Abstract
Red cell transfusions are associated with the development of acute lung injury in the critically ill. Recent evidence suggests that storage induced alterations of the red blood cell (RBC) collectively termed the “storage lesion” may be linked with adverse biologic consequences. Using a 2-event model of systemic endotoxemia followed by a secondary challenge of RBC transfusion, we investigated whether purified RBC concentrates from syngeneic C57BL/6 mice altered inflammatory responses in murine lungs. Transfusion of RBCs stored for 10 days increased neutrophil counts, macrophage inflammatory protein-2 (MIP-2) and chemokine (KC) concentrations in the airspaces, and lung microvascular permeability compared with transfusion of less than 1-day-old RBCs. Because RBCs have been shown to scavenge inflammatory chemokines through the blood group Duffy antigen, we investigated the expression and function of Duffy during storage. In banked human RBCs, both Duffy expression and chemokine scavenging function were reduced with increasing duration of storage. Transfusion of Duffy knockout RBCs into Duffy wild-type en-dotoxemic mice increased airspace neutrophils, inflammatory cytokine concentrations, and lung microvascular permeability compared with transfusion of Duffy wild-type RBCs. Thus, reduction in erythrocyte chemokine scavenging is one functional consequence of the storage lesion by which RBC transfusion can augment existing lung inflammation.
Introduction
Although red cell transfusions have been a mainstay in the treatment of critically ill patients, recent studies have shown an association between packed red blood cell (RBC) transfusion and increased morbidity and mortality in this cohort.1-4 Supported by the initial observations of Fowler et al,5 recent epidemiologic data collectively suggest that RBC transfusion is a predisposing condition for the development of lung injury in the critically ill population at risk for acute respiratory disease syndrome (ARDS). Indeed, in a cohort of critically ill patients with acute lung injury (ALI), the risk of mortality increased with the number of RBC units transfused, suggesting that each transfusion serves as another “hit” or insult perpetuating lung inflammation and injury.6 Furthermore, RBC transfusion was found to be an independent predictor for the development of and mortality from ARDS in a prospective study of patients at risk for ARDS,6 and a predictor of multiple organ failure (MOF) in a cohort of trauma patients.7 Although the present epidemiologic and observational studies highlight an important clinical association between red cell transfusion and ALI, the mechanisms underlying this association have yet to be fully elucidated
Multiple factors may contribute to the development of lung injury after transfusion in at-risk patients. Allogenic leukocytes of RBC transfusates have long been implicated as a source of immunomodulatory effects.8,9 However clinical trials of leukoreduction have shown conflicting results.8-10 Recent data from patients given packed RBCs during cardiac surgery shows that the risk of mortality rises with increasing age of RBCs being transfused.11 This lends credence to the concept that biochemical and morphologic alterations that RBCs undergo with storage (ie, the “storage lesion”) contribute to morbidity associated with RBC transfusion.
Independent of storage, prior studies illustrate that erythrocytes have the potential to induce biologic activity through various mechanisms. For example, erythrocytes form advanced glycation end products over time, convert eicosinoid mediators, generate reactive oxygen species, and possess chemokine binding activity that can modulate an existing inflammatory state.12-17 However, the role of RBC transfusates in the perpetuation or amplification of existing inflammatory responses during critical illness remains unknown.
We have previously shown that erythrocyte Duffy antigen can scavenge chemokines in whole blood stimulated with endotoxin.18 First recognized as the receptor for the parasite Plasmodium vivax, the Duffy antigen is a minor blood group antigen that binds multiple inflammatory CXC and CC chemokines with high affinity.12,14,19,20 Intracellular calcium flux does not occur upon chemokine binding to erythrocyte Duffy antigen, and chemokines bound to erythrocytes are not accessible to circulating neutrophils.12 During inflammatory states, we have also shown that erythrocyte Duffy antigen has a more prominent role than endothelial Duffy antigen in chemokine clearance from the lung microvasculature to the systemic circulation.18 Prior observations of others suggest that RBC Duffy antigen expression may be reduced under standard blood bank conditions.21 We hypothesized that storage related alterations in Duffy antigen is one mechanism by which RBC transfusion can have adverse biologic consequences by modulating existing inflammatory responses in the lung microvasculature.
Methods
Human study subjects
Written informed consent for the studies was obtained from healthy volunteers between the ages of 18 and 65 years in accordance with the Declaration of Helsinki. All experimental procedures involving human subjects or use of human packed RBCs (PRBCs) were approved by the University of Pittsburgh Institutional Review Board.
Isolation of human erythrocytes
Whole blood was obtained via venipuncture, and Citrate-Phosphate-Dextrose-Adenine (CPDA; Sigma-Aldrich, St Louis, MO) solution was added to the whole blood (1 mL CPDA: 9 mL whole blood). Erythrocytes were purified using modifications of a technique described by Beutler et al22 The whole blood was passed over 2 Sephadex G25: Microcellulose (1:3; Sigma-Aldrich) columns in tandem. The columns were washed with 10× the volume of sterile phosphate-buffered saline (PBS; Baxter Healthcare, Deerfield, IL). The eluent was centrifuged at 1000g for 4 minutes at 4°C. The erythrocyte pellet was washed 3 times with cool sterile PBS. The RBC concentrates were stored at 4°C in 50-mL polypropylene tubes (BD Biosciences, San Jose, CA).
Surface expression of erythrocyte Duffy antigen
PRBCs stored in additive solution (AS)-5 were obtained from the Institute for Transfusion Medicine (Pittsburgh, PA) and stored for various time periods (days 1-54). Human erythrocytes from healthy volunteers were purified and stored in AS-1 at 4°C for 11 and 31 days. Totals of 106 erythrocytes were suspended in fluorescence-activated cell sorting (FACS) buffer (2% fetal bovine serum [FBS]) and incubated with 1 μg anti-Fy6 mouse monoclonal antibody (BD Biosciences-Pharmingen, San Jose, CA) or IgG1 isotype control (R&D Systems, Minneapolis, MN) for 30 minutes on ice in the dark. The cells were washed 3 times and resuspended in FACS buffer. The erythrocytes were subsequently labeled with 1 μg fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse F(ab′)2 (Jackson ImmunoResearch Laboratories,West Grove, PA) for 30 minutes. The cells were again washed 3 times. FACS analysis was performed (FACSCalibur; BD Biosciences). Data were analyzed using FCS Express Software (De Novo Software, Los Angeles, CA).
Experimental animals
C57BL/6J animals were purchased from The Jackson Laboratory (Bar Harbor, ME). The generation and breeding of Duffy knockout (KO) mice has been previously described.18,23 All experimental procedures were performed in 8- to 12-week-old male mice. Animal studies were conducted in accordance with the Institutional Animal Care and Use Committee at the University of Pittsburgh. The animals were housed and maintained in a pathogen-free environment by the Department of Laboratory and Animal Research at the University of Pittsburgh.
Isolation of murine erythrocytes
C57BL/6J or KO mice were euthanized with intraperitoneal pentobarbital (120 mg/kg). Whole blood was drawn via intracardiac puncture and collected into sterile 1-mL tuberculin syringes. CPDA (Sigma-Aldrich) solution was immediately added to the whole blood (1:9). The whole blood was passed over 2 Sephadex G25: Microcellulose (1:3; Sigma-Aldrich) columns in tandem. The columns were washed with 10× the volume of sterile PBS (Baxter Healthcare Corporation). The eluent was centrifuged at 1000g for 4 minutes at 4°C. The erythrocyte pellet was washed 3 times with cool sterile PBS and concentrated to a hematocrit of 60% to 70% and stored in 50-mL polypropylene tubes (BD Biosciences) at 4°C until transfusion. Viability was assessed before transfusion using Trypan blue. For in vivo studies using less than 1-day-old versus 10-day-old stored blood, erythrocytes were purified as detailed above, and stored in AS-5 (Terumo, Somerset, NJ) at 4°C at a ratio of 3 parts RBC to 1 part AS-5.
Erythrocyte chemokine binding assays
Erythrocyte chemokine binding was assayed using modifications of the techniques of Darbonne et al.12 For studies involving human erythrocytes stored under blood bank conditions, erythrocyte samples were obtained from PRBC units containing AS-5 (Institute for Transfusion Medicine). Six units of PRBC were sampled on days 12 and 27 of storage. Just before day of experiment, RBCs were isolated and purified through sephadex-microcellulose columns as previously described. Chemokine binding studies were performed within 24 hours of RBC purification. For studies using RBCs from human volunteers, RBCs were purified as described above and stored for 1 or 13 days.
Erythrocytes were counted manually and then incubated for 30 minutes at room temperature with chemokine binding buffer (Dulbecco modified Eagle medium [DMEM] with 0.1% human serum albumin [HSA]) in the presence or absence of 2 nM murine or human recombinant CCL2, murine macrophage-inflammatory protein-2 (MIP-2), human CXCL8, or CXCL1 (PeproTech, Rocky Hill, NJ). The erythrocyte plus chemokine suspension was layered upon a 30% sucrose cushion and centrifuged for 3 minutes at 13 000g. The supernatant was collected and frozen at −80°C. The erythrocyte pellet was lysed using red cell lysis buffer (500 mM NaCl, 0.1% Triton X-100, and 0.25% BSA) and then frozen at −80°C. Chemokines contained in the supernatant or cell lysates were measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems).
Animal preparation and experimental protocol
Tail vein injection of lipopolysaccharide.
Mice were injected with 1.5 mg/kg lipopolysaccharide (LPS; from Escherichia coli 011:B4; List Biological Laboratories, Campbell, CA) via tail vein.
Transfusion of erythrocytes.
Murine PRBCs were passively warmed to room temperature before transfusion. C57BL/6J mice were transfused 8 μL/g erythrocytes, sterile PBS or sterile Hextend via tail vein. For studies involving both LPS and transfusate administration, mice received 8 μL/g erythrocytes from C57BL/6J mice (< 1-day-old RBCs, 1- to 2-day-old RBCs, or 10-day-old RBCs), KO mice (1- to 2-day-old RBCs), or 8 μL/g sterile Hextend 2 hours after systemic LPS administration. Where specified, less than 1-day-old RBCs and 10-day-old RBCs were washed twice with 20× the volume of cool sterile PBS and concentrated to a Hct of 60% immediately before transfusion.
Mouse necropsies, bronchoalveolar lavage, cell counts, and total protein measurements
Animals were killed at specified time points with 120 mg/kg intraperitoneal pentobarbital. Mouse necropsy, lung tissue processing, and bronchoalveolar lavage (BAL) have been previously described in detail.18,24 Cell counts and differentials were determined by counting 200 consecutive cells from cytospin preparations stained with Diff Quik (Fisher Scientific, Pittsburgh, PA). BAL total protein measurements were performed using the Bradford Assay (Thermo Fisher Scientific, Rockford, IL).
Lung histology.
The whole lung was inflated with 3 mL of 4% paraformaldehyde, and paraffin embedded. Hematoxylin and eosin (H&E) staining was performed on sections cut at 4 to 6 μm thick. Images were acquired using Axiocam software (Carl Zeiss MicroImaging, Thornwood, NY). Immunofluorescence was performed using rat anti–mouse GR-1 antibody (Cedarlane Laboratories, Burlington, NC), rat IgG as control (Santa Cruz Biotechnology) and goat anti–rat Alexa 488–conjugated secondary antibody. Images were acquired using an Olympus Provus Ax70 microscope (Olympus, Center Valley, PA).
Lung myeloperoxidase determination.
Lung myeloperoxidase (MPO) activity was determined using the method described by Goldblum et al.25 The left lung was homogenized in 50 mM potassium phosphate buffer (pH 6.0) with 0.5% hexadecyltrimethylammonium bromide (HTAB; Sigma-Aldrich) on ice. The homogenate was centrifuged at 3000g for 10 minutes. The pellet was rehomogenized, sonicated, and then freeze-thawed (3 times). The suspension was centrifuged at 9500g for 20 minutes. The superna-tant was assayed with 50 mM potassium phosphate buffer containing 16.7 mg/mL o-dianisidinedihydrochloride (Sigma-Aldrich) and hydrogen peroxide. Absorbance at 460 nm at 10 minutes was measured using a spectrophotometer.
Plasma and BAL cytokine determination.
Plasma and BAL cytokine concentrations were measured using a Multiplex Kit (R&D Systems).
Statistics
Results are reported as the mean plus or minus standard error of the mean (SEM). Two-tailed Student t test, Mann-Whitney rank sum, analysis of variance (ANOVA), or Spearman rank order correlation was used where appropriate to determine significance. Statistics were performed using SigmaPlot 10 software (Systat Software, Chicago, IL). P values less than or equal to .05 were determined to be significant.
Results
Transfusion of RBC concentrates does not alter lung inflammation in unstimulated mice
We developed a method of purification of erythrocytes using modifications of a technique described by Beutler et al22 that removes platelets and leukocytes. This method resulted in greater than 99% pure erythrocytes, and did not yield significant LPS contamination in PRBCs (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). We examined pH, K+, lactate, 2,3-DPG, and free hemoglobin (Hgb) levels in stored murine erythrocytes. The pH and free Hgb levels in stored murine purified RBCs were comparable to levels in banked human blood at later time points (Table S1). The 2,3-DPG levels in murine blood were higher than in human blood.
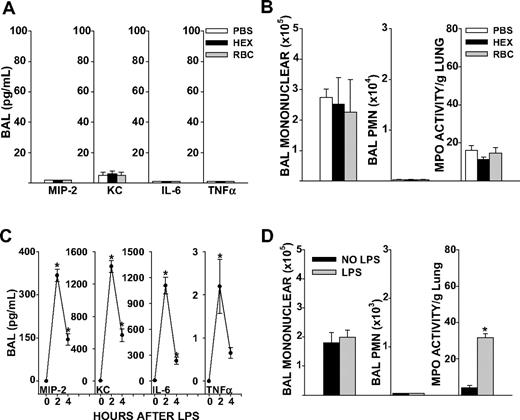
C57BL/6J mice wild-type (WT) for the Duffy antigen and naive to any prior stimulus were transfused 8 μL/g purified syngeneic erythrocytes stored for 1 to 2 days. This volume per weight of blood approximated 2 units of RBCs (560 mL/70-kg person). To control for the colloid effect of volume infused, mice were also transfused with sterile 6% Hetastarch in lactated electrolyte injection (Hextend), an infusate used for rapid volume expansion in the critical care setting. Transfusion of 1- to 2-day-old RBCs did not increase BAL cytokines, airspace neutrophil counts, or total neutrophil content in the lungs (as measured by MPO activity) of unstimulated mice 4 hours after transfusion, compared with PBS or Hextend (HEX; Figure 1A,B). Based upon these observations, we concluded that our method of RBC purification did not elicit inflammatory responses in vivo. HEX infusion elicited similar responses in lung cytokines, airspace neutrophil counts, and MPO activity as sterile PBS transfusion (Figure 1A,B). Therefore, HEX was chosen as the control infusate for subsequent experiments.
Lung inflammatory responses after RBC transfusion or systemic LPS in naive mice. (A) BAL cytokine concentrations in PBS, HEX, and RBC transfused groups in unstimulated mice (P ≤ .999 for all cytokines measured). (B) BAL total mononuclear cell counts (P = .930), total neutrophil counts (P = .815) or lung MPO activity (P = .294). (C) BAL cytokines MIP-2, KC, and IL-6 were elevated from baseline at 2 and 4 hours after LPS injection (P = .024 for MIP-2, KC, and IL-6 at 2 hours, P = .012 for MIP-2, P = .002 for KC, and P = .009 for IL-6 at 4 hours). TNFα concentrations in the BAL were significantly elevated 2 hours after LPS injection, but not at 4 hours (P ≤ .048 at 2 hours, P = .630 at 4 hours). (D) BAL total mononuclear cells (P = .658) and neutrophils 4 hours after LPS injection (P ≤ .999). Lung MPO activity was increased from baseline at 4 hours after tail vein LPS injection (P < .001). Data represent 3 independent experiments; n = 9-12 animals per group.
Lung inflammatory responses after RBC transfusion or systemic LPS in naive mice. (A) BAL cytokine concentrations in PBS, HEX, and RBC transfused groups in unstimulated mice (P ≤ .999 for all cytokines measured). (B) BAL total mononuclear cell counts (P = .930), total neutrophil counts (P = .815) or lung MPO activity (P = .294). (C) BAL cytokines MIP-2, KC, and IL-6 were elevated from baseline at 2 and 4 hours after LPS injection (P = .024 for MIP-2, KC, and IL-6 at 2 hours, P = .012 for MIP-2, P = .002 for KC, and P = .009 for IL-6 at 4 hours). TNFα concentrations in the BAL were significantly elevated 2 hours after LPS injection, but not at 4 hours (P ≤ .048 at 2 hours, P = .630 at 4 hours). (D) BAL total mononuclear cells (P = .658) and neutrophils 4 hours after LPS injection (P ≤ .999). Lung MPO activity was increased from baseline at 4 hours after tail vein LPS injection (P < .001). Data represent 3 independent experiments; n = 9-12 animals per group.
Systemic LPS increases lung cytokine responses and neutrophil entrapment but does not promote accumulation of neutrophils in the airspaces
LPS administration by tail vein produced a systemic inflammatory response marked by hypothermia and elevated systemic cytokines (Figure S2A,B). Local activation of cytokines occurred in the lungs, as marked by increases in BAL MIP-2, keratinocyte-derived chemokine (KC), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α; P < .05, Figure 1C). However, mice showed no neutrophils in the airspaces 4 hours after onset of endotoxemia, as determined by BAL polymorphonuclear (PMN) cell counts (Figure 1D). This was despite increases in lung MPO activity, a marker of total lung neutrophil content, from baseline levels. Thus, systemic LPS increased lung cytokine responses and neutrophil entrapment in the microvasculature, but an additional stimulus was required for neutrophil migration into the airspaces to occur.
Erythrocyte transfusion increases BAL chemokine MIP-2 and airspace neutrophil recruitment during endotoxemia
We next determined whether RBC transfusion can modulate existing inflammatory responses. We developed a 2-event model whereby RBC transfusion was administered after the onset of systemic endotoxemia. We transfused mice 2 hours after systemic LPS challenge with either colloid control infusion or purified RBCs stored for 1 or 2 days. While plasma concentrations of MIP-2 showed an early increase at 2 hours (994 vs 551 pg/mL, P = .044; Figure S3A) and KC showed a delayed increase at 16 hours after RBC transfusion compared with HEX control (20 741 vs 9639 pg/mL, P = .035; Figure S3B), there were no significant differences in plasma chemokine concentrations 4 hours after transfusion. In the lung compartment, however, BAL MIP-2 was higher in the RBC group compared with HEX controls 4 hours after transfusion (50 vs 15 pg/mL, P = .009; Figure 2A). The increase in BAL MIP-2 concentrations was associated with a 9-fold increase in airspace neutrophils in the 1- to 2-day-old RBC group compared with HEX controls (2433 vs 255, P < .001; Figure 2B). In contrast, total neutrophil content in the lungs, as measured by MPO activity in homogenates, was not measurably different between the 2 groups (Figure 2B). Lung microvascular permeability, as measured by BAL total protein, was higher in the RBC transfused group but this difference was not significant (411 vs 358 μg/mL, P = .122; data not shown). Immunofluorescence staining with anti-Gr1 of the lungs 4 hours after RBC transfusion confirmed increased numbers of neutrophils located within the vessels, interstitium, and airspaces compared with the HEX transfused group (Figure 2C,D). Thus, transfusion of 1- to 2-day-stored RBCs increased airspace concentrations of the Duffy ligand MIP-2 and was associated with detectable increases in neutrophil counts during endotoxemia.
BAL cytokines, airspace neutrophil counts, and lung MPO activity in endotoxemic mice 4 hours after transfusion of 1- to 2-day-old, stored RBC. (A) RBC transfusion increased BAL MIP-2 compared with HEX controls (P = .009). BAL KC, IL-6, and TNFα were not increased after RBC transfusion (P = .232, .281, and .955, respectively). (B) Total BAL mononuclear cell counts were not different between the 2 groups (P = .601). RBC transfusion increased airspace neutrophils compared with HEX (2433 vs 255, P < .001). Lung MPO activity was similar between the 2 groups. (C,D) GR-1 staining of lung sections from animals transfused with 1- to 2-day-old RBCs show increased interstitial and airspace neutrophils compared with HEX transfused controls. Magnification ×400. Data are representative of 3 independent experiments; n = 9-12 animals per group.
BAL cytokines, airspace neutrophil counts, and lung MPO activity in endotoxemic mice 4 hours after transfusion of 1- to 2-day-old, stored RBC. (A) RBC transfusion increased BAL MIP-2 compared with HEX controls (P = .009). BAL KC, IL-6, and TNFα were not increased after RBC transfusion (P = .232, .281, and .955, respectively). (B) Total BAL mononuclear cell counts were not different between the 2 groups (P = .601). RBC transfusion increased airspace neutrophils compared with HEX (2433 vs 255, P < .001). Lung MPO activity was similar between the 2 groups. (C,D) GR-1 staining of lung sections from animals transfused with 1- to 2-day-old RBCs show increased interstitial and airspace neutrophils compared with HEX transfused controls. Magnification ×400. Data are representative of 3 independent experiments; n = 9-12 animals per group.
Effect of erythrocyte storage duration on airspace PMN and lung microvascular permeability
We determined whether increased duration of RBC storage was associated with increased airspace neutrophil migration and lung microvascular permeability. It has previously been shown that rat erythrocytes stored for 1 week show storage related alterations similar to human erythrocytes stored for 4 weeks, suggesting that the RBC storage lesion occurs more rapidly in rodent erythrocytes than human erythrocytes.26 Given these concerns and our own observations of accelerated lysis of murine RBCs beyond 14 days, we chose to transfuse murine RBCs stored for 10 days. Mice transfused with 10-day-old RBCs showed increased airspace neutrophil counts, compared with mice transfused with RBC stored for less than 1 day (2748 vs 236, P = .015, Figure 3A). This increase in airspace neutrophil counts was associated with increased lung microvascular permeability (Figure 3B). Washing the purified RBCs immediately before transfusion did not abrogate either the increases in airspace neutrophil counts or lung microvascular permeability (Figure 4A,B), suggesting that the effect is related to the property of the RBC itself. Furthermore, transfusion of washed 10-day-old RBCs during endotoxemia increased BAL concentrations of the CXC chemokines MIP-2 and KC (927 vs 227 pg/mL, P = .021 for MIP-2; 4371 vs 2735 pg/mL, P < .001 for KC; Figure 4C). Collectively, these findings indicate that storage dependent alterations of the erythrocyte can promote lung injury during endotoxmia.
BAL cell counts, lung MPO activity, and microvascular permeability 4 hours after transfusion of unwashed 10-day-old RBCs in endotoxemic mice. (A) Transfusion of 10-day-old RBCs increased airspace neutrophil recruitment compared with transfusion of less than 1-day-old RBCs (P = .015). Lung MPO activity was similar between the 2 groups. (B) Transfusion of 10-day-old RBCs increased lung microvascular permeability compared with transfusion of less than 1-day-old RBCs (P = .021). Data are representative of 3 individual experiments; n = 9 animals in each group.
BAL cell counts, lung MPO activity, and microvascular permeability 4 hours after transfusion of unwashed 10-day-old RBCs in endotoxemic mice. (A) Transfusion of 10-day-old RBCs increased airspace neutrophil recruitment compared with transfusion of less than 1-day-old RBCs (P = .015). Lung MPO activity was similar between the 2 groups. (B) Transfusion of 10-day-old RBCs increased lung microvascular permeability compared with transfusion of less than 1-day-old RBCs (P = .021). Data are representative of 3 individual experiments; n = 9 animals in each group.
BAL cell counts, lung MPO activity, microvascular permeability, and BAL cytokines 4 hours after transfusion of washed 10-day-old RBCs in endotoxemic mice. Washing erythrocytes immediately before transfusion did not abrogate lung injury after transfusion of 10-day-old RBCs. (A) Transfusion of washed 10-day-old RBCs increased airspace neutrophil recruitment compared with transfusion of RBCs stored for less than 1 day (P = .007). (B) Transfusion of washed 10-day-old RBCs increased lung microvascular permeability compared with transfusion of less than 1-day-old RBCs (P = .039). (C) BAL MIP-2 and KC were increased 4 hours after transfusion of washed 10-day-old RBCs (P = .021 and P < .001 for MIP-2 and KC, respectively). Data are representative of 2 independent experiments; n = 8 animals in each group.
BAL cell counts, lung MPO activity, microvascular permeability, and BAL cytokines 4 hours after transfusion of washed 10-day-old RBCs in endotoxemic mice. Washing erythrocytes immediately before transfusion did not abrogate lung injury after transfusion of 10-day-old RBCs. (A) Transfusion of washed 10-day-old RBCs increased airspace neutrophil recruitment compared with transfusion of RBCs stored for less than 1 day (P = .007). (B) Transfusion of washed 10-day-old RBCs increased lung microvascular permeability compared with transfusion of less than 1-day-old RBCs (P = .039). (C) BAL MIP-2 and KC were increased 4 hours after transfusion of washed 10-day-old RBCs (P = .021 and P < .001 for MIP-2 and KC, respectively). Data are representative of 2 independent experiments; n = 8 animals in each group.
Transfusion of Duffy KO erythrocytes amplifies lung inflammation in endotoxemic mice
We have previously shown that the erythrocyte membrane protein Duffy antigen scavenges chemokines from the lung microvasculature during inflammatory states.18 Elevated BAL concentrations of the CXC chemokines MIP-2 and KC in mice transfused with 10-day-old RBCs suggested that stored erythrocytes do not scavenge chemokines as well as native erythrocytes.
We determined whether complete loss of chemokine scavenging on RBC transfusates (ie, transfusion of Duffy KO erythrocytes) could further augment lung inflammatory responses. Endotoxemic mice were transfused with either Duffy KO or WT RBC concentrates stored for 1 or 2 days and studied 4 hours after transfusion. In the lung compartment, transfusion with KO RBCs significantly increased BAL MIP-2, KC, IL-6, and TNF-α (P < .05 for all comparisons, Figure 5A) and airspace neutrophil counts (22 864 vs 1434 cells, P < .001, Figure 5B) compared with animals transfused with Duffy WT RBCs. This increase in airspace neutrophil migration was also associated with an increase in lung microvascular permeability (P = .034, Figure 5C). Thus, during endotoxemia, the complete loss of Duffy chemokine scavenging on RBC transfusates further augmented existing lung inflammatory responses and increased microvascular permeability.
Transfusion of WT or Duffy KO RBCs during endotoxemia. Mice were transfused with 1- to 2-day-old KO or WT RBCs 2 hours after LPS administration. (A) Transfusion of KO RBCs increased BAL cytokines 4 hours after transfusion compared with WT RBC transfusion (P = .008, .022, .022, and .013 for MIP-2, KC, IL-6, and TNF-α, respectively). (B) Total BAL mononuclear cells were similar between the KO and WT RBC transfused animals (P = .233). There was a significant increase in airspace neutrophil counts in the KO RBC transfused animals compared with WT RBC transfused animals (P < .001). (C) Transfusion of KO RBCs increased lung microvascular permeability compared with transfusion of WT RBCs (P = .034). Data are representative of 3 individual experiments; n = 12 in each experimental group.
Transfusion of WT or Duffy KO RBCs during endotoxemia. Mice were transfused with 1- to 2-day-old KO or WT RBCs 2 hours after LPS administration. (A) Transfusion of KO RBCs increased BAL cytokines 4 hours after transfusion compared with WT RBC transfusion (P = .008, .022, .022, and .013 for MIP-2, KC, IL-6, and TNF-α, respectively). (B) Total BAL mononuclear cells were similar between the KO and WT RBC transfused animals (P = .233). There was a significant increase in airspace neutrophil counts in the KO RBC transfused animals compared with WT RBC transfused animals (P < .001). (C) Transfusion of KO RBCs increased lung microvascular permeability compared with transfusion of WT RBCs (P = .034). Data are representative of 3 individual experiments; n = 12 in each experimental group.
RBC chemokine scavenging is reduced with storage
To determine whether RBC chemokine scavenging is reduced with storage, we measured the ability of RBCs of varying storage duration to scavenge soluble chemokine in vitro. Murine erythrocytes incubated with 2 nM recombinant CCL2 showed a time-dependent loss of chemokine scavenging function during storage (ie, higher soluble CCL2 concentrations; Figure 6A). To confirm alterations in soluble chemokine concentrations was Duffy-dependent, we incubated 2 nM recombinant CCL2 with Duffy KO or WT erythrocytes stored for either 11, 17, or 21 days. We did not examine surface expression of Duffy antigen on stored murine erythrocytes due to lack of a reliable antibody recognizing murine Duffy antigen. Duffy WT RBCs showed reduced chemokine scavenging (ie, higher soluble CCL2 concentrations) with increased storage time; soluble chemokine concentrations reached levels closer to those of Duffy KO RBCs (P = .006; Figure 6B). Consistent with these findings, there was a reduction in membrane associated chemokine as erythrocyte lysate chemokine concentrations decreased with storage time (P = .011, Figure 6B). Because Duffy is a promiscuous chemokine binding protein, we also tested RBC chemokine scavenging of MIP-2, a CXC neutrophilic chemokine. We noted similar reductions in chemokine scavenging (ie, higher soluble MIP-2 concentrations) by WT erythrocytes over time, with MIP-2 reaching levels closer to those of Duffy KO RBCs (Figure 6C).
Chemokine scavenging function of murine erythrocytes. (A) A total of 108 erythrocytes with differing storage times were incubated with 2 nM CCL2. CCL2 concentrations in supernatant increased with RBC storage time (P < .001). (B) A total of 108 WT or KO erythrocytes were incubated with 2 nM CCL2. For chemokine incubated with WT erythrocytes, CCL2 concentrations increase in the supernatant and decrease in the lysate over time, (+ indicates P = .006 and P = .011 for WT supernatant and lysates, respectively; P was not significant for KO). There were significant differences in CCL2 between WT and KO erythrocytes (* indicates P = .027 and P = .012 for supernatatant from 11- and 21-day-old erythrocytes; P = .057 from 17-day-old erythrocytes; lysates P < .001 for all days). (C) A total of 108 WT or KO erythrocytes were incubated with 2 nM MIP-2 for 30 minutes. (P < .001 for WT erythrocytes and P = .102 for KO erythrocytes). There were significant differences in supernatant MIP-2 between WT and KO erythrocytes that were stored for 1, 2, 9, or 12 days (P = .002, P = .019, P < .001, and P = .002, respectively), but not when erythrocytes were stored for 16, 23, or 26 days (P = .091, P = .200, P = .642, respectively).
Chemokine scavenging function of murine erythrocytes. (A) A total of 108 erythrocytes with differing storage times were incubated with 2 nM CCL2. CCL2 concentrations in supernatant increased with RBC storage time (P < .001). (B) A total of 108 WT or KO erythrocytes were incubated with 2 nM CCL2. For chemokine incubated with WT erythrocytes, CCL2 concentrations increase in the supernatant and decrease in the lysate over time, (+ indicates P = .006 and P = .011 for WT supernatant and lysates, respectively; P was not significant for KO). There were significant differences in CCL2 between WT and KO erythrocytes (* indicates P = .027 and P = .012 for supernatatant from 11- and 21-day-old erythrocytes; P = .057 from 17-day-old erythrocytes; lysates P < .001 for all days). (C) A total of 108 WT or KO erythrocytes were incubated with 2 nM MIP-2 for 30 minutes. (P < .001 for WT erythrocytes and P = .102 for KO erythrocytes). There were significant differences in supernatant MIP-2 between WT and KO erythrocytes that were stored for 1, 2, 9, or 12 days (P = .002, P = .019, P < .001, and P = .002, respectively), but not when erythrocytes were stored for 16, 23, or 26 days (P = .091, P = .200, P = .642, respectively).
Human erythrocyte chemokine scavenging and Duffy antigen expression are reduced with storage
We determined whether the observed storage dependent alterations in murine RBC chemokine scavenging occurred in human RBCs. We measured the ability of purified human RBCs stored for various time points to scavenge soluble chemokine (Figure 7A). We observed an increase in soluble CCL2 concentrations with increased erythrocyte storage time (Figure 7A). This was associated with reduced membrane-bound chemokine, as erythrocyte cell lysates showed lower CCL2 concentrations with increased storage time (Figure 7A). We next tested the ability of PRBCs obtained from the blood bank to scavenge CXC chemokines (Figure 7B). Erythrocyte chemokine scavenging of CXCL1 was significantly reduced (P = .008) where as scavenging of CXCL8 was unaltered (Figure 7B).
Chemokine scavenging function and Duffy antigen expression in stored human erythrocytes. (A) A total of 108 erythrocytes from a volunteer were incubated with 2 nM CCL2 for 30 minutes (P = .002 for supernatant and P = .021 for cell lysates). (B) A total of 108 erythrocytes from PRBC units were incubated with 2 nM CXCL1 or CXCL8. Increased soluble CXCL1 concentrations in 27-day-old RBCs compared with 12-day-old RBCs (P = .008); no difference in soluble CXCL8 concentrations (P = .999). (C) Duffy antigen expression on erythrocytes from banked blood (11 or 31 days old) compared with fresh erythrocytes isolated from healthy volunteers. (D) Storage time correlates with reduction in Duffy antigen expression on banked erythrocytes (P = .021).
Chemokine scavenging function and Duffy antigen expression in stored human erythrocytes. (A) A total of 108 erythrocytes from a volunteer were incubated with 2 nM CCL2 for 30 minutes (P = .002 for supernatant and P = .021 for cell lysates). (B) A total of 108 erythrocytes from PRBC units were incubated with 2 nM CXCL1 or CXCL8. Increased soluble CXCL1 concentrations in 27-day-old RBCs compared with 12-day-old RBCs (P = .008); no difference in soluble CXCL8 concentrations (P = .999). (C) Duffy antigen expression on erythrocytes from banked blood (11 or 31 days old) compared with fresh erythrocytes isolated from healthy volunteers. (D) Storage time correlates with reduction in Duffy antigen expression on banked erythrocytes (P = .021).
Several plausible reasons exist as to why alterations in RBC CXCL8 scavenging were not observed despite reductions in CCL2 and CXCL1 scavenging. CXCL8 may bind Duffy antigen with lower affinity than CCL2 or CXCL1. In cross-competition binding studies performed using a human endothelial cell line stably expressing the Duffy antigen, we have previously shown that the ability of CXCL1 and CCL2 to compete off radiolabeled CXCL1 from Duffy binding sites is greater than by CXCL8 (cold competitor Kd 5-7 vs 36 nM).27 Therefore, alterations in CXCL8 scavenging with storage duration may be difficult to observe without performing the study across multiple concentrations of ligand. Furthermore, reductions in RBC CXCL8 scavenging may occur either before or after the time period studied, and the comparisons made between D12 and D27 banked RBCs did not capture the difference. It is also possible that storage does not substantially reduce CXCL8 scavenging but it significantly impairs RBC binding to other neutrophilic chemokines such as CXCL1 as well as CC chemokines such as CCL2. This alternative scenario could occur if storage induces changes in the tertiary structure of the Duffy antigen (ie, oxidative modification, nonenzymatic glycosylation) that differentially affects the chemokine binding pockets for CXCL1 and CXCL8. Further studies to determine alterations in erythrocyte chemokine scavenging would necessitate formal ligand binding studies to calculate the Kd and number of receptor binding sites with duration of storage for the different chemokines mentioned.
To assess whether the observed reduction in Duffy function was associated with alterations in surface expression, we examined Duffy antigen expression on purified RBCs from volunteers or from RBC units using Fy6 monoclonal antibody (Figure 7C,D). There was detectable reduction in Duffy antigen expression on RBCs from different volunteers with increasing duration of storage (Figure 7C). Loss of Duffy antigen expression on banked RBCs from unit bags correlated with increased duration of storage (r = −0.762, P = .021; Figure 7D), although absolute reduction was modest.
Discussion
Several major conclusions can be drawn from the findings presented in this study. In the presence of systemic and local inflammatory states, transfusion of purified stored syngeneic RBCs can elicit a modest increase in lung MIP-2 concentrations and provide a secondary signal for entrapped neutrophils in the lung microvasculature to migrate into the airspaces. With increased duration of RBC storage, there is increase in BAL CXC chemokines, airspace neutrophil accumulation, and lung microvascular permeability. This response produced in the lungs after RBC transfusion appears to be related to the property of the RBC itself, as washing RBCs immediately before transfusion did not abrogate the response. Duffy antigen, a RBC transmembrane protein, showed impaired chemokine scavenging with increased duration of RBC storage. Furthermore, the complete loss of chemokine scavenging in RBC transfusates augmented existing lung inflammatory responses and promoted injury in recipients. These findings implicate the RBC storage lesion as a modulator of lung inflammatory responses and impaired erythrocyte chemokine scavenging as one functional consequence of this lesion.
Multiple aspects distinguish our model from prior studies of transfusion-related acute lung injury (TRALI). In a previous model, both the plasma and lipid fraction from stored platelets and RBC induced injury in perfused rat lungs ex vivo.28 In another model, TRALI was induced by major histocompatibility complex (MHC) class I antibodies in vivo.29 Our model is the first in vivo model to examine the unique properties of stored erythrocytes in modulating existing inflammatory states. Although our method of RBC storage and processing does not exactly replicate clinical RBC preservation conditions, our goal was to examine the properties of the RBC itself isolated from other constituents of RBC concentrates during storage and its effects in vivo after transfusion. There may be absolute time differences in duration of storage and degree of functional reduction in RBCs between mice and humans, but our findings in banked human RBC units showing reduction in chemokine scavenging with increasing duration of storage attest to the relevance of our murine model. The recent epidemiologic studies associating RBC transfusion with increased morbidity and mortality, and controversies regarding the use of stored blood in critically ill patients highlight the need for a clinically relevant model of PRBC transfusion during critical illness.11,30 Because this is not a model of severe ALI or a lethal one, our model may be a more relevant representation of what occurs in at risk populations in the critical care setting (ie, the majority of these patients are not diagnosed with TRALI by the current definition).
PRBCs undergo a series of changes collectively known as the storage lesion. Included in the storage lesion are alterations of the RBC membrane such as loss of discoid shape, vesicle formation, membrane protein oxidation, lipid peroxidation, and biochemical modifications such as loss of 2, 3-DPG.21,31-38 It has also been previously suggested that surface expression of erythrocyte Duffy antigen is decreased with RBC storage and Duffy antigen has been identified on exocytic vesicles from stored erythrocytes.39-42 Consistent with our findings, others have shown that banked human RBCs undergo loss of Duffy expression and chemokine scavenging function.21
In banked human RBC units, we found that the overall reduction in surface Duffy expression is modest and may not fully explain the significant reductions in chemokine scavenging of CXCL1 and CCL2. One possible explanation is that Duffy antigen undergoes modifications during storage which alter regions critical for chemokine binding function but that the linear epitope recognized by the Fy6 monoclonal antibody remains relatively preserved. This could also explain why scavenging of CXCL1 and CCL2 were significantly reduced but not CXCL8, as it would be expected that the chemokine binding “pocket” created by the tertiary structure is different for different ligands.
In the murine studies, the complete loss of erythrocyte chemokine scavenging function during endotoxemia results in significantly higher local chemokine concentrations. As RBCs traverse the lung microvasculature, the inability of a RBC to scavenge chemokines rapidly creates a local chemotactic gradient that favors entrapped neutrophils in the lung microvasculature to migrate into the airspaces. This is supported by significantly higher BAL chemokine concentrations in mice transfused Duffy KO erythrocytes and prior findings of Darbonne and colleagues12 that showed the ability of RBCs to deplete chemokine across the compartments of a diffusion chamber.
Loss of chemokine scavenging on stored RBCs may also account for the observed lung injury after transfusion of stored 10-day-old, washed RBCs. This is supported by the in vitro findings of impaired chemokine scavenging with increasing RBC storage time and the in vivo findings of elevated BAL chemokines in mice transfused 10-day-old RBCs compared with less than 1-day-old RBCs.
Alternatively, lung injury after RBC transfusion in endotoxemic animals may be secondary to a Duffy-independent mechanism. It is possible that biologic response modifiers other than Duffy present in the soluble fraction of RBC concentrates mediate the lung inflammatory response, as washing of RBCs immediately before transfusion may not have removed all soluble mediators present.43,44 Other storage induced alterations of the RBC may provoke an inflammatory response such as advanced glycation endproducts, which have been implicated in several disease states, and previously identified in stored RBCs.45 Free heme and hemoglobin may further incite inflammatory states in RBC-transfused animals. Heme has been shown to promote inflammatory responses in vitro and neutrophil migration in an in vivo model of peritonitis.46,47 Free hemoglobin released during hemolysis has been shown to induce endothelial cell dysfunction and vasculopathy.48-50 While we measured plasma free hemoglobin to assess the degree of hemolysis in transfused animals, we did not see significant differences in the groups examined at the 4-hour time point (data not shown). However, hemolysis byproducts in transfusates require further study, given its many biologic effects on the vascular endothelium and neutrophils.
Still another possible explanation is that the lung injury after RBC transfusion is secondary to mechanical injury and vascular occlusion. Reduced erythrocyte deformation, amplified echinocyte formation, and increased aggregability of transfused erythrocytes could lead to injury to the pulmonary vasculature. Furthermore, loss of S-nitrosohemoglobin in stored erythrocytes and an inability of transfused erythrocytes to vasodilate the local vasculature adequately may lead to increased ischemia, obstruction, and occlusion in the pulmonary microcirculation.51,52 These mechanisms are not mutually exclusive as transfusion of stored RBCs during endotoxemia may both directly incite injury (through multiple mechanisms) and sustain lung injury through an inability to clear excess chemokines.
In summary, we provide evidence that transfusion of stored erythrocytes amplify existing lung inflammation. We also demonstrate that increased duration of RBC storage can promote lung injury and implicate the loss of Duffy antigen as one functional consequence of the RBC storage lesion. RBC transfusion remains a modifiable risk factor for the development of ARDS in the critically ill. A greater understanding of the storage lesion as it relates to in vivo consequences is needed so that strategies to conserve RBC integrity during storage can be optimally addressed.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mr Frank Cornell at the Institute for Transfusion Medicine for providing the PRBCs and Drs Anuradha Ray and Prabir Ray for their critical review of this manuscript.
This work was supported by National Institutes of Health (Bethesda, MD) grants HL091644 (N.M.) and HL086884 (J.S.L.), and the University of Pittsburgh Department of Medicine Junior Scholar Award (J.S.L.).
National Institutes of Health
Authorship
Contribution: N.M. performed the research, analyzed the data, and wrote the paper; Z.X., T.O., and M.F. performed research and analyzed the data; M.H., M.R., X.H.L, and M.R. performed aspects of the research; D.T. provided vital reagents and interpreted data; A.C. analyzed and interpreted data; and J.S.L. conceived and designed the studies, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Janet S. Lee, University of Pittsburgh, NW628 MUH, 3459 Fifth Avenue, Pittsburgh, PA 15213; e-mail: leejs3@upmc.edu.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal