Abstract
Autologous stem cell transplantation (ASCT) as first-line therapy for follicular lymphoma (FL) remains controversial. The multicenter study randomized 172 patients with untreated FL for either immunochemotherapy or high-dose therapy (HDT) followed by purged ASCT. Conditioning was performed with total body irradiation (TBI) and cyclophosphamide. The 9-year overall survival (OS) was similar in the HDT and conventional chemotherapy groups (76% and 80%, respectively). The 9-year progression-free survival (PFS) was higher in the ASCT than the chemotherapy group (64% vs 39%; P = .004). A PFS plateau was observed in the HDT group after 7 years. On multivariate analysis, OS and PFS were independently affected by the per-formance status score, the number of nodal areas involved, and the treatment group. Secondary malignancies were more frequent in the HDT than in the chemotherapy group (6 secondary myelodysplastic syndrome/acute myeloid leukemia and 6 second solid tumor cancers vs 1 acute myeloid leukemia, P = .01). The occurrence of a PFS plateau suggests that a subgroup of patients might have their FL cured by ASCT. However, the increased rate of secondary malignancies may discourage the use of purged ASCT in combination with TBI as first-line treatment for FL. This trial has been registered with ClinicalTrials.gov under identifier NCT00696735.
Introduction
Follicular lymphomas (FLs) are a subgroup of B-cell non-Hodgkin lymphomas (NHLs) accounting for 15% to 30% of newly diagnosed lymphomas.1-3 The natural history of this disease is characterized by a long survival that contrasts with systematic relapses. Many chemotherapy regimens have been used for treatment but have not improved long-term outcome, which depends on initial prognostic factors such as the Follicular Lymphoma International Prognostic Index (FLIPI),4 response to first-line therapy, and minimal residual disease indicated by positivity of the bcl2 rearrangement on analysis of the polymerase chain reaction (PCR). Although new therapeutic approaches, including purine analogs and anti-CD20 monoclonal antibodies, have shown impressive response rates and prolonged progression-free survival (PFS), their effects on overall survival (OS) have yet to be confirmed. The major improvement in the management of FLs has been achieved by the use of the anti-CD20 monoclonal antibody rituximab, which improved OS in combination with chemotherapy in all 4 prospective, randomized studies published to date.5-9
Before the era of monoclonal antibodies, autologous stem cell transplantation (ASCT) was evaluated as an alternative approach to standard chemotherapy. Numerous studies have shown encouraging results for patients with relapsed follicular NHL,10-13 and the randomized European Cup Trial recently reported the superiority of ASCT in comparison with conventional chemotherapy in terms of PFS and OS.14
Only 4 randomized trials have been published to date, 3 of them comparing conventional chemotherapy without rituximab to ASCT as first-line therapy for FL.10,15,16 The fourth study, from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO), compared chemotherapy to ASCT with rituximab in both arms.17
In 2005 we published the results of the Groupe Ouest-Est d'Etude des Leucémies et Autres Maladies du Sang (GOELAMS) 064 trial, showing a higher overall response rate and longer median event-free survival (EFS) in the high-dose therapy (HDT) group then the conventional chemotherapy group after a median follow-up of 5 years, with no difference in OS but a higher rate of secondary malignancies in the HDT group.18 We report here the final results of this trial, after an extended follow-up period of 108 months (9 years).
Methods
The procedures were extensively described in the previous report of the trial17 and are briefly summarized below.
Patients
The GOELAMS 064 multicenter trial enrolled patients aged 18 to 60 years with previously untreated, histologically proven FL, classified according to the Working-Formulation criteria of the National Cancer Institute (B, C, or D lymphoma)19 and reviewed according to the Revised European-American Lymphoma (REAL) classification of the International Lymphoma Study Group (grades 1-3 center FL).20 Patients with grade 3B FL were included, whereas those displaying transformed lymphoma were excluded. Diagnostic slides were reviewed by one pathologist. Other inclusion criteria were an Ann Arbor stage of II to IV and a high tumor burden, defined according to the Groupe d'Etude des Lymphomes Folliculaires (GELF) criteria.21 Measurable disease, the absence of underlying organ dysfunction precluding the use of anthracycline or high-dose chemotherapy, and the absence of human immunodeficiency virus infection were also required. All necessary data were available and the FLIPI was calculated retrospectively at the time of analysis.4 The ethics committee of the Région Centre, Tours, France, approved the trial on April 27, 1994, and all patients provided written informed consent in accordance with the Declaration of Helsinki.
Staging, assessment of response, and follow-up
For all patients, a full review of the medical history, physical examination, routine laboratory tests and bone marrow biopsy was performed at diagnosis, as well as evaluations for abdominal and thoracic involvement by computed tomography imaging and/or ultrasonography.
The procedures used to evaluate responses were the same as those used for staging at diagnosis. Positron-emission tomographic scanning was not performed routinely. Follow-up procedures included a physical examination every 3 months for the first 2 years, every 6 months for the next 2 years, and annually thereafter. Thoracic and abdominal computed tomography was performed every 6 months during the first 2 years and annually thereafter. Bone marrow examinations were planned at the end of treatment, 6 and 12 months later, and annually thereafter. Molecular analyses were not routinely performed and were not taken into account during the staging or evaluation procedures. The report of the international workshop to standardize response criteria for NHLs22 had not been published at the time of the study design. Complete response (CR) was defined as the disappearance of all documented disease. A very good partial response (VGPR) was defined as an at least 80% reduction in the largest diameter of all measurable lesions without the persistence of bone marrow involvement except for the persistence of the sole presence of paratrabecular lymphomatous nodules. A partial response (PR) was defined as an at least 50% reduction in the largest diameter of every measurable lesion, even if bone marrow involvement persisted. In each group, treatment was considered to have failed if patients had disease progression before the end of the treatment program, and, in the HDT group, if they did not have at least a partial response before grafting. The frequency and severity of adverse effects were recorded according to the WHO (World Health Organization) classification.23
Treatment
In the standard chemotherapy group, the induction phase consisted of 6 CHVP (cyclophosphamide, doxorubicin, vepeside, prednisone)21 courses administered monthly, followed (for responders and patients with stable disease) by a maintenance phase that consisted of one cycle every 2 months for 1 year. Concomitant subcutaneous interferon alpha-2b was administered at 5 × 106 units subcutaneously 3 times per week for 18 months.
In the HDT group, patients received the VCAP (vindesine, cyclophosphamide, doxorubicin, prednisone) regimen as first-line therapy.24 Patients in CR, VGPR or PR after the second or third VCAP cycle went on to stem cell harvesting and received one course of IMVP1617 before transplantation. Patients with less than PR after the VCAP cycles received salvage therapy with 2 to 3 courses of dexamethasone, cytarabine, and cisplatin (DHAP). If at least a PR was obtained after DHAP, stem cells were harvested. Stem cell purging was offered to all patients in the 25 recruiting centers if the grafts collected contained at least 108 mononuclear cells/kg. Immunologic purging was performed either with immunomagnetic-bead negative selection or with positive selection of CD34+ cells according to the individual center procedures, as previously described.17,20 If the graft did not contain at least 104 colony-forming units–granulocyte/macrophage (CFU-GM)/kg for bone marrow samples or 2 × 104 CFU-GM/kg for peripheral blood stem cells, the procedure was considered to have failed and patients were not transplanted, but received treatment according to the local investigator's decision. The conditioning regimen started 4 to 6 weeks after the IMVP16 or the last DHAP cycle in responding patients and consisted of total body irradiation, administered in fractionated doses (200 cGy) twice daily on 3 consecutive days followed by cyclophosphamide (60 mg/kg on 2 consecutive days) in all patients. Stem cells were reinfused within 48 hours of completing the conditioning regimen.
Statistical analysis
This study was designed by the GOELAMS Lymphoma Committee with the aim of detecting a 25% absolute difference in EFS at 3 years, with an α value of 0.05 and a β value of 0.2. Secondary end points were the response rate at the end of treatment, the OS rate, and the incidence of adverse effects. Randomization was carried out centrally, and was stratified according to each center. Statistical analysis was performed with version 11.5 SPSS software (SPSS, Chicago, IL). OS, PFS, and EFS were calculated according to the Kaplan-Meier method.25 OS was measured from the time of randomization to death from any cause or the date of last contact, and PFS from the time of randomization to progression. For EFS, events were considered progression, absence of at least a partial response at the time of the intermediate evaluation, relapse and death in remission. Assuming a 5-year EFS rate of 50% in the conventional group and 75% in the HDT group, this design required the randomization of 130 patients. Data were collected by the principal investigator at each participating center, checked for accuracy by the GOELAMS research assistants, and sent to the centralized database in Tours, France. Follow-up data were updated in 2007 and data collection was stopped in July of the same year.
The log-rank test was used to compare survival in the 2 groups. The analysis was performed on an intention-to-treat basis. Multivariate analysis of survival was carried out using the Cox model and was used to identify predictors of outcome among the following variables: age at inclusion, sex, ECOG performance status (≥ 2 vs < 2), serum lactic dehydrogenase (LDH; elevated vs normal), stage (III-IV vs I-II), 10-cm bulk, hemoglobin level (< 12 g/dL vs ≥ 12), splenomegaly more than 15 cm, histology (grade I-II vs III) and treatment group. All multivariate analyses were performed on the whole population with an adjustment for sex and B symptoms. Potential interactions between treatment and risk factors were also assessed in the model; 95% confidence intervals are shown in parentheses for all data.
The French Ministry of Health (Ministère de la Santé et de la Solidarité Sociale) and Schering-Plough had no influence in the design of the protocol, the collection, analysis, and interpretation of the data, or the writing of this article.
Results
Between April 1994 and May 2001, 172 consecutive patients were enrolled at 25 centers. Six patients were excluded: 4 after the pathology review, 1 by an investigator's decision, and 1 who declined to undergo randomization. Of the 166 patients enrolled, 80 were assigned to the conventional chemotherapy group and 86 to the HDT group; 7 patients did not receive the assigned treatment. Patient characteristics are summarized in Table 1 and were similar in the 2 groups, except that there were more females and patients with B symptoms, and fewer cases of grade 3 follicular histology in the conventional therapy group than in the HDT group.
Initial characteristics of patients according to treatment group (CHVP vs HDT)
| Characteristics . | CHVP (n = 80) . | HDT (n = 86) . | P . |
|---|---|---|---|
| Median age, y (range) | 50 (29-61) | 51 (32-60) | .15 |
| Male/female, n | 47/33 | 38/48 | .017 |
| Histology, n | .03 | ||
| Grade I | 40 | 28 | |
| Grade II | 38 | 50 | |
| Grade III | 2 | 8 | |
| Ann Arbor stage, n | .1 | ||
| II | 6 | 4 | |
| III | 22 | 8 | |
| IV | 52 | 64 | |
| B symptoms present, n | 28 | 14 | .009 |
| Performance status grade, n | .1 | ||
| 0 | 44 | 39 | |
| 1 | 35 | 38 | |
| 2 or higher | 1 | 9 | |
| More than 4 nodal sites, n | 21 | 15 | .13 |
| Serum LDH higher than normal, n | 32 | 24 | .41 |
| FLIPI*, n | .4 | ||
| Low | 23 | 26 | |
| Intermediate | 31 | 40 | |
| High | 26 | 20 | |
| Mass > 10 cm, n | 28 | 25 | .77 |
| Characteristics . | CHVP (n = 80) . | HDT (n = 86) . | P . |
|---|---|---|---|
| Median age, y (range) | 50 (29-61) | 51 (32-60) | .15 |
| Male/female, n | 47/33 | 38/48 | .017 |
| Histology, n | .03 | ||
| Grade I | 40 | 28 | |
| Grade II | 38 | 50 | |
| Grade III | 2 | 8 | |
| Ann Arbor stage, n | .1 | ||
| II | 6 | 4 | |
| III | 22 | 8 | |
| IV | 52 | 64 | |
| B symptoms present, n | 28 | 14 | .009 |
| Performance status grade, n | .1 | ||
| 0 | 44 | 39 | |
| 1 | 35 | 38 | |
| 2 or higher | 1 | 9 | |
| More than 4 nodal sites, n | 21 | 15 | .13 |
| Serum LDH higher than normal, n | 32 | 24 | .41 |
| FLIPI*, n | .4 | ||
| Low | 23 | 26 | |
| Intermediate | 31 | 40 | |
| High | 26 | 20 | |
| Mass > 10 cm, n | 28 | 25 | .77 |
FLIPI was assessed retrospectively.
In the chemotherapy group, 18 patients did not complete the scheduled regimen for the following reasons: toxicity (5), disease relapse or progression (5), stable disease or treatment failure (6), patient refusal (1), and early transformation (1). In the HDT group, 9 patients did not receive the planned ASCT for the following reasons: insufficient CD34+ cell yield (4), patient refusal (1), consent withdrawal (1), disease progression (1), toxicity (1), investigator's decision to proceed to allogeneic transplantation (1). Treatment was completed by 77% percent of the patients assigned to the chemotherapy group and 90% of those in the HDT group. In the HDT group, collection of hematopoietic stem cells was performed in 77 patients and purging was achieved in all but 9 patients: immunologic purging with immunomagnetic beads in 10 patients and positive selection of CD34+ cells in the 58 other patients. As previously described,18 the overall response rate at the end of treatment was 69% for the chemotherapy group and 81% for the HDT group after transplantation.
Overall survival
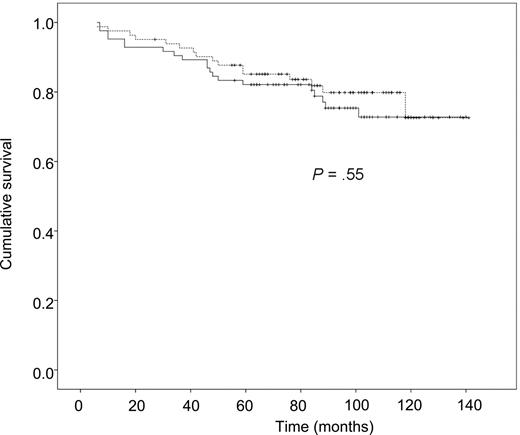
During the follow-up period, 36 of the 166 patients died, 20 in the HDT group and 16 in the chemotherapy group. The estimated 9-year survival rates were 76% (67%-85%) and 80% (72%-89%), respectively, with no significant difference (Figure 1). Seven deaths in the HDT group were related to secondary cancers. On multivariate analysis, OS was independently affected by ECOG performance status and the number of nodal areas involved (Table 2). No effect of the FLIPI on OS was observed.
Overall survival as a function of treatment group. The solid line indicates the HDT group and the dashed line, the CHVP group.
Overall survival as a function of treatment group. The solid line indicates the HDT group and the dashed line, the CHVP group.
Prognostic factors of outcome by multivariate analysis
| . | . | ORR . | ORR 95% CI . |
|---|---|---|---|
| Overall survival | |||
| ECOG | ≥ 2 vs < 2 | 2.05 | 1.28-3.28 |
| Nodal sites | > 4 vs ≤ 4 | 1.18 | 1.02-1.36 |
| Event-free survival | |||
| ECOG | ≥ 2 vs < 2 | 1.61 | 1.13-2.27 |
| Nodal sites | > 4 vs ≤ 4 | 1.21 | 1.11-1.33 |
| Progression-free survival | |||
| ECOG | ≥ 2 vs < 2 | 1.61 | 1.12-2.31 |
| Nodal sites | > 4 vs ≤ 4 | 1.19 | 1.08-0.30 |
| ITT group | CHVP vs HDT | 1.82 | 1.14-2.91 |
| . | . | ORR . | ORR 95% CI . |
|---|---|---|---|
| Overall survival | |||
| ECOG | ≥ 2 vs < 2 | 2.05 | 1.28-3.28 |
| Nodal sites | > 4 vs ≤ 4 | 1.18 | 1.02-1.36 |
| Event-free survival | |||
| ECOG | ≥ 2 vs < 2 | 1.61 | 1.13-2.27 |
| Nodal sites | > 4 vs ≤ 4 | 1.21 | 1.11-1.33 |
| Progression-free survival | |||
| ECOG | ≥ 2 vs < 2 | 1.61 | 1.12-2.31 |
| Nodal sites | > 4 vs ≤ 4 | 1.19 | 1.08-0.30 |
| ITT group | CHVP vs HDT | 1.82 | 1.14-2.91 |
CI indicates confidence interval; ECOG, ECOG performance status; and ITT, intention-to-treat.
Event-free survival
With a median follow-up of 9 years, 38 events were observed in the HDT group and 50 in the reference chemotherapy group. The probabilities of 9-year EFS for the whole group, HDT, and chemotherapy groups, respectively, were 48% (40%-55%), 56% (45%-67%), and 39% (28%-50%), with a significant difference between the 2 treatment groups (P = .03; Figure 2). On multivariate analysis, EFS was independently affected by ECOG performance status and the number of nodal areas involved (Table 2). No effect of the FLIPI on EFS was found.
Event-free survival as a function of treatment group. The solid line indicates the HDT group and the dashed line, the CHVP group.
Event-free survival as a function of treatment group. The solid line indicates the HDT group and the dashed line, the CHVP group.
Progression-free survival
Eighty patients experienced disease progression during treatment or relapse. In the HDT group, only 2 additional relapses have occurred since the last publication18 and a plateau was observed after 7 years of follow-up. The estimated 9-year PFS rate was 64% (54%-75%) for the HDT group and 39% (28%-50%) for the chemotherapy group (P = .004; Figure 3). On multivariate analysis, PFS was independently affected by ECOG performance status, the number of nodal areas involved, and allocation to the chemotherapy group (Table 2). In an unplanned subgroup analysis, patients with a low FLIPI were found to have a longer PFS when treated by ASCT, whereas those who had an intermediate or high FLIPI did not (Figure 4). The median survival after progression or relapse was 38 months in the HDT group and 50 months in the reference chemotherapy group (P = .43). Among the 30 patients with relapses in the HDT group, 10 died of progression or subsequent relapse, and 3 died of second cancers while in second CR. One patient received no treatment for the relapse and is still alive. Six patients were treated with rituximab alone, of whom 4 achieved a second CR. Seventeen patients were treated with various combinations of chemotherapy, rituximab, and radiation therapy.
Progression-free survival as a function of treatment group. The solid line indicates the HDT group and the dashed line, the CHVP group.
Progression-free survival as a function of treatment group. The solid line indicates the HDT group and the dashed line, the CHVP group.
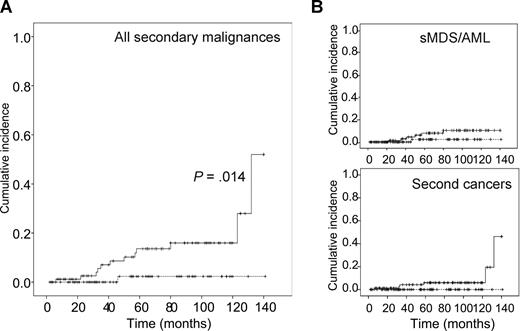
Incidence of secondary cancers. (A) Incidence of all secondary cancers as a function of treatment group. (B) Breakdown of the incidence of secondary MDS/AML (sMDS/AML; top panel) and second solid cancers (bottom panel). The solid line indicates the HDT group, the dashed line, the CHVP group.
Incidence of secondary cancers. (A) Incidence of all secondary cancers as a function of treatment group. (B) Breakdown of the incidence of secondary MDS/AML (sMDS/AML; top panel) and second solid cancers (bottom panel). The solid line indicates the HDT group, the dashed line, the CHVP group.
In the chemotherapy group, 50 patients experienced a relapse, 35 of whom were treated with various chemotherapy-based treatment regimens, 2 were treated with rituximab, 1 was treated with ibritumomab tiuxetan, and 2 were left untreated. Treatment was unknown for the remaining 10 patients. Among the 35 patients treated for relapse with chemotherapy, 15 underwent autologous stem cell transplantation. All of them achieved a second CR, except 1 for whom the response was unknown. Two patients who went on to allogeneic bone marrow transplantation in remission died of toxicity.
Five patients developed transformation to high-grade lymphoma: 2 were in the HDT group and 3 in the chemotherapy group.
Secondary cancers
Two more cancers have occurred in the HDT group since the first publication, 1 breast cancer and 1 metastatic lung cancer, 123 and 132 months after inclusion, respectively. A total of 12 secondary cancers therefore occurred after HDT: 3 cases of acute myeloid leukemia (AML), 3 of myelodysplastic syndrome (MDS), 3 of breast cancer, 1 of renal cancer, 1 of prostate cancer, and 1 of lung cancer. Five cancers occurred in CR patients, and there were 7 cases of a fatal secondary cancer. These secondary cancers occurred between 12 and 133 months after ASCT with an actuarial risk of 55% (35%-75%) at 140 months. Four cases of leukemia or myelodysplasia occurred after purging with B-cell depletion and the other 2 cases after positive selection of CD34+ cells. The breakdown of the secondary malignancies according to the purging methods and source of stem cells is described in Table 3. There was a significantly higher rate of secondary malignancies in the group with negative selection than in the group with positive selection (P = .02). One case of secondary acute promyelocytic leukemia occurred in the conventional chemotherapy group 46 months after inclusion. The patient is still alive and the disease in remission.
Second cancers and stem cell collection/purging procedures used for patients in the HDT arm (84 patients)
| Purging method . | HSC source . | No. of patients . | Secondary malignancies . | |
|---|---|---|---|---|
| No. and type . | % . | |||
| CD34+ | PBSC | 55 | 1 BC, 1 RC, 1 PC, 1 AML, 1 MDS | 9 |
| BM | 1 | 0 | ||
| BM + purged PBSC | 1 | 0 | ||
| CD34− | PBSC | 4 | 1 MDS | 33* |
| BM | 8 | 2 MDS, 1 BC | ||
| Unpurged | PBSC | 3 | 1 RC | |
| BM | 1 | 0 | ||
| BM + PBSC | 1 | 0 | ||
| No transplant | NA | 9 | 1 LC, 1 MDS | |
| Allo-SCT | Allogeneic PBSC | 1 | 0 | |
| Purging method . | HSC source . | No. of patients . | Secondary malignancies . | |
|---|---|---|---|---|
| No. and type . | % . | |||
| CD34+ | PBSC | 55 | 1 BC, 1 RC, 1 PC, 1 AML, 1 MDS | 9 |
| BM | 1 | 0 | ||
| BM + purged PBSC | 1 | 0 | ||
| CD34− | PBSC | 4 | 1 MDS | 33* |
| BM | 8 | 2 MDS, 1 BC | ||
| Unpurged | PBSC | 3 | 1 RC | |
| BM | 1 | 0 | ||
| BM + PBSC | 1 | 0 | ||
| No transplant | NA | 9 | 1 LC, 1 MDS | |
| Allo-SCT | Allogeneic PBSC | 1 | 0 | |
HSC indicates hematopoietic stem cell; Allo-SCT, allogeneic stem cell transplantation; PBSC, peripheral blood stem cells; BC, breast cancer; RC, renal cancer; PC, prostate cancer; BM, bone marrow; LC, lung cancer; and NA, not applicable.
For CD34+ versus CD34− purging, P = .02.
Discussion
The median survival of patients with FL varies from 5 to 10 years depending on prognostic factors and response to treatment.1-3 Although FL is typically an indolent disease, it is rarely curable with conventional chemotherapy.
The place of ASCT in the therapeutic strategy for FL has not been clearly determined to date.26,27 Practice guidelines for the management of nodal indolent NHLs were published in 2005 by the Italian Society of Hematology, and it is recommended that patients less than 65 years of age with extended relapses after first-line therapy containing either anthracyclines or fludarabine should be treated with HDT and ASCT. ASCT should be undertaken after achieving at least partial remission with an appropriate cytoreductive treatment, and it is recommended that any procedure capable of producing a lymphoma-free graft be used.28 The randomized CUP trial indicated a considerable benefit of ASCT over conventional chemotherapy in terms of DFS (P = .004) and OS (P = .079) in 89 relapsing patients with FL and a median follow-up of 6 years.14
Apart from our study, only 2 other large phase 3 studies have tested the value of ASCT as first-line therapy for patients with FL in the prerituximab era. The German Low-Grade Lymphoma Study Group compared ASCT to interferon-α as maintenance therapy after conventional induction chemotherapy combining cyclophosphamide-doxorubicin-vincristine-prednisone or mitoxantrone-chlorambucil-prednisone. The 5-year PFS rate was 64.7% in the ASCT arm and 33.3% in the interferon-α arm (P < .001) but the follow-up was short and OS data are not available.16
The Groupe d'Etude des Lymphomes de l'Adulte (GELA) recently reported the results of the GELF-94 study which compared the same chemotherapy and interferon regimen (CHVP-Interferon) as those used in our study, with cyclophosphamide, vincristine, 2doxorubicin, prednisone (CHOP) followed by unpurged stem cell transplantation conditioned by VP16/cyclophosphamide and total body irradiation. The inclusion criteria were the same as ours; 401 patients were randomized. With a median follow-up of 92 months, there was no difference between the 2 arms for OS (estimated 7-year OS of 76% vs 71%, P = .53) nor EFS (estimated 7-year EFS of 38% vs 28%, P = .11).15
We report here the results of HDT followed by ASCT as first-line therapy for FL with high tumor burden in a randomized study with the longest follow-up published to date. We show that HDT followed by ASCT with purged stem cells was able to provide a high response rate and long-term control of the disease in more than 50% of patients. However, HDT was not superior to conventional chemotherapy in terms of OS, because of the better survival rate of relapsing patients in the chemotherapy group and the occurrence of more secondary cancers in the HDT group. However, at the time of this report, 10 of the 32 patients with relapses in the HDT group are in long-lasting (> 4 years) second complete remission, after rituximab alone in many cases, suggesting that ASCT has changed the natural history of the disease in some patients.
Studies reporting very long follow-up after conventional chemotherapy fail to show a plateau suggesting cure.29,30 One important question is whether alternative therapeutic approaches such as ASCT or anti-CD20 monoclonal antibody therapy are able to cure FL. Few studies have already described a plateau in FL after ASCT. Horning et al31 reported on 37 patients grafted with purged bone marrow after VP16/cyclophosphamide/TBI-conditioning regimen. In that study, with a median follow-up of 6.5 years, the estimated PFS at 10 years was 70% with a plateau. Corradini et al32 recently reported on 70 patients with indolent lymphoma (40 with follicular, 14 with small lymphocytic, and 16 with mantle cell lymphomas) treated with intensified high-dose chemotherapy followed by peripheral blood progenitor cell autografting. With a median follow-up of 75 months, 26 patients (20 with FL and 6 with non-FL) were long-term survivors with no sign of clinical or molecular disease.
In this study, no relapse occurred later than 75 months after inclusion, with a PFS plateau curve at 64% in the HDT group. Surprisingly, after a longer period of follow-up, we also observed that patients with the lower FLIPIs seemed to draw greater benefit from ASCT. These results suggest ASCT has changed the natural history of the disease, and that some patients might be cured by this procedure.
The OS of the whole group is very high (78% at 10 years), although most of the patients had poor prognostic factors (63% with FLIPI ≥2) at diagnosis. This result is comparable with the survival rate observed with first-line ASCT reported in the retrospective European Group of Blood and Bone Marrow Transplantation (EBMT) trial.33 A very high rate of secondary malignancies occurred in the HDT group, including MDS (n = 3), AML (n = 3), and solid tumors (n = 6). The 10-year estimated incidence of these secondary cancers is approximately 16%, which is greater than the incidence reported to date.10,26,34 An excess of incidence of secondary malignancies was not reported in the HDT arm of the GELA study, which was very comparable with ours in terms of chemotherapy and the conditioning regimen, which used TBI without purging.15 One explanation could be the use of stem cell purging in our study, removing the immunocompetent component of the bone marrow possibly involved in tumor control. Negative purging was associated with a higher rate of secondary malignancies. Even though the numbers are small, these results strongly suggest a role of stem cell purging in the occurrence of second cancers. In a retrospective study of HDT with purging and TBI in relapsed FL, 15 deaths in 121 patients were reported.35 Recent publications suggest an antitumor effect of the autologous immune system recovery against malignancies treated with ASCT,36 and report a prognostic value of absolute lymphocyte count at diagnosis on OS in FLs.37 It could be postulated that in vitro purging affects post-ASCT immune reconstitution, thereby favoring the incidence of secondary tumors. However, purged ASCT has the potential benefit of removing remaining tumor cells. In a large retrospective analysis of the International Bone Marrow Transplantation Registry (IBMTR), multivariate analysis showed that purged ASCT patients had a 26% lower recurrence risk than unpurged ASCT patients.27 Moreover, the Dana-Farber group demonstrated the prognostic value of PCR-negative grafts on the freedom-from-relapse rate in patients grafted for relapsed FL.38
Another possible explanation for excessive second malignancies could be the pretransplant chemotherapy, delivered at a high dose-intensity in the HDT group, as suggested by the occurrence of 1 MDS and 1 lung cancer in 2 patients who did not receive the planned transplantation. Our results suggest recommending stopping in vitro purging and using instead in vivo purging with rituximab, which is widely used in the treatment of CD20+ NHL and is effective for in vivo purging.39-41
Progress has been achieved in the treatment of FLs, with the use of polychemotherapy and rituximab combinations.5,6,42,43 Therefore, despite a high CR rate and prolonged PFS, ASCT cannot be considered as the standard first-line treatment for FL patients with high tumor burden as defined in our study. The GITMO Italian group recently reported the results of a randomized trial comparing CHOP-rituximab to high-dose chemotherapy with rituximab (R-HDS) in high-risk FL. Despite improved PFS and EFS, OS was not different in the 2 arms (82% vs 79% at 4 years, with a median follow-up of 50 months) because of the high level of effectiveness of R-HDS as salvage therapy.17 Therefore, if ASCT has to be recommended for disease progression or relapsing patients, our encouraging results with a plateau suggest a potential benefit of ASCT for a small subgroup of patients with specific prognostic factors at diagnosis, which remain to be determined or which could be the absence of clinical or molecular remission after conventional first-line therapy. Although in vitro purging has to be stopped, the results of ASCT may be improved by combination with in vivo purging with rituximab, maintenance treatment with rituximab, and/or radioimmunotherapy as part of the conditioning regimen.
The online version of this article contains a data supplement.
Presented in part in oral sessions at the 43rd Annual Meeting of the American Society of Hematology, Orlando, FL, December 10, 2001; and the 45th Annual Meeting of the American Society of Hematology, San Diego, CA, December 9, 2003.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank M. C. Chamard for her technical assistance.
The work presented here was partly supported by grants of the French Ministry of Health (Paris, France) and the Schering-Plough Corporation (Kenilworth, NJ).
Authorship
Contribution: All authors participated in designing and performing the research and checked the final version of the manuscript. P.M. centralized the pathology review; E.G., E.D., P.B., R.D., and P.C. controlled and analyzed the data; E.D., E.G., and P.C. wrote the report; and C.F., S.L.G., C.B., H.M., V.D., R.G., P.Q., J.-P.V., B.D., J.J., J.-F.R., N.A., A.T., C.M.-C., and N.M. designed and performed research, and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Groupe Ouest-Est des Leucémies et des Autres Maladies du Sang (GOELAMS) appears in the Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Eric Deconinck, Inserm U-645/Université de Franche-Comté/IFR 133, Hématologie-CHU Jean Minjoz, 2 bvd Fleming, 25030 Besançon, France; e-mail: edeconinck@chu-besancon.fr.