Platelets can be recruited by an ultra-large multimer fraction of von Willebrand factor extending from the endothelial surface into the plasma flow as long strings. In this issue of Blood, Huang and colleagues investigate the complex structure of these fishing lines and uncover a role for αvβ3 integrin as their anchor to the endothelial surface.
Immediately after activation, endothelial cells release long strings of highly-multimerized von Willebrand factor (VWF) that recruit platelets to form “beads on a string” (see figure). Previously documented both in vitro1 and in vivo,2-4 these strings described a dramatic new mechanism by which platelets can be recruited. However, not much is known about the strings. Both structural and functional questions of fundamental importance are still unanswered, although their structure does explain the need to store VWF coiled into tubules within Weibel-Palade bodies (an orderly unfurling is required to effectively cast a fishing line), neatly explaining the unique rod-like shape of this organelle.
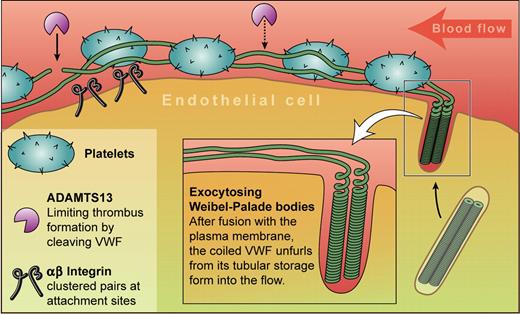
An endothelial cell releases von Willebrand factor (VWF) by exocytosis of Weibel-Palade bodies. Their fusion to the plasma membrane triggers unfurling of the coiled VWF into the blood where it collects platelets to form the characteristic “beads on a string.” Anchor points containing αvβ3 integrins occur at intervals, and ADAMTS13 cuts the strings, reducing their prothrombotic potential. Professional illustration by Kenneth X. Probst.
An endothelial cell releases von Willebrand factor (VWF) by exocytosis of Weibel-Palade bodies. Their fusion to the plasma membrane triggers unfurling of the coiled VWF into the blood where it collects platelets to form the characteristic “beads on a string.” Anchor points containing αvβ3 integrins occur at intervals, and ADAMTS13 cuts the strings, reducing their prothrombotic potential. Professional illustration by Kenneth X. Probst.
How are the strings anchored to the endothelial surface? Huang et al5 provide a new set of data showing that αvβ3 integrins play an important role, in particular by using lentivirus-expressed shRNA-mediated knockdown of αvβ3 in human umbilical vein endothelial cells. Anchorage is important because stretching of VWF under flow exposes the cleavage sites used by the TTP-associated protease ADAMTS13.1,6 Since this cleavage is needed to cut the dangerously prothrombotic ultra-large VWF into safer fragments, and stretching requires a firm anchor, this is of real importance. However, conclusive identification of string-anchoring molecules has proven difficult. Targeting of P-selectin to WPB by its binding of VWF plus blockage of in vitro string formation by anti–P-selectin antibodies or soluble P-selectin7 suggest a role for this receptor as an anchor. However, intravital microscopy within mesenteric venules shows that mice deficient in P-selectin can still make strings. A role in string-anchoring for αvβ3 was also ruled out by Padilla et al7 in vitro, and by Andre et al2 and Chauhan et al3 in vivo.
What underlies these differences? Perhaps at the lower shear stress found within venules, adhesion by integrins and by P-selectin may be less important than at the higher shear stress occurring in arterioles that was used in the in vitro experiments. This emphasizes differences in string behavior between types of endothelia.1 In addition, variability between the experimental conditions that have been used in binding studies are likely involved.
Less controversially, all studies suggest a small number of anchorage points per cell, and Huang et al suggest that changes in direction of flow can lead to formation of new attachment sites. Light microscopy from Huang et al5 shows αvβ3 in patches along the strings. Whether these are all tethering points is not yet clear.
How are such long strings made? Since the very largest VWF multimers are the most efficient at recruiting platelets, size definitely matters. The simplest question here is whether a single multimer is equivalent to 1 string. This was unlikely since strings can be up to several millimeters long, whereas structural studies8 suggested that a multimer coiled into a tubule running the length of a very large Weibel-Palade body (5 μm) could extend to about 250 μm. Self-association of VWF has been previously described as well as the formation of multistranded bundles3,9 but in their paper, Huang et al5 use high-resolution scanning electron microscopy of acutely-secreted VWF to reveal a variety of forms of self-associated molecules, forming twisted bundles and networks that likely account for the observed length of the strings.
Finally, one entirely unexpected finding by Huang et al5 is that a significant subset of strings carry no platelets at all. The only real clue as to why this might be is that an increase in flow does slightly increase the fraction of platelet-bearing strings, suggesting that the structure of strings changes to reveal interior platelet-binding sites, but whether changes in turbulence and shear within the vasculature are sufficient to raise the fraction to one, or whether some strings just have a different function remains unanswered for now.
How important is all this? The VWF released from endothelial cells in immediate response to secretagogue-stimulation is the most prothrombotic, spontaneously platelet-binding form of this molecule, implying that it must play an important role in recruitment of platelets. However, the strings seem to be short-lived, disappearing within minutes. Despite this, and assuming a simple relationship between the ultra-large VWF seen on multimer gels and VWF in strings, the hemostatic significance of ultra-large multimers as shown by von Willebrand disease mutations that reduce this fraction of VWF suggests that ultra-large VWF is indeed important. Just how this “μfishing” is carried out is therefore of high interest.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal