Abstract
Endothelial cells regulate thrombosis, hemostasis, and inflammatory responses by supplying the vasculature with several factors that include procoagulant von Willebrand factor (VWF) and fibrinolytic tissue-type plasminogen activator (tPA). Both proteins can be secreted in a Ca2+-regulated manner after endothelial activation but exhibit opposing physiologic effects. In search for factors that could modulate endothelial responses by selectively affecting the secretion of procoagulant or anticoagulant proteins, we identify here phospholipase D1 (PLD1) as a specific regulator of VWF secretion. PLD1 is translocated to the plasma membrane upon stimulation of endothelial secretion, and this process correlates with the generation of phosphatidic acid (PA) in the plasma membrane. Histamine-evoked secretion of VWF, but not tPA, is inhibited by blocking PLD-mediated production of PA, and this effect can be attributed to PLD1 and not PLD2. Thus, different mechanisms appear to control the agonist-induced secretion of VWF and tPA, with only the former requiring PLD1.
Introduction
Vascular endothelial cells (ECs) secrete a variety of procoagulant, anticoagulant, and inflammatory factors that regulate blood clotting and local immune responses. The tight regulation of these events permits endothelial cells to present an anticoagulant surface under normal conditions and to help promoting thrombus formation at sites of vessel injury. Secreted factors mediating these responses are procoagulant and proinflammatory proteins like von Willebrand factor (VWF) and P-selectin and anticoagulant proteins like tissue-type plasminogen activator (tPA) and protein S.1,2
VWF and P-selectin are stored in Weibel-Palade bodies (WPbs), large, elongated secretory granules that constitute a unique morphologic hallmark of endothelial cells.3,4 Their exocytosis generally requires an intraendothelial rise in Ca2+ or cAMP and can be induced by several agonists, such as thrombin, histamine, and other mediators of thrombosis or inflammation.5,6 The anticoagulant tPA has been localized to small vesicular structures, which differ from WPbs in size and morphology but are also released after intraendothelial Ca2+ elevation.7,8 Furthermore, it has been reported that the acute secretion of WPbs and smaller granules, possibly those containing tPA, occurs with different kinetics.9 Storage of VWF and tPA in different granules and their selective release after cell stimulation would enable the endothelium to specifically respond to a given (patho)physiologic situation by locally elevating the levels of either procoagulant (VWF) or anticoagulant factors (tPA). Thus, specific release machineries and specific signaling pathways triggering the acute secretion of either WPbs or tPA granules are likely to exist. However, very little, if any, is known about the underlying mechanisms.
Phospholipase D (PLD) enzymes have emerged recently as major players in a varied set of secretory events. In mammals, 2 phosphatidylcholine (PC)–selective PLDs, termed PLD1 and PLD2, catalyze the hydrolysis of PC to release choline and yield phosphatidic acid (PA). PLD2 exhibits relatively high basal activity, but PLD1 activation requires stimulation by small GTPases, including those of the ADP-ribosylation factor (Arf), Rho and Ral families, and protein kinase C.10-12 RalA and its guanine nucleotide exchange factor RalGDS also participate in the regulation of WPB exocytosis,13,14 and RalA can be found associated with WPBs.15 Furthermore, Rho family GTPase have been implicated in acute endothelial exocytosis.16 Given this involvement of PLD1 regulators in WPB exocytosis, we decided to explore whether PLD enzymes themselves participate in exocytotic processes in endothelial cells. We show here that both, PLD1 and PLD2, are expressed in human umbilical vein endothelial cells (HUVECs) and that endogenous PLD activity and PA production at the plasma membrane are markedly increased in endothelial cells stimulated for exocytosis. Inhibition of PLD-mediated production of PA leads to a drastic reduction of histamine-evoked secretion of VWF, but not tPA. Our findings indicate that endothelial lipid modification by PLD1 specifically regulates the Ca2+-induced exocytosis of WPbs and thus provide evidence for the existence of distinct mechanisms underlying the acute release of procoagulant and anticoagulant factors from endothelial cells.
Methods
Antibodies and plasmids
WPbs were stained with mouse monoclonal (clone F8/86) and rabbit polyclonal anti-human VWF antibodies (Dako, Glostrup, Denmark). Rabbit polyclonal antibodies against human PC-PLD1 (H-160) and PC-PLD2 (H-133) were from Santa Cruz Biotechnology (Heidelberg, Germany), mouse monoclonal antibodies against hemagglutinin (HA)–Tag (6E2) from Cell Signaling Technology (Danvers, MA), mouse monoclonal antibodies against syntaxin 4 (clone 46) from BD Pharmingen (San Diego, CA), and mouse monoclonal antibodies against human LAMP3/CD63 (clone H5C6) from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA). Mouse monoclonal anti-green fluorescent protein (GFP) antibodies (clone JL-8) were obtained from Clontech (Palo Alto, CA), and mouse monoclonal antibodies to vimentin (clone V9) were obtained from Immunotech (Hamburg, Germany). Secondary anti-mouse antibodies coupled to Texas Red or Cy2 were from Dianova (Hamburg, Germany), and Alexa (488,568)–conjugated secondary anti-rabbit antibodies were from Molecular Probes (Eugene, OR).
Wild-type human PLD1b and mouse PLD217 were cloned as N-terminally enhanced GFP (EGFP)–tagged version and used for transient transfections. Small interfering RNA (siRNA)–mediated down-regulation was performed using short hairpin RNA (shRNA) plasmids consisting of an H1 promoter and siRNA sequences, nucleotides (nt) 547-565 of human PLD1 and nt 1145-1164 or nt 723-741 of mouse PLD2 (a mixture of 2 shRNA plasmids was used in case of PLD2), cloned into a dsRed2 plasmid.18 This allowed independent visualization of transfected cells via the dsRed fluorescence. In rescue experiments, cells were cotransfected with shRNA constructs and pCGN plasmids encoding HA-tagged mutated rescue PLD constructs resistant to siRNA degradation.19 Raf1 PA-binding domain (PABD) in a pEGFP vector was used to visualize PA.20
Cell culture and transfection
Primary cultures of human umbilical vein endothelial cells (HUVECs) were either established from umbilical cord veins as described21 or purchased as cryoconserved cells (Lonza, Basel, Switzerland). Cell culture and transfections were as described.22 All experiments used nearly confluent cells between passages 2 and 4. To stimulate regulated secretion, cells were treated with 100 μM histamine (H7125; Sigma-Aldrich, St Louis, MO) in basal medium for the time indicated.
Reverse-transcription polymerase chain reaction for PLD1 and PLD2
Reverse-transcription polymerase chain reaction (RT-PCR) was used to establish whether PLD1 and PLD2 are expressed in HUVECs. Total cellular RNA isolated from HUVECs was kindly provided by E. Aareskjold (Institute of Medical Biochemistry, Muenster, Germany). Total RNA (2 μg) was used as templates for the RT reaction using random hexamers and Superscript Reverse Transcriptase II (Invitrogen, Karlsruhe, Germany) according to the manufacturer's instructions. PCR amplification was performed with 30 cycles of 30 seconds at 94°C, 30 seconds at 54°C, and 2 minutes at 72°C. Oligonucleotide pairs were 5′-GTGGGCAGTGTGAAGCGGGTC-3′ and 5′-CTATTCCTTTCAGTTTTGAATCCACATC-3′ for human PLD1 and 5′-CTCTTCCCCACTGGGGACGAA-3′ and 5′-GACAGAATACAGAGTG CAGGTTCCCAC-3′ for human PLD2. The predicted lengths of the products expected in the reaction were 166 and 246 bp, respectively. RNA samples, which were not reverse-transcribed, were used as negative control. PCR products were resolved on 1% agarose gels, stained with ethidium bromide, and photographed.
Determination of PLD activity
Thirty-six hours after transfection, HUVECs were washed 3 times with phosphate-buffered saline (PBS) and then incubated overnight in basal medium containing 5 mg/mL bovine serum albumin (BSA) and antibiotics. HUVECs were stimulated by treatment with histamine containing basal medium for 10 min. Control experiments used basal medium without any addition to measure the level of constitutive PLD activity that was set at 100%. After 10 minutes, cells were washed once with ice-cold 50 mM Tris, pH 8.0, containing 1 mM EGTA and complete protease inhibitor cocktail without ethylenediaminetetraacetic acid (EDTA; Roche, Rotkreuz, Switzerland). The washing solution was then replaced by 300 μL ice-cold 50 mM Tris, pH 8.0, containing a complete protease inhibitor cocktail without EDTA. Subsequently, the cells were lysed by 3 freeze-thaw cycles. Samples were collected, mixed with an equal amount of the Amplex Red reaction buffer (Amplex Red Phospholipase D assay kit; Molecular Probes), and PLD activity was measured after 30-minute incubation at 37°C in a Lambda Fluoro 320 fluorometer (MWG-Biotech, Ebersberg, Germany).
Quantification of VWF and tPA secretion
HUVECs grown to confluency in collagen-coated 24-well plates were serum-starved overnight. Ca2+-regulated exocytosis of WPbs was induced by treatment with 200 μL histamine-containing stimulation medium (basal medium, 2% BSA, 100 μM histamine) at 37°C for 20 min. Control experiments used 2% BSA in basal medium without the addition of histamine to measure the levels of constitutive exocytosis occurring at the respective condition. In butanol inhibition experiments, cells were incubated with 25 mM tert-butanol (control) or 25 mM n-butanol (inhibition of PLD) in basal medium for 10 minutes before stimulation, followed by treatment with stimulation medium containing the respective concentrations of butanol. Collection of the culture supernatant and quantification of VWF secretion by enzyme-linked immunosorbent assay (ELISA)23 was carried out as described previously.24 Briefly, high binding ELISA plates (Greiner Bio One, Essen, Germany) were first coated overnight at 4°C with rabbit anti-human VWF antibodies (Dako) diluted 1:1000 in coating buffer (50 mM carbonate, pH 9.6). Subsequently, wells were washed with 0.1% Tween 20 in PBS, blocked for nonspecific binding with 1% BSA in PBS/Tween 20 for 1 hour, washed again (3 times), and incubated for 2 hours at room temperature with 100 μL of the culture supernatants. The plates were then washed with PBS/Tween 20 and incubated with a rabbit anti-human VWF peroxidase conjugate (Dako), diluted 1:4000 in 1% BSA/PBS/Tween 20 for 1 hour. For detection and visualization of the antigen-antibody conjugates, 100 μL of the ImmunoPure TMB Substrate kit (Pierce, Rockford, IL) were added to each well after additional 6 washes. The peroxidase-reaction was stopped by the addition of 100 μL of 2 M sulfuric acid per well, and reaction products were detected photometrically at 450 nm in a Lambda K Elx808 microplate reader (MWG-Biotech).
Analysis of tPA secretion used a slightly modified protocol for the collection of culture supernatants. Briefly, serum-starved HUVECs grown to confluency in 12-well plates were incubated for 5 minutes with PBS containing 0.5% BSA and 25 mM tert-butanol or 25 mM n-butanol, respectively. To induce regulated exocytosis of tPA, cells were then treated with the above mixture either with or without 100 μM histamine at 37°C for 5 min. Cell culture supernatants were collected and directly subjected to ELISAs to determine the amount of secreted tPA. For detection of tPA, a matched-pair antibody set for ELISA of human tPA (Affinity Biologicals, Ancaster, ON) was used following the manufacturer's protocol. In brief, high-binding ELISA plates were incubated at 4°C overnight with 100 μL primary anti-tPA antibodies in coating buffer (50 mM carbonate, pH 9.6). Wells were then washed with 0.5% Tween 20 in PBS, blocked for nonspecific binding with 200 μL blocking solution (5% sheep serum, 0.5% Tween 20 in PBS) for 1 hour, washed again (3 times), and incubated for 2 hours at room temperature with 150 μL of the culture supernatants. Thereafter, the washing step was repeated, followed by incubation with 100 μL horseradish peroxidase (HRP)–conjugated detection antibodies diluted 1:500 in blocking solution. After a final wash, tPA was quantified using the ImmunoPure TMB Substrate kit as described in the previous paragraph.
To exclude variations, we always used the same batch of HUVECs when comparing histamine-induced and constitutive secretion. Results obtained with different batches of cells were then compared with one another by calculating relative values for the stimulated secretion. Therefore, the constitutive secretion occurring in a given time interval was set to 100%, and the values obtained with histamine-treated cells of the same batch were calculated in percent as the sum of constitutive and regulated secretion. The relative amounts of regulated secretion are given in the figures.
Quantification of PLD1 and PLD2 knockdown efficiency
Forty-eight hours after transfection, cells expressing either nontargeting shRNA plasmids or shRNA plasmids targeted at PLD1 or PLD2 were lysed in ice-cold lysis buffer containing 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), pH 7.4, 150 mM NaCl, 1% Triton X-100, 1.5 mM phenylmethylsulfonyl fluoride (PMSF) with added protease inhibitor cocktail (Roche) by passage through a 23-gauge needle. Postnuclear supernatants (PNS) were prepared by centrifugation of the lysates for 10 minutes at 1000g. Equal protein amounts of the PNS prepared from shRNA-transfected and control cells were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and subsequent immunoblotting using rabbit polyclonal anti-human PC-PLD1 and PC-PLD2 antibodies. Detection of vimentin using monoclonal anti-vimentin antibodies was included as internal loading control. Signal intensities were quantified by densitometric analysis using the ImageJ software (National Institutes of Health [NIH]). Statistical significance of the results obtained from 3 independent experiments was evaluated by unpaired Student t test.
Confocal microscopy
Cells destined for immunofluorescence labeling were fixed for 10 minutes at room temperature with 4% paraformaldehyde (PFA)/PBS, followed by a 2-minute permeabilization step in PBS containing 0.2% Triton X-100. Samples were then blocked with 10% fetal calf serum (FCS) before incubation with primary and the appropriate secondary antibodies, each applied for 1 hour at room temperature. All primary antibodies used were diluted 1:100 in 2% BSA/PBS. All secondary mouse antibodies, used as Cy2 or Texas Red conjugates, were diluted 1:200 in 2% BSA in PBS. All secondary rabbit antibodies coupled to Alexa dyes were diluted 1:400 in 2% BSA in PBS. Stained cells were analyzed by confocal microscopy (LSM510; Zeiss, Jena, Germany).
Fluorescence intensities were quantified using the Zeiss LSM 510 instrument software 3.2. Average fluorescence intensities of plasma membrane versus cytosolically localized PABD-GFP were determined by randomly choosing areas of defined size covering the plasma membrane and a cytosolic region of identical size 5 μm away from the plasma membrane. Ratios of plasma membrane to cytosolic pixel fluorescence intensity were then calculated and subjected to statistical analysis.
Statistical analysis
Graphs and associated statistics were generated using GraphPad Prism version 4.00 for Windows software (GraphPad Software, San Diego, CA). Data were compared for significant differences using either the unpaired Student t test (in the case of 2 groups) or an analysis of variance (1-way ANOVA, for more than 2 groups). Once 1-way ANOVA identified a significant difference within several groups, comparison between 2 individual groups within that set was again carried out by the unpaired Student t test. A value of P less than .01 was considered significant, and results were expressed as mean values plus or minus SEM.
Results
Expression of PLD isoforms in primary endothelial cells
RT-PCR revealed the presence of both PLD1 and PLD2 mRNAs in HUVECs with the a and b isoform of PLD1 being expressed (not shown). PLD1 localized to perinuclear vesicles of resting HUVECs, the majority of which were positive for the tetraspanin CD63, a marker for late endosomes/lysosomes (Figure 1A). Similar intracellular localizations of PLD1 are also seen in other cell types, for instance COS-7 cells, in which PLD1 is found on perinuclear endosomes.25 Infrequently, a small amount of PLD1 was also present at the plasma membrane, whereas hardly any enzyme was found at early endosome antigen 1 (EEA1)–positive early endosomes (data not shown). No significant association of PLD1 with either WPbs or tPA-containing granules was observed (Figure 1A). The same distribution to internal vesicles of resting HUVECs was seen for ectopically expressed GFP-PLD1 (data not shown). In contrast, a substantial fraction of GFP-PLD2 associated with the plasma membrane of resting HUVECs, colocalizing with the plasma membrane t-SNARE syntaxin 4 (Figure 1B).
Localization of PLD isoforms in HUVECs. (A) Subcellular localization of endogenous PLD1 in resting HUVECs. Fixed and permeabilized HUVECs were stained with antibodies against PLD1 and anti-human LAMP3/CD63 (top panels) or anti-human VWF antibodies (middle panels). In the bottom panels, the anti-PLD1 staining is compared with the distribution of exogenously expressed tPA-GFP. (B) Colocalization of GFP-tagged PLD2 and syntaxin 4 in resting HUVECs. Bars represent 10 μm.
Localization of PLD isoforms in HUVECs. (A) Subcellular localization of endogenous PLD1 in resting HUVECs. Fixed and permeabilized HUVECs were stained with antibodies against PLD1 and anti-human LAMP3/CD63 (top panels) or anti-human VWF antibodies (middle panels). In the bottom panels, the anti-PLD1 staining is compared with the distribution of exogenously expressed tPA-GFP. (B) Colocalization of GFP-tagged PLD2 and syntaxin 4 in resting HUVECs. Bars represent 10 μm.
Stimulation of endothelial secretion triggers PLD activation
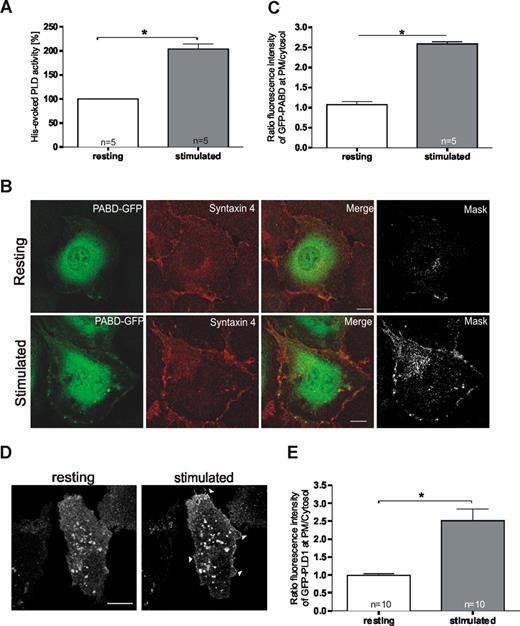
We next assessed whether endothelial PLD activity is affected by cell stimulation that triggers secretory events. Histamine was used as an established agonist known to evoke a transient increase in intraendothelial Ca2+ concentration and the subsequent Ca2+-regulated secretion of different factors including VWF and tPA.5 As shown in Figure 2A, PLD activity was significantly increased in histamine-stimulated compared with resting HUVECs. To determine whether this increased activity results in the production of PA at the principal site of exocytosis (ie, the plasma membrane), we visualized PA by transfecting cells with the PABD of Raf1 expressed as a GFP-fusion.20 As illustrated in Figure 2B, the PABD showed a general cellular distribution in resting cells (top panels), whereas a significant fraction of the PA sensor was recruited to the plasma membrane upon stimulation of exocytosis by histamine (bottom panels, statistical evaluation in Figure 2C). Elevation of plasma membrane PA levels was already observed at 5 minutes of histamine stimulation (not shown) thus preceding the acute release of VWF, but probably not that of tPA.24
Cellular PLD activity and plasma membrane PA levels increase upon stimulation of endothelial secretion. (A) PLD activity in resting and histamine-stimulated HUVECs. After a 10-minute treatment with histamine-free or histamine-containing medium, cells were lysed and assayed for PLD activity. The effect on PLD activity was quantified in sets of independent experiments (number of experiments shown at the base of each column) and statistical significance (*P < .01) was calculated by unpaired Student t test. Bars represent mean plus or minus SEM. (B) PA generation at the plasma membrane (PM) in response to histamine-stimulation. HUVECs were transfected with the PABD of Raf1 fused to EGFP (PABD-GFP). Six hours after transfection, cells were incubated for 10 minutes with basal medium either without (top panels) or with (bottom panels) 100 μM histamine. Stimulation of exocytosis triggered the recruitment of a fraction of the PA sensor to the plasma membrane as visualized by costaining with syntaxin 4. In the mask images, only the double-labeled pixels are displayed, visualizing areas of colocalization of PABD-GFP with syntaxin 4. Bars represent 10 μm. (C) The fluorescence intensity of GFP-PABD at the PM relative to that in the cytosol was determined in 5 representative cells from independent experiments using the Zeiss LSM instrument software 3.2. Average fluorescence intensities were determined by randomly choosing areas of defined size covering the plasma membrane and a cytosolic region of identical size 5 μm away from the plasma membrane. Ratios of plasma membrane to cytosolic pixel fluorescence intensity were then calculated, and statistical significance (*P < .01) of the results was evaluated by unpaired Student t tests. Bars represent mean plus or minus the standard error of the mean. (D) Stimulation with histamine triggers a recruitment of PLD1 to the plasma membrane. HUVECs were transiently transfected with a PLD1-GFP expression plasmid. Six hours after transfection, cells were stimulated by addition of 100 μM histamine to the culture medium. Confocal images show a live cell before (resting) and 5 minutes after addition of histamine (stimulated). Note the plasma membrane localization in stimulated cells (arrowheads). Bar represents 10 μm. (E) The fluorescence intensity of GFP-PLD1 at the PM relative to that in the cytosol at 5 minutes after histamine stimulation was determined in 10 representative cells from independent experiments using the Zeiss LSM instrument software 3.2. Bars represent mean plus or minus SEM, and statistical significance (*P < .01) was calculated by unpaired Student t test.
Cellular PLD activity and plasma membrane PA levels increase upon stimulation of endothelial secretion. (A) PLD activity in resting and histamine-stimulated HUVECs. After a 10-minute treatment with histamine-free or histamine-containing medium, cells were lysed and assayed for PLD activity. The effect on PLD activity was quantified in sets of independent experiments (number of experiments shown at the base of each column) and statistical significance (*P < .01) was calculated by unpaired Student t test. Bars represent mean plus or minus SEM. (B) PA generation at the plasma membrane (PM) in response to histamine-stimulation. HUVECs were transfected with the PABD of Raf1 fused to EGFP (PABD-GFP). Six hours after transfection, cells were incubated for 10 minutes with basal medium either without (top panels) or with (bottom panels) 100 μM histamine. Stimulation of exocytosis triggered the recruitment of a fraction of the PA sensor to the plasma membrane as visualized by costaining with syntaxin 4. In the mask images, only the double-labeled pixels are displayed, visualizing areas of colocalization of PABD-GFP with syntaxin 4. Bars represent 10 μm. (C) The fluorescence intensity of GFP-PABD at the PM relative to that in the cytosol was determined in 5 representative cells from independent experiments using the Zeiss LSM instrument software 3.2. Average fluorescence intensities were determined by randomly choosing areas of defined size covering the plasma membrane and a cytosolic region of identical size 5 μm away from the plasma membrane. Ratios of plasma membrane to cytosolic pixel fluorescence intensity were then calculated, and statistical significance (*P < .01) of the results was evaluated by unpaired Student t tests. Bars represent mean plus or minus the standard error of the mean. (D) Stimulation with histamine triggers a recruitment of PLD1 to the plasma membrane. HUVECs were transiently transfected with a PLD1-GFP expression plasmid. Six hours after transfection, cells were stimulated by addition of 100 μM histamine to the culture medium. Confocal images show a live cell before (resting) and 5 minutes after addition of histamine (stimulated). Note the plasma membrane localization in stimulated cells (arrowheads). Bar represents 10 μm. (E) The fluorescence intensity of GFP-PLD1 at the PM relative to that in the cytosol at 5 minutes after histamine stimulation was determined in 10 representative cells from independent experiments using the Zeiss LSM instrument software 3.2. Bars represent mean plus or minus SEM, and statistical significance (*P < .01) was calculated by unpaired Student t test.
Of the 2 PLD isoforms expressed in HUVECs, only PLD2 showed a significant association with the plasma membrane in resting cells. However, PLD2 is known to display a high constitutive activity that is often not affected in a significant manner when cells are stimulated.26 On the other hand, PLD1 has low basal activity that is increased upon cell stimulation. Thus, PLD1 represents a likely candidate for mediating the histamine-evoked stimulation of PA production in the plasma membrane. To test this idea, we examined whether the predominant intracellular localization of PLD1 was affected by endothelial stimulation. Indeed, a significant fraction of PLD1 translocated to the plasma membrane already after 5 minutes of histamine stimulation of HUVECs (Figure 2D,E), most likely providing the regulated enzyme activity required for the histamine-induced generation of PA in the plasma membrane.
PLD activity is required for regulated secretion of VWF, but not tPA
To establish a direct link between the histamine-triggered increase in PLD activity and the histamine-induced and Ca2+-dependent exocytotic events in endothelial cells, we measured the acute release of thrombogenic VWF and fibrinolytic tPA under conditions where PLD-mediated PA production was blocked. The latter was achieved using primary alcohols that serve as shunt substrates for PLD; tertiary alcohols that are not PLD substrates were used as experimental controls. Figure 3A reveals that the histamine-evoked release of VWF was profoundly inhibited by n-butanol but not tert-butanol. No effect was seen on basal (ie, constitutive) secretion (data not shown). A similar inhibition by n-butanol is observed when VWF secretion from HUVECs is induced by raising the intracellular cAMP levels through forskolin treatment (not shown). In marked contrast, the histamine-induced release of tPA was not affected significantly by the addition of n-butanol (Figure 3B). These observations indicate that the inhibition of PLD-mediated PA production selectively blocks the secretion of VWF, but not that of tPA, in line with the view that different mechanisms govern the regulated exocytosis of WPbs and tPA granules.
Histamine-induced secretion of VWF, but not tPA, is affected by inhibition of PLD-mediated PA production. (A,B) Quantification of VWF and tPA secretion after PLD inhibition. HUVECs grown to confluency were treated for 10 minutes in culture medium with n-butanol to inhibit the PLD-mediated generation of PA. Control experiments used culture medium alone (control) or culture medium containing tert-butanol that does not serve as a substrate for PLD. Subsequently, the cells were subjected to histamine stimulation for 20 minutes (A) or 5 minutes (B), respectively, and the acute release of VWF (A) and tPA (B) into the cell culture supernatant was then determined by specific ELISAs. The relative histamine-triggered increase of VWF and tPA secretion exceeding the constitutive level is given in percentage as the mean value plus or minus SEM. The effect on secretion was quantified in sets of independent experiments (number of experiments shown at the base of each column). One-way ANOVA with unpaired Student t test was performed to evaluate statistical significance (*P < .01).
Histamine-induced secretion of VWF, but not tPA, is affected by inhibition of PLD-mediated PA production. (A,B) Quantification of VWF and tPA secretion after PLD inhibition. HUVECs grown to confluency were treated for 10 minutes in culture medium with n-butanol to inhibit the PLD-mediated generation of PA. Control experiments used culture medium alone (control) or culture medium containing tert-butanol that does not serve as a substrate for PLD. Subsequently, the cells were subjected to histamine stimulation for 20 minutes (A) or 5 minutes (B), respectively, and the acute release of VWF (A) and tPA (B) into the cell culture supernatant was then determined by specific ELISAs. The relative histamine-triggered increase of VWF and tPA secretion exceeding the constitutive level is given in percentage as the mean value plus or minus SEM. The effect on secretion was quantified in sets of independent experiments (number of experiments shown at the base of each column). One-way ANOVA with unpaired Student t test was performed to evaluate statistical significance (*P < .01).
Regulated WPb exocytosis depends on PLD1, but not PLD2
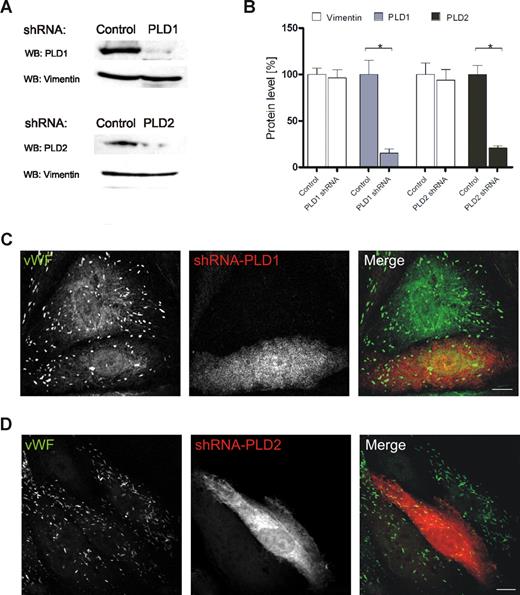
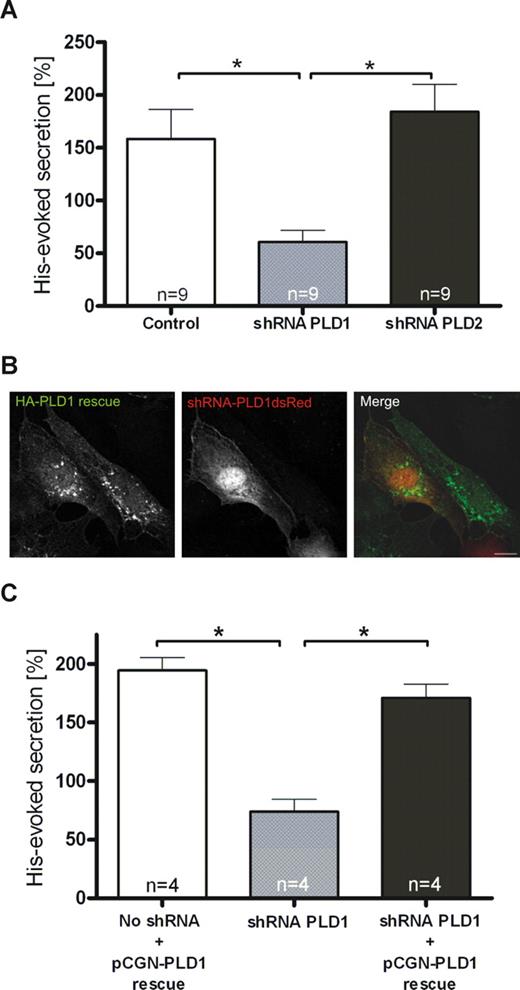
To determine which PLD isoform is involved in regulated exocytosis of WPbs, we transfected HUVECs with shRNA plasmids to down-regulate the expression of PLD1 and PLD2. When compared with nontargeting shRNAs, shPLD1 and shPLD2 reduced the level of their respective PLD targets by approximately 80% and 75%, respectively, as revealed by Western blot analysis (Figure 4A,B) and immunofluorescence (not shown) of the transfected HUVECs. In line with the lack of colocalization between both PLD isoforms and WPbs or tPA granules, neither PLD1 nor PLD2 down-regulation affected the number, morphologic appearance, and distribution of WPbs (Figure 4C,D) and tPA granules (data not shown). However, reduction of endogenous PLD1 expression markedly inhibited the secretion of VWF from histamine-stimulated HUVECs (Figure 5A). PLD2 knockdown, on the other hand, had no effect. To confirm that PLD1 is the key cellular PLD isoform required to permit efficient secretagogue-stimulated VWF release, we investigated if secretion in PLD1 knockdown cells can be rescued by a shRNA-insensitive PLD1 variant (Figure 5B). Expression of this shRNA-insensitive construct in PLD1-depleted HUVECs fully restored histamine-evoked VWF secretion (Figure 5C). Thus, PLD1 has an essential function in the histamine-induced release of WPbs, but not tPA-containing vesicles, from endothelial cells.
siRNA-mediated down-regulation of PLD isoforms does not affect the number or appearance of WPbs. HUVECs were transiently transfected with either nontargeting shRNA plasmids or shRNA plasmids targeted at PLD1 or PLD2. (A) Forty-eight hours after transfection, PNS were prepared and analyzed by Western blot analysis using rabbit polyclonal anti–PC-PLD1 and anti–PC-PLD2 as well as monoclonal anti-vimentin antibodies (loading controls). (B) Quantification of PLD1 and PLD2 down-regulation. Signal intensities were quantified by densitometric analysis using the ImageJ software (NIH). Statistical significance (*P < .01) of the results obtained from 3 independent experiments was evaluated by unpaired Student t test. (C,D) WPb distribution in shRNA-transfected cells. Transfected cells were identified by fluorescence of the plasmid encoded dsRed and WPbs were visualized by labeling with monoclonal anti-human VWF antibodies. Transfection rates typically were in the range of 70% to 90%. Images shown here were chosen to show nontransfected and transfected cells in the same frame. Note that the anti-VWF staining is not affected by PLD1 (C) or PLD2 (D) depletion. Bars represent 10 μm.
siRNA-mediated down-regulation of PLD isoforms does not affect the number or appearance of WPbs. HUVECs were transiently transfected with either nontargeting shRNA plasmids or shRNA plasmids targeted at PLD1 or PLD2. (A) Forty-eight hours after transfection, PNS were prepared and analyzed by Western blot analysis using rabbit polyclonal anti–PC-PLD1 and anti–PC-PLD2 as well as monoclonal anti-vimentin antibodies (loading controls). (B) Quantification of PLD1 and PLD2 down-regulation. Signal intensities were quantified by densitometric analysis using the ImageJ software (NIH). Statistical significance (*P < .01) of the results obtained from 3 independent experiments was evaluated by unpaired Student t test. (C,D) WPb distribution in shRNA-transfected cells. Transfected cells were identified by fluorescence of the plasmid encoded dsRed and WPbs were visualized by labeling with monoclonal anti-human VWF antibodies. Transfection rates typically were in the range of 70% to 90%. Images shown here were chosen to show nontransfected and transfected cells in the same frame. Note that the anti-VWF staining is not affected by PLD1 (C) or PLD2 (D) depletion. Bars represent 10 μm.
Depletion of endothelial PLD1, but not PLD2, interferes with histamine-evoked secretion of VWF. (A) Quantification of VWF secretion from histamine-stimulated HUVECs depleted for PLD1 or PLD2. Forty-eight hours after transfection with shRNA plasmids specific for PLD1 or PLD2 or a nontargeting shRNA control, HUVECs were subjected to histamine treatment for 20 minutes. The amount of VWF released into the cell culture supernatant was then determined as described in “Methods.” The effect on secretion was quantified in sets of 9 independent experiments, and 1-way ANOVA with unpaired Student t test was performed to evaluate statistical significance (*P < .01). Bars represent mean plus or minus SEM. (B,C) Expression of a shRNA-insensitive PLD1 construct restores secretory responsiveness. Twenty-four hours after transfection with shRNA plasmids targeted at PLD1, cells were transfected with a pCGN-PLD1 rescue plasmid expressing a wobble position-mutated HA-tagged PLD1 cDNA insensitive to PLD1 shRNA. Twenty-four hours after the second transfection, HUVECs were used in immunofluorescence experiments or stimulated for secretion. (B) HA-tagged PLD1 was labeled using mouse monoclonal anti-HA antibodies, and cells expressing the shRNA constructs were identified by expression of plasmid-encoded dsRed. Note that the PLD1 rescue construct is stable in the shRNA-expressing cell. Bar represents 10 μm. (C) Cells transfected with shRNA and rescue plasmids were subjected to histamine treatment, and the amount of VWF released into the cell culture supernatant was determined as described in “Methods.” The effect on secretion was quantified in sets of 4 independent experiments, and 1-way ANOVA with unpaired Student t test was performed to evaluate statistical significance (*P < .01). Bars represent mean plus or minus SEM. Note that histamine-evoked secretion of VWF is restored by coexpression of the pCGN-PLD1 rescue plasmid.
Depletion of endothelial PLD1, but not PLD2, interferes with histamine-evoked secretion of VWF. (A) Quantification of VWF secretion from histamine-stimulated HUVECs depleted for PLD1 or PLD2. Forty-eight hours after transfection with shRNA plasmids specific for PLD1 or PLD2 or a nontargeting shRNA control, HUVECs were subjected to histamine treatment for 20 minutes. The amount of VWF released into the cell culture supernatant was then determined as described in “Methods.” The effect on secretion was quantified in sets of 9 independent experiments, and 1-way ANOVA with unpaired Student t test was performed to evaluate statistical significance (*P < .01). Bars represent mean plus or minus SEM. (B,C) Expression of a shRNA-insensitive PLD1 construct restores secretory responsiveness. Twenty-four hours after transfection with shRNA plasmids targeted at PLD1, cells were transfected with a pCGN-PLD1 rescue plasmid expressing a wobble position-mutated HA-tagged PLD1 cDNA insensitive to PLD1 shRNA. Twenty-four hours after the second transfection, HUVECs were used in immunofluorescence experiments or stimulated for secretion. (B) HA-tagged PLD1 was labeled using mouse monoclonal anti-HA antibodies, and cells expressing the shRNA constructs were identified by expression of plasmid-encoded dsRed. Note that the PLD1 rescue construct is stable in the shRNA-expressing cell. Bar represents 10 μm. (C) Cells transfected with shRNA and rescue plasmids were subjected to histamine treatment, and the amount of VWF released into the cell culture supernatant was determined as described in “Methods.” The effect on secretion was quantified in sets of 4 independent experiments, and 1-way ANOVA with unpaired Student t test was performed to evaluate statistical significance (*P < .01). Bars represent mean plus or minus SEM. Note that histamine-evoked secretion of VWF is restored by coexpression of the pCGN-PLD1 rescue plasmid.
Discussion
Endothelial cells are capable of responding to agonist-induced intracellular Ca2+ elevation with the acute release of intracellularly stored factors that control homeostasis in the vascular system. The underlying exocytotic processes can be recorded by high resolution capacitance measurements that reveal different types of secretory events most likely corresponding to the fusion of large granules (VWF-containing WPbs) and smaller non-WPb vesicles.9 The latter have not been identified, but they could, at least in part, correspond to tPA-containing granules previously shown to be morphologically distinct and much smaller than WPbs.7 Interestingly, secretion of WPbs appeared delayed after photolysis-induced intracellular Ca2+ elevation and after endothelial stimulation with low histamine concentrations, whereas the small vesicular components were immediately available for secretion.9,27 Based on the present observation that histamine-evoked formation of PA in the plasma membrane precedes WPb, but not tPA exocytosis, the delay in WPb secretion could reflect the dependence on PLD1 recruitment and activation at the plasma membrane. Together, the capacitance measurements and our functional analyses strongly suggest that endothelial cells are capable of selective cargo release from different types of granules enabling them to mount distinct physiologic responses (eg, prothrombotic effects by WPb secretion and profibrinolytic responses by tPA granule release). The predicted mechanistic differences in the exocytotic release mediating WPb and tPA granule secretion reflect themselves in the PLD1-requirement for the former but not the latter secretory event.
Two phospholipase isoforms, PLD1 and PLD2, are expressed in the human endothelial cells analyzed in this study. In line with observations in other cell types, PLD1 is mainly present on internal structures of resting HUVECs, some of which colocalize with late endosomes, whereas PLD2 is found at the plasma membrane. Several arguments support the view that PLD1, and not PLD2, is the isoform responsible for the acute generation of PA in the plasma membrane occurring in response to histamine-induced endothelial secretion. First, PLD1 is the regulated isoform known to be activated after cell stimulation (eg, downstream of Rho and Arf GTPases).28 Second, PLD1 has been shown to participate in dense core granule exocytosis in neuroendocrine chromaffin and PC12 cells that is accompanied by the regulated generation of PA in the plasma membrane.17 Third, our imaging of live HUVECs reveals that cell stimulation with histamine triggers a recruitment of PLD1 to the plasma membrane coinciding in time with the production of PA. Finally, down-regulation of PLD1, but not PLD2, specifically interferes with the histamine-evoked exocytosis of WPbs. This secretory event is directly linked to the presence of elevated PA levels in the plasma membrane, since overexpression of the PABD of Raf1 inhibits histamine-triggered VWF release, possibly due to some steric hindrance (J.D. and V.G., unpublished observation, May 2008). The observation that regulated WPb secretion requires PA elevation at the plasma membrane suggests that the delay in VWF compared with the rapid tPA release might at least partially reflect the dependence on PLD1 recruitment and activation at the site of exocytosis.
PLD1 has recently emerged as a major actor in various exocytotic events, and it has been proposed that it represents a general promoter of membrane fusion by providing fusogenic cone-shaped lipids like PA.10-12 Upstream activation of the regulated PLD1 isoform is known to occur by various means. In endothelial cells, RalA could serve an important role as an upstream activator. Agonists raising both, endothelial Ca2+ (histamine, thrombin) and cAMP levels (epinephrine), trigger the activation of RalA through the nucleotide exchange factor RalGDS, and this activation has recently been shown to be required for regulated WPb exocytosis.14 Thus, RalA could represent an important link between agonist-induced stimulation of different G protein-coupled receptors (GPCRs), a resulting elevation in intracellular Ca2+ and/or cAMP, and the activation of PLD1. Based on these considerations, we propose the following order of events that underlies the acute release of thrombogenic factors from endothelial cells. Histamine stimulation triggers a Ca2+-dependent RalA activation and induces plasma membrane translocation and activation of PLD1. At the plasma membrane, PLD1 catalyzes the hydrolysis of PC and thus triggers the generation of PA-enriched domains. PA, in turn, is a membrane component required for docking and/or fusion of mature WPbs that deliver a substantial amount of additional membrane easily discernible in capacitance recordings.9 Whether PA functions primarily as the cone-shaped lipid forming the membrane curvatures required for fusion or whether it serves as a recruitment factor for additional, yet to be identified components of the WPb secretion apparatus remains to be determined. Regardless of this mode of action, the PLD1-dependent increase in plasma membrane-resident PA is only required for the regulated secretion of prothrombotic VWF and not for the regulated secretion of profibrinolytic tPA. This provides strong support for the idea that molecularly distinct machineries supervise different Ca2+-triggered secretion events in endothelial cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Inés Rojo Pulido and Klaus Ebnet for discussions and comments on the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 629; GK 1050) and the Interdisciplinary Center for Clinical Research of the Münster Medical School.
Authorship
Contribution: J.D. performed research, analyzed data, and wrote parts of the manuscript; N.V. and M.-F.B. prepared vital reagents and wrote parts of the manuscript; and V.G. designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Volker Gerke, Institute of Medical Biochemistry, Centre for Molecular Biology of Inflammation, University of Münster, Von-Esmarch-Str 56, 48149 Münster, Germany; e-mail: gerke@uni-muenster.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal