Abstract
Heat-shock protein 90 (Hsp90) acts as a molecular chaperone required for maintaining the conformational stability of client proteins regulating cell proliferation, survival, and apoptosis. Here we investigate the biologic significance of Hsp90 inhibition in multiple myeloma (MM) and other hematologic tumors using an orally available novel small molecule inhibitor SNX-2112, which exhibits unique activities relative to 17-allyamino-17-demethoxy-geldanamycin (17-AAG). SNX-2112 triggers growth inhibition and is more potent than 17-AAG against MM and other malignancies. It induces apoptosis via caspase-8, -9, -3, and poly (ADP-ribose) polymerase cleavage. SNX-2112 inhibits cytokine-induced Akt and extracellular signal-related kinase (ERK) activation and also overcomes the growth advantages conferred by interleukin-6, insulin-like growth factor-1, and bone marrow stromal cells. Importantly, SNX-2112 inhibits tube formation by human umbilical vein endothelial cells via abrogation of eNOS/Akt pathway and markedly inhibits osteoclast formation via down-regulation of ERK/c-fos and PU.1. Finally, SNX-2112, delivered by its prodrug SNX-5422, inhibits MM cell growth and prolongs survival in a xenograft murine model. Our results indicate that blockade of Hsp90 by SNX-2112 not only inhibits MM cell growth but also acts in the bone marrow microenvironment to block angiogenesis and osteoclastogenesis. Taken together, our data provide the framework for clinical studies of SNX-2112 to improve patient outcome in MM and other hematologic malignancies.
Introduction
Multiple myeloma (MM) is a B-cell malignancy characterized by excess abnormal plasma cells in the bone marrow (BM), bone lesions, and immunodeficiency. Despite treatment with high-dose chemotherapy and stem cell transplantation as well as novel agents including bortezomib, thalidomide, and lenalidomide, MM remains incurable.1,2
Heat shock protein 90 (Hsp90) is an important chaperone required for protein folding as well as assembly and maintenance of conformational stability for a suite of proteins (clients) involved in intracellular signaling.3 These client proteins and Hsp90-dependent pathways include Akt, Raf, and Her2/neu, with downstream molecules, such as extracellular signal-related kinase (ERK), pS6, and nuclear factor-κB (NF-κB), which regulates cell survival and proliferation.3-5 Because Hsp90 inhibition induces degradation of its client proteins, it is considered an attractive target for anticancer drugs.6 Geldanamycin and its analog 17-allylamino-17-demethoxy-geldanamycin (17-AAG) inhibit the protein function of Hsp90 and induce apoptosis in various tumor cells.4,7-10 17-AAG also shows antitumor activity in an array of human tumor xenograft models11,12 and is now undergoing clinical trials.8,10 Importantly, previous reports have demonstrated that 17-AAG inhibits proliferation and survival of MM cells, associated with down-regulation of insulin-like growth factor 1 receptor (IGF-1R) and interleukin-6 receptor (IL-6R) signaling (eg, IKK/NF-κB, PI-3K/Akt, and Raf/MAPK) as well as downstream molecules (eg, proteasome, telomerase, and HIF-1-α activities).13 Phase 1 clinical trials using 17-AAG in patients with relapsed or refractory MM and other advanced malignancies showed that its toxicity was clinically manageable.13-15 Moreover, we have shown that combined Hsp90 inhibitor and proteasome inhibitor treatment induces synergistic MM cell death in preclinical studies,13 and clinical trials show that the combination of Hsp90 inhibitor tanespimysin and bortezomib can achieve responses, even in patients resistant to bortezomib alone.16
Although efficacious, these natural product–derived Hsp90 inhibitors are limited in dosing frequency by lack of oral availability and concerns surrounding the chemical reactivity of the quinone moiety at the core of the geldanamycin analogs.17 Recently, a novel true small molecule class of Hsp90 inhibitor was reported, exemplified by SNX-2112 (Figure 1A).18-20 SNX-2112 competitively binds to the N-terminal adenosine triphosphate binding site of Hsp90, is highly orally bioavailable when delivered via its prodrug SNX-5422, and is highly potent against various cancers in vitro and in vivo.18-20 Three phase 1 clinical studies of SNX-5422 are currently recruiting participants in refractory hematologic and solid tumor malignancies (National Institutes of Health Clinical Trials website, http://www.cancer.gov/clinicaltrials). Here we demonstrate that SNX-2112 exhibits more potent activity than 17-AAG against MM as well as other hematologic tumor lines and evaluate the mechanism of this enhanced activity. We further characterize the role of Hsp90 in promoting growth and survival of MM as well as effects on angiogenesis and osteoclastogenesis in the BM microenvironment, and also evaluate the molecular consequences of targeting Hsp90 function. We demonstrate that SNX-2112 induces cytotoxicity, associated with inhibition of Akt and ERK pathways, in MM cell lines as well as patient MM cells. MM cell apoptosis triggered by SNX-2112 is mediated via caspase-8, -9, -3, and poly (ADP-ribose) polymerase (PARP) cleavage. In addition, SNX-2112 overcomes the growth stimulatory effects of exogenous cytokines, such as IL-6 and IGF-1, as well as inhibits growth of MM cells adherent to bone marrow stromal cells (BMSCs). Importantly, Hsp90 inhibition by SNX-2112 targets not only MM cells but also inhibits tubule formation by human umbilical vein endothelial cells (HUVECs) and osteoclast (OCL) formation, associated with down-regulation of Akt and ERK signaling. Importantly, SNX-5422 induces in vivo tumor growth inhibition and prolongs survival in a murine xenograft model of human MM, associated with down-regulation of Akt and ERK pathways. Therefore, these data demonstrate that targeting Hsp90 by small molecule inhibitors blocks tumor cell growth, angiogenesis, and osteoclastogenesis, providing the preclinical rationale for its clinical evaluation to improve patient outcome in MM and other hematologic cancers.
Methods
Reagents
Hsp90 inhibitor SNX-2112 and its prodrug SNX-5422 were provided by Serenex (Durham, NC). These compounds are representatives of a synthetic, novel class of small molecule inhibitors that competitively bind to the N-terminal adenosine triphosphate binding site of hsp90 and are orally bioavailable.18-20 They are pan-selective for the Hsp90 and its family members that bind to Hsp90α, Hsp90β, Grp94, and Trap-1.20 SNX-2112 was dissolved in dimethyl sulfoxide at 10 mM stock solution and stored at −20°C for in vitro study. SNX-5422 was dissolved in 1% carboxy methylcellulose/0.5% Tween 80 at 10 mg/mL and stored at 4°C for in vivo study. Recombinant human IL-1β, IL-6, and IGF-1 (R&D Systems, Minneapolis, MN) were reconstituted with sterile phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin.
Cells
Dex-sensitive (MM.1S) and -resistant (MM.1R) human MM cell lines were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL). RPMI-8226, U266, and NCI-H929 human MM cell lines were obtained from ATCC (Manassas, VA). RPMI 8226-Dox40 (doxorubicin-resistant) human MM cells were kindly provided by Dr William Dalton (Lee Moffitt Cancer Center, Tampa, FL). OPM1 and OPM2 were provided by Dr P. Leif Bergsagel (Mayo Clinic, Scottsdale, AZ). INA-6 cell line was kindly provided by Dr Martin Gramatzki (University of Erlangen-Nuernberg, Erlangen, Germany). Each of these cell lines was cultured in RPMI 1640 containing 10% fetal bovine serum (FBS; Sigma Chemical, St Louis, MO), 2 μM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Grand Island, NY). A375 melanoma cell line and AU565 breast cancer cell line were also obtained from ATCC but were maintained in Dulbecco modified Eagle medium/F12 medium supplemented with 10% FBS. HUVECs and endothelial growth medium were obtained from Lonza Walkersville (Walkersville, MD). Fresh peripheral blood mononuclear cells (PBMNCs) were obtained from healthy volunteers by separation using Ficoll-Hypaque (GE Healthcare, Piscataway, NJ) density sedimentation.
Primary MM cells and BMSCs from MM patients
Tumor cells from MM patients and plasma cells from healthy volunteers were purified by CD138+ selection using CD138 (Syndecan-1) Micro Beads and the Auto MACS magnetic cell sorter (Miltenyi Biotec, Auburn, CA). BM mononuclear cells separated by Ficoll-Hypaque were cultured in Dulbecco modified Eagle medium (Sigma-Aldrich) supplemented with 15% FBS, 2 μM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin for 3 to 6 weeks to generate BMSCs. Approval for these studies was obtained from the Dana-Farber Cancer Institute Institutional Review Board. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Growth inhibition assay
The growth inhibitory effect of SNX-2112 or 17-AAG in MM cell lines, PBMNCs, and BMSCs was assessed by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT; Sigma-Aldrich) dye absorbance. Cells were incubated in 96-well microtiter plates (Corning Costar, Cambridge, MA) for 24 or 48 hours, with MTT added to each well for the last 4 hours. The absorbance was measured at 570/630 nm using a spectrophotometer (Molecular Devices, Sunnyvale, CA).
To measure proliferation of MM cells and BMSCs, the rate of DNA synthesis was measured, as described previously.21 MM cells were incubated in 96-well culture plates in the presence of SNX-2112 and/or IL-6 or IGF-1 or BMSCs for 48 hours. Cells were pulsed with 0.5 μCi/well of [3H]-thymidine (PerkinElmer Life and Analytical Sciences, Waltham, MA) during the last 8 hours of culture, harvested onto glass filters with an automatic cell harvester (Cambridge Technology, Cambridge, MA), and counted using the LKB Betaplate scintillation counter (Wallac, Gaithersburg, MD). Inhibition of proliferation by test compounds in solid tumor cell lines was measured in 96-well plates after 72 hours of treatment with Cyquant (Invitrogen) DNA binding dye. AML, LCL, and K562 cell line proliferation rates were measured after 72 hours of compound treatment with CellTiter-Glo (Promega, Madison, WI).
Immunoblotting
MM cells were harvested and lysed using lysis buffer: 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 10 mM sodium pyrophosphate, 5 mM ethylenediaminetetraacetic acid, 1 mM ethyleneglycoltetraacetic acid, 2 mM Na3VO4, 5 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 5 μg/mL leupeptin, and 5 μg/mL aprotinin, as described previously.22 The whole-cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA), and immunoblotted with anti-PARP, caspase-8, caspase-3, caspase-9, phosphorylated-Akt, Akt, PU.1, phosphorylated-p38 mitogen-activated protein kinase (MAPK), p38MAPK, phosphorylated-endothelial nitric oxide synthases (eNOS), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and c-fos antibodies (Abs; Cell Signaling Technology, Beverly, MA); as well as with antiphosphorylated-ERK 1/2, ERK 1/2, hsp90α/β, and α-tubulin Abs (Santa Cruz Biotechnology, Santa Cruz, CA).
Assessment of apoptotic cells by flow cytometric analysis
For detection of apoptotic cells, mitochondria protein staining was performed with phycoerythrin-labeled anti Apo2.7 Ab (Immunotec, Marseille, France). Cells were treated with SNX-2112 for 48 hours and then incubated with Apo2.7 for 30 minutes. Apo2.7 staining was analyzed using the RXP cytomics software on an Epics flow cytometer (Beckman Coulter, Fullerton, CA).
Hsp90 client assay and immunohistochemical analysis
Antiphosphorylated ribosomal protein S6 (pS6), p-ERK, and p-AKT antibodies were obtained from Cell Signaling Technology, anti-Her2 antibody from Millipore (Billerica, MA), and anti-NF-κB antibody from Santa Cruz Biotechnology. For treatments, cells were seeded into 96-well plates and allowed to adhere overnight, followed by incubation with compound for an additional 24 hours. After treatment, cells were fixed and permeabilized, followed by addition of primary antibodies. Primary antibody binding was detected using appropriate secondary antibodies conjugated with either fluorescein isothiocyanate or tetramethylrhodamine isothiocyanate (Invitrogen) and measuring staining intensities with either a Cellomics Array Scan 4.5 (Pittsburgh, PA) or Becton Dickinson 435 (BD Biosciences, San Jose, CA) high content analysis instrument. After a 30-minute treatment with 1 ng/mL of IL-1β, AU565 cells were used to measure p-ERK and Her2 levels; A375 cells were used for measuring p-S6, p-ERK, and p-Akt levels; and HUVECs were used to measure NF-κB translocation. Levels of p-AKT were measured after a 15-minute activation with 100 ng/mL of IGF-1. NCI-H929 cells were also used to measure Akt, p-signal transducers and activators of transcription 3, p-ERK, and p-MAPK kinase (MEK) levels.
Angiogenesis assay
The antiangiogenic effect of SNX-2112 was determined using an in vitro Angiogenesis Assay Kit (Chemicon, Temecula, CA). HUVECs were cultured with or without SNX-2112 on polymerized matrix gel at 37°C. After 12 hours, tube formation was evaluated using Leica DM IL microscopy (Leica Microsystems, Wetzlar, Germany) and processed with IM50 software (Leica). We also used vascular endothelial growth factor (VEGF)–free medium and protein kinase C inhibitor enzaustaurin (LY317615.HCl; Eli Lilly, Indianapolis, IN) as a negative or positive control, respectively.23 Cellular proteins obtained from HUVECs were analyzed by Western blotting.
Osteoclast formation assay
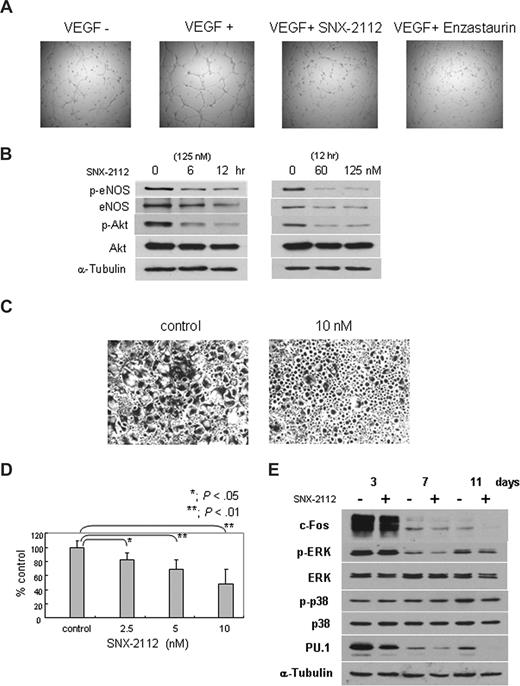
PBMNCs from healthy volunteers were cultured in 6-well or 96-well plates (0.5 × 106 cells/cm2), and OCLs were generated by culturing adherent cells for 3 weeks in α-minimum essential medium containing 10% FBS, and 1% penicillin-streptomycin (Mediatech, Herndon, VA), as well as 50 ng/mL of macrophage colony stimulating factor (M-CSF; R&D Systems) and receptor activator of NF-κB ligand (RANKL; PeproTech, Rocky Hill, NJ), in the presence or absence of SNX-2112. After 3 weeks, cells were fixed with citrate-acetone solution and stained for tartrate-resistant acid phosphatase (TRAP) using an acid phosphatase leukocyte staining kit (Sigma-Aldrich). TRAP-positive multinucleated OCLs containing 3 or more nuclei per cell were enumerated using inverted microscopy. PBMNCs cultured with M-CSF and RANKL, with or without SNX-2112, were collected at days 3, 7, and 11; cell lysates were analyzed by Western blotting.
Xenograft murine model
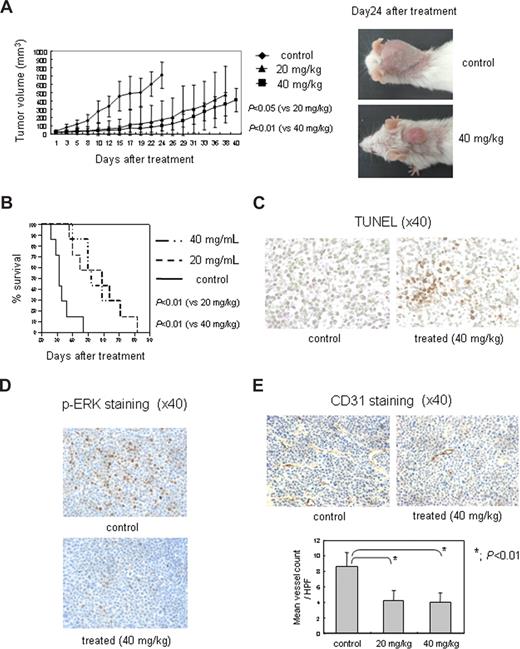
Fox Chase SCID mice (6-7 weeks old) were purchased from Charles River Laboratories (Wilmington, MA). All animal studies were conducted according to protocols approved by the Animal Ethics Committee of the Dana-Farber Cancer Institute. The mice were irradiated (200 cGy) and 5 × 106 MM.1S cells inoculated subcutaneously on the next day. When tumors were measurable, mice were assigned into 2 treatment groups receiving oral SNX-5422 (20 mg/kg or 40 mg/kg, 3 times per week, total 3 weeks) or a control group receiving oral vehicle alone (1% carboxy methylcellulose/0.5% Tween 80). Caliper measurements of the longest perpendicular tumor diameters were performed every alternate day to estimate the tumor volume, using the following formula representing the 3-dimensional volume of an ellipse: 4/3 × (width/2)2 × (length/2). Animals were killed when tumors reached 2 cm in diameter or if the mice appeared moribund, to prevent unnecessary morbidity. Survival was evaluated from the first day of treatment until death. Tumor growth was evaluated using caliper measurements from the first day of treatment until day of first death, which was day 24 for control mice, day 38 for 20 mg/kg SNX-5422 treated mice, and day 40 for 40 mg/kg SNX-5422 treated mice. For analysis of tumor tissues, mice in both control and treatment groups were killed at day 24 after treatment with SNX-5422; these mice were excluded from statistical analysis for survival. Tumors excised from mice were evaluated by TdT-mediated d-UTP nick end labeling (TUNEL) assay and immunohistochemical analysis using p-ERK and p-Akt staining. In addition, microvessel density (MVD), assessed by immunohistochemical analysis for CD31 expression in vivo, was performed as described previously.24
Statistical analysis
Statistical significance of differences observed in drug-treated compared with control cultures was determined using the Mann-Whitney test. The minimal level of significance was a P value less than .05. Tumor volumes of mice were compared using the Kruskal-Wallis test and Dunn's multiple comparison test. Overall survival was assessed using Kaplan-Meier curves and log-rank analysis. All statistical analysis was performed using GraphPad Prism software (GraphPad Software, San Diego, CA).
Results
SNX-2112 inhibits growth of solid and hematologic tumor cells
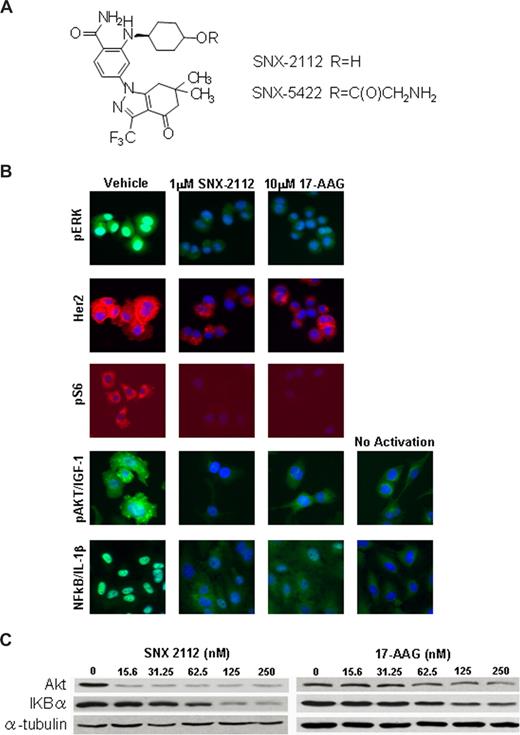
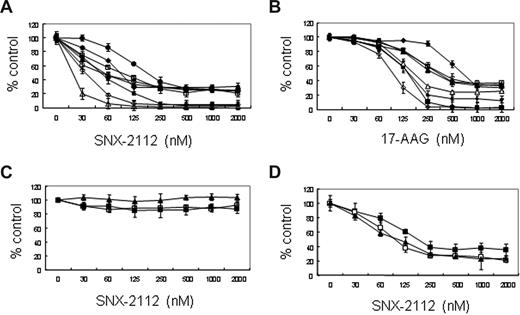
Figure 1A shows the formal chemical structure of SNX-2112 and its prodrug SNX-5422. We first analyzed the effect of Hsp90 inhibition by SNX-2112 and 17-AAG against a panel of solid and hematologic tumor cell lines using high content proliferation assay (Table 1; Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Although SNX-2112 exhibits superior potency against most solid tumors tested (Table S1), the magnitude of this difference is greater in hematologic tumors, including several lymphoblastoid lines (IM9, ARH77, MCCAR, HS Sultan), an erythroleukemia line (K562), and the c-kit+ acute myeloid leukemia line (Kasumi-1) (Table 1). SNX-2112 maintains low nanomolar potency against both solid and hematologic tumors, whereas 17-AAG is much less potent in hematologic tumors, with IC50 ranging from 128 nM for K562 to low micromolar levels against IM9, ARH77, and HS Sultan.
SNX-2112 targets degradation of Hsp90 client protein. (A) The formal chemical structure of SNX-2112 and SNX-5422. (B) High content analysis images of various Hsp90 client proteins and phosphorylated proteins regulated by Hsp90 clients. Levels of p-ERK and Her2 were measured concurrently in proliferating AU565 cells using species specific fluorescein isothiocyanate- and tetramethylrhodamine isothiocyanate-conjugated secondary antibodies, and images from each treatment condition are from identical cells. Exposure times for each channel were identical for all 3 treatment conditions to allow for direct comparison of staining intensities between treatment conditions. Levels of p-S6 were also measured concurrently in treated A375 cells. Levels of p-Akt were measured in serum-starved A375 cells activated with 100 ng/mL of IGF-1 after 24 hours of compound treatment. For measuring NF-κB translocation, HUVECs were activated with 1 ng/mL of IL-1β for 30 minutes after a 24-hour compound treatment, and nuclear staining intensity was quantified by segmenting individual nuclei based on Hoechst staining. For all images shown, individual cells were identified by Hoescht blue staining of nuclei. (C) Hsp90 client assay in MM cells. MM.1S cell were treated with SNX-2112 or 17-AAG for 24 hours at indicated dose, and the levels of Akt and IKBα protein were evaluated by Western blotting.
SNX-2112 targets degradation of Hsp90 client protein. (A) The formal chemical structure of SNX-2112 and SNX-5422. (B) High content analysis images of various Hsp90 client proteins and phosphorylated proteins regulated by Hsp90 clients. Levels of p-ERK and Her2 were measured concurrently in proliferating AU565 cells using species specific fluorescein isothiocyanate- and tetramethylrhodamine isothiocyanate-conjugated secondary antibodies, and images from each treatment condition are from identical cells. Exposure times for each channel were identical for all 3 treatment conditions to allow for direct comparison of staining intensities between treatment conditions. Levels of p-S6 were also measured concurrently in treated A375 cells. Levels of p-Akt were measured in serum-starved A375 cells activated with 100 ng/mL of IGF-1 after 24 hours of compound treatment. For measuring NF-κB translocation, HUVECs were activated with 1 ng/mL of IL-1β for 30 minutes after a 24-hour compound treatment, and nuclear staining intensity was quantified by segmenting individual nuclei based on Hoechst staining. For all images shown, individual cells were identified by Hoescht blue staining of nuclei. (C) Hsp90 client assay in MM cells. MM.1S cell were treated with SNX-2112 or 17-AAG for 24 hours at indicated dose, and the levels of Akt and IKBα protein were evaluated by Western blotting.
SNX-2112 inhibits in vitro cell proliferation in hematologic malignancies
| Hematologic malignancy . | IC50, nM . | |
|---|---|---|
| SNX-2112 . | 17-AAG . | |
| Kasumi-1 (c-kit; AML) | 0.9 ± 0.3 | 305 ± 113 |
| IM9 (LCL) | 3 ± 1 | 1533 ± 34 |
| ARH77 (LCL) | 2 ± 1 | 1059 ± 206 |
| MCCAR (LCL) | 0.9 ± 0.4 | 210 ± 79 |
| HS SULTAN (LCL) | 9 ± 4 | 2036 ± 53 |
| K562 (erythroleukemia) | 5 ± 0.8 | 128 ± 81 |
| Hematologic malignancy . | IC50, nM . | |
|---|---|---|
| SNX-2112 . | 17-AAG . | |
| Kasumi-1 (c-kit; AML) | 0.9 ± 0.3 | 305 ± 113 |
| IM9 (LCL) | 3 ± 1 | 1533 ± 34 |
| ARH77 (LCL) | 2 ± 1 | 1059 ± 206 |
| MCCAR (LCL) | 0.9 ± 0.4 | 210 ± 79 |
| HS SULTAN (LCL) | 9 ± 4 | 2036 ± 53 |
| K562 (erythroleukemia) | 5 ± 0.8 | 128 ± 81 |
Various cell lines of hematologic malignancies were cultured with SNX-2112 or 17-AAG for 72 hours, and cell growth was evaluated by CellTiter-Glo luminescent cell viability assay. Values shown are means plus or minus SD of quadruplicate determinations. LCL indicates lymphoblastoid cell lines.
SNX-2112 potently inhibits Hsp90 clients
High content cell imaging assays were applied to identify the specific Hsp90 clients that may be responsible for the increased potency of SNX-2112 compared with 17-AAG (Figure 1B; Table 2). Although 17-AAG induces the degradation of Her2 at low nanomolar level, it is much weaker than SNX-2112 in various cells at blocking signaling of most other clients, including Akt, ERK, and NF-κB pathways (Figure 1B; Table 2). In addition, significantly more highly degradation of Hsp90 clients, including Akt, ERK, and NF-κB cascade proteins, was triggered in MM cells by SNX-2112 than 17-AAG (Figure 1C; Table 2). These results suggest that SNX-2112 more potently targets Hsp90 clients than 17-AAG in MM and other malignancies.
SNX-2112 in vitro activity for Hsp90 clients
| Hsp90 client assays . | IC50, nM . | |
|---|---|---|
| SNX-2112 . | 17-AAG . | |
| p-ERK degradation (A375: melanoma) | 48 ± 8 | 434 ± 196 |
| p-ERK degradation (AU565: breast) | 10 ± 6 | 22 ± 6 |
| EphA2 degradation (A375) | 133 ± 22 | 367 ± 49 |
| Her2 degradation (SKBR3: breast) | 6 ± 0.5 | 0.4 ± 0.3 |
| Her2 degradation (AU565) | 10 ± 0.8 | 4 ± 1 |
| p-S6 degradation (A375) | 4 ± 0.2 | 308 ± 32 |
| AKT degradation (A375) | 163 ± 27 | 189 ± 53 |
| p-AKT degradation (A375-IGF-1) | 70 ± 15 | 120 ± 32 |
| NF-κB translocation (HUVEC-IL-1β) | 175 ± 33 | 539 ± 124 |
| B-Raf degradation (A375) | 319 ± 63 | 315 ± 74 |
| p-c-JUN (HUVEC-IL-1β) | 327 ± 84 | 623 ± 143 |
| AKT degradation (NCI-H929: myeloma) | 39 ± 0.6 | 684 ± 141 |
| p-STAT3 degradation (NCI-H929) | 41 ± 0.8 | 920 ± 10 |
| p-ERK degradation (NCI-H929) | 30 ± 1 | 813 ± 17 |
| p-MEK degradation (NCI-H929) | 40 ± 0.1 | 897 ± 66 |
| Hsp90 client assays . | IC50, nM . | |
|---|---|---|
| SNX-2112 . | 17-AAG . | |
| p-ERK degradation (A375: melanoma) | 48 ± 8 | 434 ± 196 |
| p-ERK degradation (AU565: breast) | 10 ± 6 | 22 ± 6 |
| EphA2 degradation (A375) | 133 ± 22 | 367 ± 49 |
| Her2 degradation (SKBR3: breast) | 6 ± 0.5 | 0.4 ± 0.3 |
| Her2 degradation (AU565) | 10 ± 0.8 | 4 ± 1 |
| p-S6 degradation (A375) | 4 ± 0.2 | 308 ± 32 |
| AKT degradation (A375) | 163 ± 27 | 189 ± 53 |
| p-AKT degradation (A375-IGF-1) | 70 ± 15 | 120 ± 32 |
| NF-κB translocation (HUVEC-IL-1β) | 175 ± 33 | 539 ± 124 |
| B-Raf degradation (A375) | 319 ± 63 | 315 ± 74 |
| p-c-JUN (HUVEC-IL-1β) | 327 ± 84 | 623 ± 143 |
| AKT degradation (NCI-H929: myeloma) | 39 ± 0.6 | 684 ± 141 |
| p-STAT3 degradation (NCI-H929) | 41 ± 0.8 | 920 ± 10 |
| p-ERK degradation (NCI-H929) | 30 ± 1 | 813 ± 17 |
| p-MEK degradation (NCI-H929) | 40 ± 0.1 | 897 ± 66 |
Cells were cultured with SNX-2112 or 17-AAG for 48 hours, and in vitro activity of SNX-2112 against various hsp90 clients was evaluated by high content cell imaging assay. IC50 values shown are means plus or minus SD of triplicate determinations.
SNX-2112 inhibits MM cell growth
Based on this high potency of SNX-2112 in various tumor cells, we next investigated the biologic significance of Hsp90 inhibition in MM using this compound. We first analyzed the effect of SNX-2112 and 17-AAG on growth of MM cell lines using an MTT assay. SNX-2112 inhibits the growth of MM.1S, U266, INA-6, RPMI8226, OPM1, OPM2, MM.1R, and Dox40 MM cell lines, with IC50 at 48 hours of 52, 55, 19, 186, 89, 67, 93, and 53 nM, respectively (Figure 2A; Table S2). All MM cell lines were more sensitive to SNX-2112 than 17-AAG (Figure 2A,B; Table S2). On the other hand, SNX-2112 does not induce cytotoxicity in PBMNCs from 3 healthy volunteers (Figure 2C). Importantly, SNX-2112 inhibits the growth of CD138+ MM cells (IC50, 42-187 nM) isolated from 3 patients whose disease was refractory to prior therapies, including dexamethasone, melphalan, thalidomide, or bortezomib (Figure 2D). These results indicate that SNX-2112 induces cytotoxicity selectively and potently in MM cell lines as well as primary MM cells, even those resistant to conventional and novel chemotherapy.
SNX-2112 induces cytotoxicity against MM cells. (A,B) MM.1S (■), MM.1R (□), U266 (▴), INA-6 (Δ) RPMI8226 (●), RPMI Dox40 (○) OPM1(♦), and OPM2 (◇) MM cell lines. (C) Normal PBMNCs (1 (■), 2 (□), 3 (▴), n = 3); and (D) MM patient cells (1 (■), 2 (□), 3 (▴), n = 3) were cultured for 48 hours in the presence of SNX-2112 or 17-AAG. Cell growth was assessed by MTT assay, and data represent mean plus or minus SD of triplicate cultures.
SNX-2112 induces cytotoxicity against MM cells. (A,B) MM.1S (■), MM.1R (□), U266 (▴), INA-6 (Δ) RPMI8226 (●), RPMI Dox40 (○) OPM1(♦), and OPM2 (◇) MM cell lines. (C) Normal PBMNCs (1 (■), 2 (□), 3 (▴), n = 3); and (D) MM patient cells (1 (■), 2 (□), 3 (▴), n = 3) were cultured for 48 hours in the presence of SNX-2112 or 17-AAG. Cell growth was assessed by MTT assay, and data represent mean plus or minus SD of triplicate cultures.
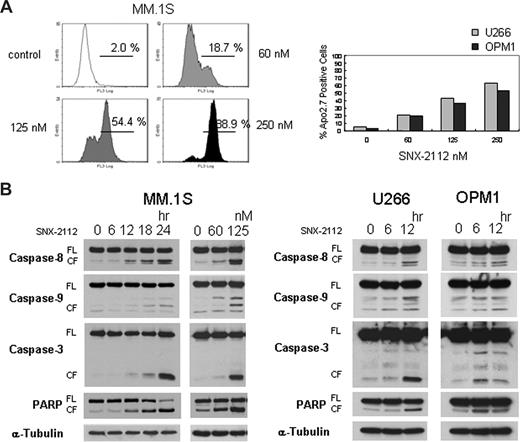
SNX-2112 induces apoptosis in MM cells
To investigate the mechanism of cytotoxicity in MM cells triggered by Hsp90 inhibition, we performed Apo2.7 analysis and immunoblotting in MM cells. The percentage of apoptotic cells increased after treatment with SNX-2112, assessed by flow-cytometric analysis for Apo2.7 staining (Figure 3A). In addition, it triggered caspase-8, -9, -3, and PARP cleavage in these MM cells (Figure 3B). These results suggest that SNX-2112-induced cytotoxicity is mediated by apoptosis via caspase-8, -9, and -3, and PARP activation.
SNX-2112 induces apoptosis in MM cells. (A) MM.1S, U266, and OPM1 cells were cultured for 48 hours with SNX-2112 at indicated doses. Apoptosis was determined by flow-cytometric analysis for Apo2.7 staining. (B) MM.1S, U266, and OPM1 cells were cultured with SNX-2112 (125 nM) for the indicated times, and with SNX-2112 for 24 hours at the indicated doses. Cell lysates were analyzed by Western blotting. FL indicates full-length; CF, cleaved form.
SNX-2112 induces apoptosis in MM cells. (A) MM.1S, U266, and OPM1 cells were cultured for 48 hours with SNX-2112 at indicated doses. Apoptosis was determined by flow-cytometric analysis for Apo2.7 staining. (B) MM.1S, U266, and OPM1 cells were cultured with SNX-2112 (125 nM) for the indicated times, and with SNX-2112 for 24 hours at the indicated doses. Cell lysates were analyzed by Western blotting. FL indicates full-length; CF, cleaved form.
SNX-2112 inhibits Akt and ERK signaling triggered by cytokines
We next evaluated the potential mechanisms underlying the enhanced potency of SNX-2112 in MM cells. Because ERK and Akt signaling cascades mediate cell proliferation and drug resistance in MM cells,25 we examined whether Hsp90 inhibition suppresses these signaling cascades induced by cytokines. SNX-2112 significantly inhibits p-ERK and p-Akt in a time-dependent manner in MM cells (Figure 4A). Phosphorylation of ERK and Akt is induced by IL-6 in MM.1S cells; conversely, pretreatment with SNX-2112 (125 nM for 12 hours) markedly inhibits IL-6–induced ERK and Akt phosphorylation in a dose-dependent manner (Figure 4B left). Similarly, pretreatment with SNX-2112 (125 nM for 12 hours) also inhibits IGF-1–induced phosphorylation of ERK and Akt in MM.1S cells (Figure 4B right). In addition, simultaneous exposure to SNX-2112 and IL-6 or IGF-1 for 12 hours markedly inhibits IL-6- or IGF-1-induced Akt and ERK phophorylation (Figure S1). These data demonstrate that targeting Hsp90 by SNX-2112 potently inhibits cytokine-induced phosphorylation of ERK and Akt in MM cells.
SNX-2112 inhibits Akt and ERK pathway and overcomes the growth stimulatory effects of IL-6, IGF-1, and BMSCs on MM cell growth. (A) MM.1S, U266, and OPM1 cells were cultured with SNX-2112 (125 nM) for the indicated times. (B) MM.1S cells were cultured with or without SNX-2112 (0-250 nM) for 12 hours and then stimulated with IL-6 (10 ng/mL) or IGF-1 (50 ng/mL) for 10 minutes. Cell lysates were analyzed by Western blotting (A,B). (C) MM.1S cells were cultured for 48 hours with indicated concentrations: 0 nM (□), 5 nM ( ) 10 nM (■) of SNX-2112, in the presence or absence of IL-6 (1 or 10 ng/mL; left) or IGF-1 (10 or 50 ng/mL; right). (D) MM.1S cells were cultured with BMSCs for 48 hours with the indicated concentrations: 0 nM (□), 10 nM (
) 10 nM (■) of SNX-2112, in the presence or absence of IL-6 (1 or 10 ng/mL; left) or IGF-1 (10 or 50 ng/mL; right). (D) MM.1S cells were cultured with BMSCs for 48 hours with the indicated concentrations: 0 nM (□), 10 nM ( ), 20 nM (
), 20 nM ( ), 40 nM (■) of SNX-2112. (E) SNX-2112 treatment for 48 hours does not affect cell viability of BMSCs: BMSC-1 (■), BMSC-2 (□), BMSC-3 (▴) (n = 3). Cell growth was assessed by [3H]-thymidine uptake (C,D) and MTT assay (E). Values represent mean plus or minus SD of quadruplicate (C,D) or triplicate cultures (E).
), 40 nM (■) of SNX-2112. (E) SNX-2112 treatment for 48 hours does not affect cell viability of BMSCs: BMSC-1 (■), BMSC-2 (□), BMSC-3 (▴) (n = 3). Cell growth was assessed by [3H]-thymidine uptake (C,D) and MTT assay (E). Values represent mean plus or minus SD of quadruplicate (C,D) or triplicate cultures (E).
SNX-2112 inhibits Akt and ERK pathway and overcomes the growth stimulatory effects of IL-6, IGF-1, and BMSCs on MM cell growth. (A) MM.1S, U266, and OPM1 cells were cultured with SNX-2112 (125 nM) for the indicated times. (B) MM.1S cells were cultured with or without SNX-2112 (0-250 nM) for 12 hours and then stimulated with IL-6 (10 ng/mL) or IGF-1 (50 ng/mL) for 10 minutes. Cell lysates were analyzed by Western blotting (A,B). (C) MM.1S cells were cultured for 48 hours with indicated concentrations: 0 nM (□), 5 nM ( ) 10 nM (■) of SNX-2112, in the presence or absence of IL-6 (1 or 10 ng/mL; left) or IGF-1 (10 or 50 ng/mL; right). (D) MM.1S cells were cultured with BMSCs for 48 hours with the indicated concentrations: 0 nM (□), 10 nM (
) 10 nM (■) of SNX-2112, in the presence or absence of IL-6 (1 or 10 ng/mL; left) or IGF-1 (10 or 50 ng/mL; right). (D) MM.1S cells were cultured with BMSCs for 48 hours with the indicated concentrations: 0 nM (□), 10 nM ( ), 20 nM (
), 20 nM ( ), 40 nM (■) of SNX-2112. (E) SNX-2112 treatment for 48 hours does not affect cell viability of BMSCs: BMSC-1 (■), BMSC-2 (□), BMSC-3 (▴) (n = 3). Cell growth was assessed by [3H]-thymidine uptake (C,D) and MTT assay (E). Values represent mean plus or minus SD of quadruplicate (C,D) or triplicate cultures (E).
), 40 nM (■) of SNX-2112. (E) SNX-2112 treatment for 48 hours does not affect cell viability of BMSCs: BMSC-1 (■), BMSC-2 (□), BMSC-3 (▴) (n = 3). Cell growth was assessed by [3H]-thymidine uptake (C,D) and MTT assay (E). Values represent mean plus or minus SD of quadruplicate (C,D) or triplicate cultures (E).
SNX-2112 overcomes the growth stimulatory effects of cytokines and inhibits MM cell growth in coculture with BMSCs
Because we and others have demonstrated that IL-6 and IGF-1 induce both growth and inhibition of apoptosis in MM cells,26-28 we next examined whether Hsp90 inhibition can overcome these effects of exogenous IL-6 and IGF-1. Both IL-6 and IGF-1 trigger increased MM.1S cell growth, which is inhibited by SNX-2112 (P < .05; Figure 4C). Because we previously have shown that the BM microenvironment confers growth and drug resistance in MM cells,25 we next examined the effect of Hsp90 inhibition on MM cell growth in the BM milieu. We first tested the direct cytotoxicity of SNX-2112 on patient BMSCs using MTT assay: no significant growth inhibition in BMSCs is triggered by SNX-2112 (Figure 4E). MM.1S cells were then cultured for 48 hours with or without BMSCs, in the presence or absence of SNX-2112. MM cell adherence to BMSCs enhances [3H]-thymidine uptake in MM.1S cells, which is inhibited by SNX-2112 (P < .05; Figure 4D). These results indicate that SNX-2112 also blocks the growth stimulatory effect of the BM microenvironment on MM cells.
SNX-2112 inhibits HUVEC tubule formation
We next investigated the effect of Hsp90 inhibition in the BM microenvironment, specifically on angiogenesis. HUVECs were treated with 125 nM of SNX-2112 for 12 hours, and tube formation by endothelial cells was evaluated. Figure 5A shows that the number of capillaries does not change markedly with SNX-2112 treatment; however, vessels become thin and immature, even at drug concentrations (125 nM) that do not affect viability of HUVECs (Figure S2). We also confirmed the inhibitory effect of tube formation treated with 10 μM of enzastaurin or VEGF free medium as a positive or negative control, respectively (Figure 5A). These results indicate that in vitro capillary-like tube formation is significantly inhibited by SNX-2112 treatment. Next we analyzed the eNOS/Akt pathway, implicated in angiogenesis. The phosphorylation and expression of both eNOS and Akt are markedly down-regulated by SNX-2112 treatment (Figure 5B). These results suggest that Hsp90 inhibition by SNX-2112 blocks angiogenesis via down-regulation of eNOS/Akt pathway.
SNX-2112 acts in the BM microenvironment to inhibit angiogenesis and osteoclastogenesis. (A) HUVECs were cultured with or without 125 nM SNX-2112 for 12 hours, and tube formation was assessed using microscopy. VEGF free medium and protein kinase C inhibitor enzaustaurin were used as a negative or positive control for growth inhibition (original magnification ×4). (B) HUVECs were cultured with SNX-2112 (125 nM) for indicated times (left panel) and cultured for 12 hours with indicated doses of SNX-2112 (right panel). Cell lysates were analyzed by immunoblotting. (C,D) Osteoclastogenesis was determined by TRAP assay. PBMNCs were cultured for 3 weeks in the presence of 50 ng/mL of M-CSF and RANKL, with or without SNX-2112. SNX-2112 decreased multinucleated TRAP-positive cells (P < .01). Images were obtained using a Leica DMIL microscope equipped with a 4×, 10×/0.22, and 40×/0.60 numeric aperture objective lens (Leica Microsystems, Wetzlar, Germany) and acquired through IM50 software (Leica Microsystems Imaging Solutions, Cambridge, United Kingdom). Original magnification ×4. (E) PBMNCs were cultured with M-CSF and RANKL for the indicated days, in the presence or absence of 10 nM of SNX-2112. Cell lysates were analyzed by Western blotting.
SNX-2112 acts in the BM microenvironment to inhibit angiogenesis and osteoclastogenesis. (A) HUVECs were cultured with or without 125 nM SNX-2112 for 12 hours, and tube formation was assessed using microscopy. VEGF free medium and protein kinase C inhibitor enzaustaurin were used as a negative or positive control for growth inhibition (original magnification ×4). (B) HUVECs were cultured with SNX-2112 (125 nM) for indicated times (left panel) and cultured for 12 hours with indicated doses of SNX-2112 (right panel). Cell lysates were analyzed by immunoblotting. (C,D) Osteoclastogenesis was determined by TRAP assay. PBMNCs were cultured for 3 weeks in the presence of 50 ng/mL of M-CSF and RANKL, with or without SNX-2112. SNX-2112 decreased multinucleated TRAP-positive cells (P < .01). Images were obtained using a Leica DMIL microscope equipped with a 4×, 10×/0.22, and 40×/0.60 numeric aperture objective lens (Leica Microsystems, Wetzlar, Germany) and acquired through IM50 software (Leica Microsystems Imaging Solutions, Cambridge, United Kingdom). Original magnification ×4. (E) PBMNCs were cultured with M-CSF and RANKL for the indicated days, in the presence or absence of 10 nM of SNX-2112. Cell lysates were analyzed by Western blotting.
SNX-2112 inhibits osteoclast formation
We next examined the effect of Hsp90 inhibition on osteoclastogenesis. PBMNCs cultured with M-CSF and RANKL for 3 weeks differentiate to osteoclasts (Figure 5C, control). SNX-2112 markedly inhibits osteoclast formation (Figure 5C). The total number of multinucleated cells is decreased, confirmed by TRAP assay (P < .01; Figure 5D), even at concentrations that do not affect the viability of osteoclasts (data not shown). Western blot analysis shows that p-ERK and c-fos, client proteins of Hsp90 mediating osteoclastogenesis, are also down-regulated (Figure 5E; Table S3). In addition, PU.1, an important transcription factor in osteoclastogenesis, is also down-regulated by SNX-2112 (Figure 5E; Table S3). These results indicate that Hsp90 inhibition by SNX-2112 suppresses osteoclast formation, associated with down-regulation of ERK/c-fos and PU.1.
SNX-5422 (prodrug of SNX-2112) inhibits human MM cell growth in vivo
In light of the in vitro effects of SNX-2112 on critical pathways in MM cells, we next tested the effects of this drug on human MM cell growth in vivo. SNX-5422 significantly reduced MM tumor growth in the treatment group (n = 7) compared with control mice (n = 7) (Figure 6A). Previous studies have determined that the MTD on this regimen is approximately 75 mg/kg, with efficacy observed at doses as low as 5 mg/kg in some xenograft models (P.S., unpublished data, October 2006). Comparison of tumor volumes showed statistically significant differences between control versus treatment groups (vs 20 mg/kg, P < .05; vs 40 mg/kg, P < .01; Figure 6A). Kaplan-Meier curves and log-rank analysis showed a mean overall survival of 32 days (95% confidence interval, 24-47 days) in control mice versus 57 days (95% confidence interval, 38-82 days) and 58 days (95% confidence interval, 40-82 days) in the 20 mg/kg and 40 mg/kg SNX-5422-treated groups, respectively (Figure 6B). Statistically significant prolongation in mean overall survival compared with control mice was also observed in treatment groups (vs 20 mg/kg, P < .01; vs 40 mg/kg, P < .01; Figure 6B); however, there was no significant difference between the 2 treatment groups. Although body weight loss of 8.9% and 7.3% was observed at day 24 in the 20 mg/kg and 40 mg/kg SNX-5422 treatment groups, respectively, compared with 0.2% in the control group, all mice recovered by 30 days.
SNX-5422 inhibits human MM cell growth and angiogenesis in vivo. (A) SNX-5422 significantly inhibits MM tumor growth compared with controls: control (♦), 20 mg/kg (▴), and 40 mg/kg (■) SNX-5422 (n = 7); control versus 20 mg/kg (P < .05); and control versus 40 mg/kg (P < .01). Error bars represent mean plus or minus SD. (B) In Kaplan-Meier curve and log-rank analysis, SNX-5422 markedly prolongs overall survival of treated groups compared with the control group (P < .01). (C) SNX-5422 significantly induces MM cell apoptosis in treated mice (40 mg/kg) compared with control mice, evidenced by TUNEL assay. (D) SNX-5422 significantly inhibits p-ERK in treated mice (40 mg/kg), evidenced by immunohistochemical analysis of p-ERK staining. (E) Effect of SNX-5422 on MVD in tumors. MVD was evaluated by immunohistochemical analysis for CD31 expression. Inhibition of blood vessel formation was observed in SNX-5422-treated mice compared with control mice (P < .01). Blood vessels (mean ± SD of 5 separate HPFs) were enumerated at ×40. Images in panels C-E were obtained using a Leica DMIL microscope equipped with a 4×, 10×/0.22, and 40×/0.60 numeric aperture objective lens and acquired through IM50 software. Original magnification ×40.
SNX-5422 inhibits human MM cell growth and angiogenesis in vivo. (A) SNX-5422 significantly inhibits MM tumor growth compared with controls: control (♦), 20 mg/kg (▴), and 40 mg/kg (■) SNX-5422 (n = 7); control versus 20 mg/kg (P < .05); and control versus 40 mg/kg (P < .01). Error bars represent mean plus or minus SD. (B) In Kaplan-Meier curve and log-rank analysis, SNX-5422 markedly prolongs overall survival of treated groups compared with the control group (P < .01). (C) SNX-5422 significantly induces MM cell apoptosis in treated mice (40 mg/kg) compared with control mice, evidenced by TUNEL assay. (D) SNX-5422 significantly inhibits p-ERK in treated mice (40 mg/kg), evidenced by immunohistochemical analysis of p-ERK staining. (E) Effect of SNX-5422 on MVD in tumors. MVD was evaluated by immunohistochemical analysis for CD31 expression. Inhibition of blood vessel formation was observed in SNX-5422-treated mice compared with control mice (P < .01). Blood vessels (mean ± SD of 5 separate HPFs) were enumerated at ×40. Images in panels C-E were obtained using a Leica DMIL microscope equipped with a 4×, 10×/0.22, and 40×/0.60 numeric aperture objective lens and acquired through IM50 software. Original magnification ×40.
Importantly, tumor harvested from treated mice showed increased apoptosis by TUNEL assay (Figure 6C). In addition, we also confirmed by immunohistochemical analysis that SNX-5422 significantly inhibits p-ERK (Figure 6D) and p-Akt (Figure S3), consistent with in vitro studies. Finally, SNX-5422 treatment significantly decreased the percentage of CD31+ cells and MVD, consistent with an inhibitory effect on angiogenesis in vivo (P < .01; Figure 6E). These data demonstrate that targeting Hsp90 by SNX-5422 significantly inhibits MM tumor growth and inhibits microvessel formation in vivo as well as prolongs host survival.
Discussion
In this study, we demonstrate that Hsp90 inhibition by SNX-2112 potently induces cytotoxicity in drug-sensitive and drug-resistant MM and other hematologic tumor cell lines, as well as in primary patient MM cells. SNX-2112 was uniformly more potent than 17-AAG in these assays and, in some cases, was more than 100-fold more potent. The basis for this appears to be the weak activity of 17-AAG against hematologic tumor lines compared with solid tumor lines, although these lines are not reported to carry the NQ01 mutation shown to confer 17-AAG resistance. Profiling of numerous compounds in these assays rules out effects of efflux pumps contributing to the loss in potency for 17-AAG (data not shown). In contrast, we observed no cytotoxicity in PBMNCs, suggesting selective cytotoxicity against tumor cells. The detailed mechanism of differential selectivity of hsp90 inhibitors against normal cells versus MM cells remains obscure; however, our data indicate that the expression of hsp90 in MM cells is much higher than in normal cells (Figure S4). Moreover, the unfolded protein response is constitutively activated in MM cells, consistent with greater dependency on hsp90.29
Using high content cell imaging to assay for Hsp90-mediated effects, we determined that the antiproliferative effects of SNX-2112 versus 17-AAG correlated with the ability of the compounds to block signaling via Akt and ERK in these cells, with SNX-2112 exhibiting superior potency against these Hsp90-dependent endpoints. Our imaging assays evaluated both direct Hsp90 clients and downstream components of the signaling cascades because the presence of client protein does not indicate whether the signaling pathway is competent. As expected given this activity, we show that SNX-2112 potently triggered MM cell apoptosis and caspase-8, -9, and -3 induction, as well as PARP cleavage. MM cell apoptosis was also observed in vivo by TUNEL assay. The increased potency of SNX-2112 with respect to MM and other hematologic tumor cell antiproliferation and apoptosis, coupled with the high oral bioavailability of SNX-2112 delivered via its prodrug SNX-5422, strongly suggest its potential utility in these tumor types, especially with respect to overcoming conventional drug resistance in MM. Our data are consistent with reports by Chandarlapaty et al showing that SNX-2112 has antitumor activity against Her kinase-dependent tumors, including breast, ovary, and lung cancers.20 Importantly, SNX-2112 and its prodrugs with improved solubility and pharmacologic properties may have potential tolerability advantages over geldanamycin derivatives, such as 17-AAG.20
In light of the activity of SNX-2112 against MM cells, the biologic effects of Hsp90 inhibition using this drug were investigated further. The BM microenvironment confers growth, survival, and drug resistance in MM cells.25 Cell adhesion-mediated drug resistance is induced by adhesion of MM cells to fibronectin and BMSCs.30 Moreover, MM cells in the BM milieu trigger cytokines (ie, IL-6, IGF-1, and VEGF), which in turn activate phosphatidylinositol 3-kinase/Akt, MEK/ERK, and/or Janus kinase 2/signal transducers and activators of transcription 3 signaling cascades to mediate drug resistance and MM cell growth.31,32 Importantly, although Dex-induced apoptosis of MM cells is significantly inhibited by IL-6 and IGF-1 via activation of the phosphatidylinositol 3-kinase/Akt pathway,32 SNX-2112 triggers MM cell cytotoxicity via down-regulation of Akt pathway, even in the presence of exogenous IL-6, IGF-1, or BMSCs, suggesting that this compound can overcome cell adhesion-mediated drug resistance in the BM milieu. Similarly, MEK/ERK signaling mediating MM cell proliferation,31,33 triggered by IL-6 and IGF-1,33 is inhibited by SNX-2112. Therefore, our study demonstrates that inhibition of Hsp90 by SNX-2112 can overcome the protective effects of cytokines or BMSCs via inhibition of Akt and ERK signaling, which depend on client proteins of Hsp90.3
We next examined the effect of SNX-2112 on BM microenvironment, specifically angiogenesis because MVD in the BM is associated with disease progression and poor prognosis in MM.34-36 Hsp90 modulates eNOS and Akt activity associated with endothelial cell growth and angiogenesis.37-41 Prior reports show that 17-AAG inhibits angiogenesis,42,43 and we here demonstrate that SNX-2112 blocks in vitro capillary-like tube formation, associated with down-regulation of eNOS and Akt expression and phosphorylation. In addition, we confirm the inhibitory effect of SNX-2112 on MVD in vivo by immunohistochemical analysis for CD31 expression. These results suggest that targeting Hsp90 by SNX-2112 inhibits angiogenesis via suppression of the eNOS/Akt pathway.
Osteolytic bone destruction is a common complication of MM.35 Both RANKL and M-CSF induce osteoclast differentiation44,45 via induction of ERK, p38MAPK, and c-Jun N-terminal kinase signaling cascades.44,45 Transcription factors, including PU.1 and c-fos, are also necessary for OCL formation46,47 ; PU.1-deficient mice show a failure in macrophage development at an early stage and decreased osteoclastogenesis, whereas c-fos-deficient mice develop osteopetrosis, indicating that c-fos plays a critical role in late stages of OCL differentiation.46-48 In addition, both M-CSF and αVβ3 integrin induce activation of ERK pathway and maintain c-fos expression, thereby promoting differentiation of OCL precursors.49 Previous reports show that 17-AAG inhibits OCL formation,43 and we here demonstrate that inhibition of Hsp90 by SNX-2112 at 7 and 11 days completely abrogates M-CSF- and RANKL-induced OCL differentiation, associated with down-regulation of p-ERK and c-fos. Transient ERK activation is associated with c-fos instability,50 consistent with our data that ERK inactivation mediated by SNX-2112 is associated with c-fos down-regulation. Although p-p38MAPK is slightly down-regulated after 11 days of treatment by SNX-2112, the level is not dramatically changed after 3 and 7 days, confirming that p-ERK is the main regulator of c-fos expression in OCL precursors. Interestingly, the inhibitory effects of SNX-2112 on OCL formation are at the early stage of differentiation of hematopoietic progenitor cells, resulting from the down-regulation of the PU.1 transcription factor. It remains unclear whether PU.1 is a client protein of Hsp90, but our data strongly support this possibility. Our results therefore indicate that SNX-2112 targets various important molecules in osteoclastogenesis, including ERK/c-fos and PU.1.
In conclusion, inhibition of Hsp90 by novel small molecule inhibitor SNX-2112 potently abrogates signaling via Akt and ERK and, as a consequence, is highly potent against all hematologic tumor cells tested. In MM, it not only targets cell growth but also acts in the BM microenvironment to block angiogenesis and osteoclastogenesis, associated with down-regulation of Akt and ERK signaling. Importantly, SNX-5422, a highly orally bioavailable means to deliver SNX-2112, both induces tumor growth inhibition and prolongs survival in a murine xenograft model of human MM. These data therefore show that targeting Hsp90 by SNX-2112 inhibits tumor cell growth, angiogenesis, and osteoclastogenesis in the BM milieu, providing the framework for clinical studies of this agent to improve patient outcome in MM and other hematologic cancers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the National Institutes of Health Specialized Programs of Research Excellence (grants IP50 CA10070-01, PO-1 CA78378, and RO-1 CA50947), the Multiple Myeloma Research Foundation (T.H., K.C.A.), and the LeBow Family Fund to Cure Myeloma (K.C.A.).
National Institutes of Health
Authorship
Contribution: Y.O., T.H., and P.S. designed, performed, and analyzed research and wrote the paper; S.V., S.H., K.H., J.R., A.B., B.F., H.I., N.R., T.K., H.Y., and S.E. performed and analyzed research; and K.C.A. participated in design, coordination, and performance of the study, assisted in writing the paper, and funded the study.
Conflict-of-interest disclosure: P.S., S.H., K.H., J.R., A.B., and B.F. are employees of Serenex, a subsidiary of Pfizer. The other authors declare no competing financial interests.
Correspondence: Kenneth C. Anderson, Dana-Farber Cancer Institute, Mayer 557, 44 Binney Street, Boston, MA 02115; e-mail: kenneth_anderson@dfci.harvard.edu; or Yutaka Okawa, Dana-Farber Cancer Institute, Mayer 549, 44 Binney Street, Boston, MA 02115; e-mail: yutaka_okawa@dfci.harvard.edu.

![Figure 4. SNX-2112 inhibits Akt and ERK pathway and overcomes the growth stimulatory effects of IL-6, IGF-1, and BMSCs on MM cell growth. (A) MM.1S, U266, and OPM1 cells were cultured with SNX-2112 (125 nM) for the indicated times. (B) MM.1S cells were cultured with or without SNX-2112 (0-250 nM) for 12 hours and then stimulated with IL-6 (10 ng/mL) or IGF-1 (50 ng/mL) for 10 minutes. Cell lysates were analyzed by Western blotting (A,B). (C) MM.1S cells were cultured for 48 hours with indicated concentrations: 0 nM (□), 5 nM () 10 nM (■) of SNX-2112, in the presence or absence of IL-6 (1 or 10 ng/mL; left) or IGF-1 (10 or 50 ng/mL; right). (D) MM.1S cells were cultured with BMSCs for 48 hours with the indicated concentrations: 0 nM (□), 10 nM (), 20 nM (), 40 nM (■) of SNX-2112. (E) SNX-2112 treatment for 48 hours does not affect cell viability of BMSCs: BMSC-1 (■), BMSC-2 (□), BMSC-3 (▴) (n = 3). Cell growth was assessed by [3H]-thymidine uptake (C,D) and MTT assay (E). Values represent mean plus or minus SD of quadruplicate (C,D) or triplicate cultures (E).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/4/10.1182_blood-2008-04-151928/4/m_zh80020929490004.jpeg?Expires=1769098563&Signature=KKobbXrvGXo2A-PBmBq0RwKxNp3U6vVLtK2TefV2i1rc~5PRWVEVrrLquKEXXp8K3LGvz6QCQnVJP6R0Owlrnpk-DUKyj9jk3vmk3wZdYPeUuSCMFCthjGE2-0ZiyNBeVvFHGKtdkpc6~o71rOly1ioUz3TK62etim~SlckdZRBSCDKkScrK619Ogdv0KHJA6TqBi~TIz906s~~3Bu4g3MGhixZOEICLNjT8lEPkVnxx6cro7mTQwh43tBFhfMeHpflx6ZKpjmbV22haQ3omNRmbb0Qge1HKlA997U353fJFX-skrnK1jeLmaxPFoCWnvUEfiVViFPsW6x9zYupzuQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)