,E,E′). Upon sectioning embryos, it could be seen that all blood cells present in the yolk sacs are LacZ+, surrounded by endothelial cells which are also LacZ+ (Figure 1C,C′,D,D′). We found there was both strong (entire cell stains blue) and weak (staining confined to cytoplasm) LacZ staining in nearly every type of cell that stained positively. It is likely that the processes underlying X-gal staining, including fixation, tissue permeabilization, and stain penetration, could affect the uniformity of staining within these cells. At the single-cell level, about 97% (average, 96.73% ± 3.27%; n = 8) of the erythroid cells (Ter119+) and 97% (average, 97.12 %± 2.22%; n = 8) of the macrophage (Mac1+) of the E9.5 Flk1+/Cre;Rosa26R-EYFP yolk sac cells were EYFP+ (Figure 1F,G). About 2.7% of the E9.5 yolk sac cells were CD45+, which were also EYFP+ (data not shown). Within the developing embryo proper, the paired dorsal aortas (DAs) were present and visible in the E9.5 Flk1+/Cre;Rosa26R-LacZ mice, and the endothelial cells lining the DAs were clearly LacZ+ (Figure 1H,H′). All blood cells visible in the DA were LacZ+ when E9.5 Flk1+/Cre;Rosa26R-LacZ mice were examined (Figure 1H,H′).
A Flk-1+ origin for embryonic blood cells of Flk1+/Cre;Rosa26R-LacZ embryos. (A-E′) E8.5 embryos. (F,F′) E9.5 embryo. (A) Whole-mount image of 3 representative Flk1+/Cre;Rosa26R-LacZ E8.5 embryos. *Plane of major blood islands in yolk sacs. (A′) Whole-mount high-power magnification of an embryo showing stained dorsal aorta (◀, ▶). (B) Control E8.5 embryos display no LacZ+ cells in their yolk sacs or embryo proper. *Plane of major blood islands in yolk sacs. (C,D) Five-micrometer sections through Flk1+/Cre;Rosa26R-LacZ E8.5 embryos. (C′,D′) High-power views of blood islands from embryos in panels C and D, respectively. (E,E′) Sections of a control embryo showing an absence of LacZ+ cells in the embryo, yolk sac, or blood islands. (F,G) Representative FACS plots of E9.5 R26R-EYFP and E9.5 Flk1+/Cre;Rosa26R-EYFP yolk sacs staining for the erythroid cell marker Ter119 and macrophage marker Mac1, respectively (y-axis) and EYFP (x-axis) to assess the origin of these lineage cells within the E9.5 yolk sac. On the right panel, summary data of multiple experiments (n = 8) analyzing the yolk sacs of Flk1+Cre;Rosa26R-EYFP mice. Numbers indicate the percentage of Ter119+ and Mac1+ cells that are either EYFP− or EYFP+, respectively. The bars represent SD of the mean percentage of total Ter119+ and Mac1+ cells, respectively. (H) Transverse section through an E9.5 embryo. LPC indicates left pericardial-peritoneal cavity; LVV, left vitelline vein; NT, neural tube; RPC right pericardial-peritoneal cavity; and RVV, right viteline vein. (H′) Magnification of region containing paired dorsal aorta demonstrating uniformity of LacZ+ blood cells in the embryonic circulation. (A-H') For whole mount embryo images, embryos were bathed in 1× PBS and photographed with an Olympus (Center Valley, PA) DP25 camera attached to an Olpmpus BH-2 dissecting microscope at either 25× or 63× magnification. The same camera was used with an Olympus BX-51 upright microscope to document sectioned embryos with either 40× or 90× oil immersion lenses. Images were captured with Olympus DP2-BSW software and processed with Adobe Photoshop CS 10.0.1.
A Flk-1+ origin for embryonic blood cells of Flk1+/Cre;Rosa26R-LacZ embryos. (A-E′) E8.5 embryos. (F,F′) E9.5 embryo. (A) Whole-mount image of 3 representative Flk1+/Cre;Rosa26R-LacZ E8.5 embryos. *Plane of major blood islands in yolk sacs. (A′) Whole-mount high-power magnification of an embryo showing stained dorsal aorta (◀, ▶). (B) Control E8.5 embryos display no LacZ+ cells in their yolk sacs or embryo proper. *Plane of major blood islands in yolk sacs. (C,D) Five-micrometer sections through Flk1+/Cre;Rosa26R-LacZ E8.5 embryos. (C′,D′) High-power views of blood islands from embryos in panels C and D, respectively. (E,E′) Sections of a control embryo showing an absence of LacZ+ cells in the embryo, yolk sac, or blood islands. (F,G) Representative FACS plots of E9.5 R26R-EYFP and E9.5 Flk1+/Cre;Rosa26R-EYFP yolk sacs staining for the erythroid cell marker Ter119 and macrophage marker Mac1, respectively (y-axis) and EYFP (x-axis) to assess the origin of these lineage cells within the E9.5 yolk sac. On the right panel, summary data of multiple experiments (n = 8) analyzing the yolk sacs of Flk1+Cre;Rosa26R-EYFP mice. Numbers indicate the percentage of Ter119+ and Mac1+ cells that are either EYFP− or EYFP+, respectively. The bars represent SD of the mean percentage of total Ter119+ and Mac1+ cells, respectively. (H) Transverse section through an E9.5 embryo. LPC indicates left pericardial-peritoneal cavity; LVV, left vitelline vein; NT, neural tube; RPC right pericardial-peritoneal cavity; and RVV, right viteline vein. (H′) Magnification of region containing paired dorsal aorta demonstrating uniformity of LacZ+ blood cells in the embryonic circulation. (A-H') For whole mount embryo images, embryos were bathed in 1× PBS and photographed with an Olympus (Center Valley, PA) DP25 camera attached to an Olpmpus BH-2 dissecting microscope at either 25× or 63× magnification. The same camera was used with an Olympus BX-51 upright microscope to document sectioned embryos with either 40× or 90× oil immersion lenses. Images were captured with Olympus DP2-BSW software and processed with Adobe Photoshop CS 10.0.1.
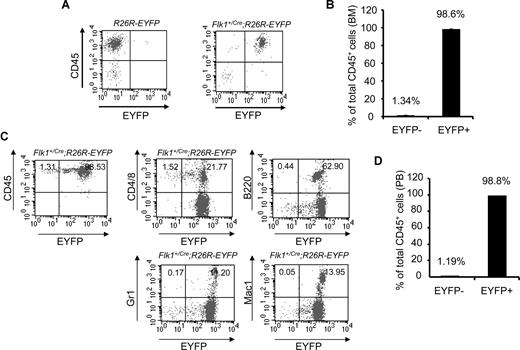
To examine the hematopoietic tissues of adult mice, we analyzed the BM of Flk1+/Cre;Rosa26R-EYFP mice. More than 98% (average, 98.6% ± 0.098%; n = 3) of CD45+ BM cells were EYFP+, whereas fewer than 2% of CD45+ cells were EYFP− (Figure 2A,B). More importantly, more than 98% (average, 98.8% ± 0.528%; n = 4) of CD45+ peripheral blood cells were EYFP+ (Figure 2C,D). B220+, Gr1+, and Mac1+ peripheral blood cells were all EYFP+. With reasons unclear, a small fraction of the peripheral T cells (CD4+ or CD8+) were EYFP− (Figure 2C). Potentially, inactivation of the Rosa locus in some T cells could contribute to this minor CD45+EYFP− cell population. The data strongly argue that all blood cells found in embryos and in adult mice originate from Flk-1+ mesodermal cells.
An Flk-1+ origin for blood cells of adult Flk1+/Cre;Rosa26R-EYFP mice. (A) Representative FACS plots of BM staining for the hematopoietic marker CD45 (y-axis) and EYFP (x-axis) to assess the origin of hematopoietic cells within the BM of 3-month-old Flk1+/Cre;Rosa26R-EYFP mice. (B) Summary data of multiple experiments analyzing BM of Flk1+/Cre;Rosa26R-EYFP mice. Numbers above bars indicate percentage of CD45+ cells that are either EYFP+ or EYFP−. The bars represent SD of the mean percentage of total CD 45+ cells.(C) Representative FACS plots of PB staining for the hematopoietic marker CD45 (y-axis) and EYFP (x-axis) to assess the origin of hematopoietic cells within the PB. Individual lineage markers were also assessed to examine if all mature blood lineages are indeed Flk-1+ in origin. Hematopoietic lineages analyzed include CD4 and CD8, T cells; B220, B cells; Gr1, granulocytes; and Mac1, macrophages. (D) Summary data of multiple experiments (n = 4) analyzing PB of Flk1+Cre;Rosa26R-EYFP mice. Numbers above bars indicate percentage of CD45+ cells that are either EYFP− or EYFP+, respectively. The bars represent SD of the mean percentage of total CD45+ cells.
An Flk-1+ origin for blood cells of adult Flk1+/Cre;Rosa26R-EYFP mice. (A) Representative FACS plots of BM staining for the hematopoietic marker CD45 (y-axis) and EYFP (x-axis) to assess the origin of hematopoietic cells within the BM of 3-month-old Flk1+/Cre;Rosa26R-EYFP mice. (B) Summary data of multiple experiments analyzing BM of Flk1+/Cre;Rosa26R-EYFP mice. Numbers above bars indicate percentage of CD45+ cells that are either EYFP+ or EYFP−. The bars represent SD of the mean percentage of total CD 45+ cells.(C) Representative FACS plots of PB staining for the hematopoietic marker CD45 (y-axis) and EYFP (x-axis) to assess the origin of hematopoietic cells within the PB. Individual lineage markers were also assessed to examine if all mature blood lineages are indeed Flk-1+ in origin. Hematopoietic lineages analyzed include CD4 and CD8, T cells; B220, B cells; Gr1, granulocytes; and Mac1, macrophages. (D) Summary data of multiple experiments (n = 4) analyzing PB of Flk1+Cre;Rosa26R-EYFP mice. Numbers above bars indicate percentage of CD45+ cells that are either EYFP− or EYFP+, respectively. The bars represent SD of the mean percentage of total CD45+ cells.
The Cre/loxP system is an ideal way to trace cell lineage in vivo without complicated manipulations.17 Using Flk1+/Cre and Rosa26 reporter mice, we have demonstrated for the first time that adult blood cells are of an Flk-1+ origin. Although our findings that primitive blood cells are of an Flk-1+ origin are consistent with current literature, recent work reported that blood cells present in the yolk sac have a heterogeneous cellular origin, Flk-1+ or Flk-1−.13 In this report, the authors transfected Flk1+/Cre ES cells with floxed-STOP reporter vectors driving expression of fluorescent proteins. The transfected cells were then injected into blastocysts, and the blood islands of the chimeric embryos were analyzed. Based on the presence of fluorescent cells, they argued that the majority of blood cells have an Flk-1− rather than Flk-1+ origin. This study falls short for a number of reasons. By transfecting Flk1+/Cre ES cells and relying on the excision of the floxed-STOP sequences in the fluorescent reporter vector, the authors ignore both the inherent difficulty in transfecting ES cells as well as the confounding issue of integration site effect, the latter compounded by the failure to select for stable clones allowing for nontransfected ES cell to be further analyzed. Second, the composition of ES cells in a chimeric embryo may be extremely low, varied, or result in an embryo that will fail to develop properly to parturition.18 Third, the authors compare the presence of fluorescent endothelial cells to that of hematopoietic cells with no regard for possible differences in the rates of proliferation between the 2 cell types. Last, as the blood island is a 3-dimensional structure, the proper way to look for populations of clonal cells would be via 3-dimensional reconstructions, not a 2-dimensional plane of section. In short, these complications could account for large numbers of nonfluorescent Flk-1+ cells in the embryos that could lead to the spurious conclusion that some blood cells arise from a Flk-1− origin. In contrast, our study used a “genuine” cell lineage–tracing system and examined the hematopoietic cells of embryos and adult mice with minimal technical complications such as transfection efficiency or ES cell clonality. We clearly demonstrate that from the first hematopoietic cells in the yolk sac blood islands to the resident hematopoietic cells in the adult bone marrow, all blood cells are derived from Flk-1+ mesodermal precursors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Tom Sato (Cornell University, Ithaca, NY) and Frank Costantini (Columbia University, New York, NY) for the Flk1+/Cre and Rosa26 reporter mice, respectively.
J.J.L. was supported by the American Heart Association predoctoral fellowship. This work was supported by National Institutes of Health, National Heart, Lung and Blood Institute (NHLBI) grants HL63736 and HL55337 (K.C.).
National Institutes of Health
Authorship
Contribution: J.J.L., C.P., and Y.D.M. designed and performed experiments and wrote the manuscript; and K.C. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kyunghee Choi, Washington University School of Medicine, Department of Pathology and Immunology, 660 S Euclid Avenue, Campus Box 8118, St Louis, MO 63110; e-mail: kchoi@wustl.edu.
References
Author notes
*J.J.L. and C.P. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal