The mechanical fragility and healing problems in patients with EB provide interesting paradigms for tissue integrity and wound healing. In this issue of Blood, Tolar and colleagues report on how RDEB can be helped by specific subpopulations of cells (CD150+CD48−) derived from bone marrow.
Epidermolysis bullosa (EB) is a very heterogeneous genetic disease.1 Careful genetic analysis, immunomapping, and electron microscopy have yielded very detailed knowledge about the epithelial, basement membrane zone, and dermal molecules responsible for anchoring the cutaneous and mucosal epithelium to the underlying cells and structures. For example, intraepidermal mutations in keratins 5/14, plectin, can lead to EB simplex, with cleavage through the epidermis and milder disease. Mutations in laminin-332 (formerly laminin-5), type XVII collagen, and α6β4 integrin cause an often lethal form (junctional EB), with cleavage in the lamina lucida. Recessive dystrophic EB (RDEB) is due to mutations in type VII collagen (COL7A1), a molecule produced by keratinocytes and fibroblasts that comprises most of the fibrils anchoring the epidermis to the underlying dermis.
In individuals with RDEB, even mild trauma leads to the rapid development of blisters and wounds. Severe scarring is the rule, and invasive, metastasizing squamous cell carcinomas are common. Treatments are palliative and remain unsatisfactory. During the past few years, very exciting developments have occurred in the potential therapeutic approach to RDEB. Many of these findings have been surprising in that, when administered intradermally or systemically, the corrective collagen type VII protein appears to find its way to the subepidermal area of anchoring fibrils.2-4 However, Tolar et al make a valid point in stating that, because of the generalized nature of RDEB, the focus should remain on a systemic treatment approach. They provide a useful proof of principle that the administration of congenic bone marrow CD150+CD48− cells in a murine model of RDEB can partially rescue the affected animals, which normally die within 2 weeks after birth. The results are intriguing. The authors demonstrate clinical efficacy in RDEB mice and histologic evidence that this hematopoietic cell (HC) subpopulation, or possibly cells copurified with it, homes to the subepidermal area and helps reconstitute the anchoring fibrils. Mesenchymal stem cells (MSCs), which also produce type VII collagen, were not able to achieve positive results in RDEB mice. However, this conclusion is weakened by the fact that 8 to 10 times more CD150+CD48− cells as compared to MSCs were used. A similar argument can be made with the negative results the authors obtained with epidermal stem cells and transient-amplifying cells because these cell types were also used at a lower dose. The authors readily acknowledge these shortcomings; in some cases, cell size may have precluded larger doses because of infusional toxicity. Of course, one could make the argument that cell number, and not necessarily the cell subpopulation, is the critical factor. In fact, we have found that the dose of cultured autologous MSCs delivered topically to human chronic leg wounds directly correlates with healing; doses lower than 1.5×106 cells/cm2 are not effective.5 The possibility of a dose threshold for engraftment and effectiveness is real. Still, Tolar et al do show very interesting evidence for engraftment of CD150+CD48-, a subpopulation that contains true stem cells and is capable of giving rise to both HC and non-HC progeny.6 More work is needed to expand on these promising findings.
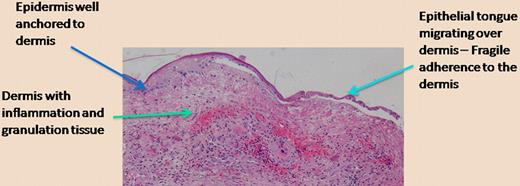
This type of work, while focused on a rare genetic disease that results in mechanical skin fragility and difficult-to-heal wounds, has ramifications for other aspects of tissue integrity and wound healing. A delicate balance exists between anchoring of epithelia (like in skin) to underlying structures, the necessity for cells to migrate upon tissue injury, and the traction required for a more “temporary” anchoring of cells as they migrate.7 The figure is offered as a telling histologic photomicrograph of a chronic wound in which the epidermis is finally migrating over the underlying dermis. As shown on the left side of the figure, away from the wound where the epidermis is thicker, one can expect that the anchoring of the epidermis to the dermis, for example, through type VII collagen, is strong. However, as the epidermis migrates, the keratinocytes need to free themselves from the hemidesmosomes and the structural proteins (including laminin-332, type VII collagen) that are so vital for tissue integrity. We believe that, given the emerging role of bone marrow cells in wound healing, we may ultimately be able to manipulate the contribution of specific marrow cells and alter this delicate balance as required.
Epidermal migration over a chronic wound. The photomicrograph shows the histology of a chronic wound in which the epidermis migrates over the inflamed and vascular dermis. The separation of the epithelial tongue from the underlying dermis is an artifact from tissue preparation, but it properly suggests that the anchoring is diminished in order to allow keratinocyte migration to occur. (H&E; ×200.)
Epidermal migration over a chronic wound. The photomicrograph shows the histology of a chronic wound in which the epidermis migrates over the inflamed and vascular dermis. The separation of the epithelial tongue from the underlying dermis is an artifact from tissue preparation, but it properly suggests that the anchoring is diminished in order to allow keratinocyte migration to occur. (H&E; ×200.)
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal