Abstract
Defects in apoptosis contribute to poor outcome in pediatric acute lymphoblastic leukemia (ALL), calling for novel strategies that counter apoptosis resistance. Here, we demonstrate for the first time that small molecule inhibitors of the antiapoptotic protein XIAP cooperate with TRAIL to induce apoptosis in childhood acute leukemia cells. XIAP inhibitors at subtoxic concentrations, but not a structurally related control compound, synergize with TRAIL to trigger apoptosis and to inhibit clonogenic survival of acute leukemia cells, whereas they do not affect viability of normal peripheral blood lymphocytes, suggesting some tumor selectivity. Analysis of signaling pathways reveals that XIAP inhibitors enhance TRAIL-induced activation of caspases, loss of mitochondrial membrane potential, and cytochrome c release in a caspase-dependent manner, indicating that they promote a caspase-dependent feedback mitochondrial amplification loop. Of note, XIAP inhibitors even overcome Bcl-2–mediated resistance to TRAIL by enhancing Bcl-2 cleavage and Bak conformational change. Importantly, XIAP inhibitors kill leukemic blasts from children with ALL ex vivo and cooperate with TRAIL to induce apoptosis. In vivo, they significantly reduce leukemic burden in a mouse model of pediatric ALL engrafted in non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice. Thus, XIAP inhibitors present a promising novel approach for apoptosis-based therapy of childhood ALL.
Introduction
Apoptosis, or programmed cell death, occurs in various physiological and pathological situations and plays a critical role in maintaining tissue homeostasis in multicellular organisms.1,2 Apoptosis may be initiated through different entry sites, such as death receptors or mitochondria, resulting in activation of effector caspases.1,2 Ligation of death receptors of the tumor necrosis factor (TNF) receptor superfamily such as CD95 (APO-1/Fas) or the agonistic TNF-related apoptosis-inducing ligand (TRAIL) receptors TRAIL-R1 and TRAIL-R2 by their cognate ligands or agonistic antibodies results in caspase-8 activation at the death inducing signaling complex (DISC) that induces direct cleavage of downstream effector caspases such as caspase-3.3 In the mitochondrial pathway, the release of apoptogenic factors such as cytochrome c, apoptosis inducing factor (AIF), second mitochondria-derived activator of caspase (Smac)/direct IAP binding protein with low PI (DIABLO), or Omi/high temperature requirement protein A2 (HtrA2) from mitochondria into the cytosol results in caspase-3 activation via formation of the cytochrome c/Apaf-1/caspase-9–containing apoptosome complex.4 Signals originating from death receptors may be linked to mitochondria via Bcl-2 family proteins such as Bid, which causes cytochrome c release upon cleavage by caspase-8, thereby initiating a mitochondrial amplification loop.5 The mitochondrial contribution to death receptor signaling is of special relevance in type II cells, for example some leukemias.6,7 Although caspase-8 is activated upon death receptor ligation at the DISC in quantities sufficient to directly activate downstream effector caspases such as caspase-3 in type I cells, type II cells rely on the apoptotic function of mitochondria for full activation of effector caspases and apoptosis.6
Resistance to apoptosis is one of the hallmarks of human cancers and promotes cancer development and progression, for example in leukemia.8,9 In addition, evasion from apoptosis represents one of the leading causes of failure of antileukemic therapy, since many anticancer treatments act by triggering apoptosis in cancer cells.2 The concept of inducing apoptosis in cancer cells by ligation of death receptors is of special interest for cancer therapy, since death receptors are directly linked to the cell's intrinsic death program.3 The death-inducing ligand TRAIL is a prime candidate for clinical development, because it has been reported to induce apoptosis in a wide spectrum of cancer cell lines with no or minimal toxicity on normal human cells.10,11 Recombinant TRAIL or fully human monoclonal antibodies that specifically target one of the 2 agonistic TRAIL receptors are currently being evaluated in early clinical trials.10,11 However, many human tumors including hematologic malignancies are partially or completely resistant to monotherapy with TRAIL, limiting its therapeutic utility.10-13 In childhood acute lymphoblastic leukemia (ALL), we previously reported that 50% of pretreatment samples are resistant to TRAIL-induced apoptosis and that TRAIL even stimulates survival and proliferation in some of the resistant patients.14 This highlights the demand to develop TRAIL-based combination therapies that counter resistance mechanisms of acute leukemia cells toward TRAIL to ensure the success of TRAIL in the clinic. The molecular details that cause resistance of human cancers to TRAIL are still only partially understood.10,11,15 Increasing evidence suggests that high levels of inhibitor of apoptosis proteins (IAPs) may represent a key antiapoptotic mechanism in cancer cells including acute leukemia.16-18 Among the IAP family members, it is especially X-linked inhibitor of apoptosis (XIAP) that is known for its antiapoptotic function.19,20
Since XIAP blocks apoptosis at a central point via inhibition of effector caspases,19 there is currently much interest to exploit XIAP as a molecular target in human cancers. We previously demonstrated proof-of-concept in a glioblastoma model that Smac peptides that antagonize XIAP can be used to enhance TRAIL-induced killing in vitro and in vivo.21 However, the question whether targeting XIAP is a suitable strategy to prime acute leukemia cells for TRAIL-induced apoptosis has not yet been answered. Therefore, we investigated the effect of small molecule XIAP inhibitors on TRAIL-mediated antitumor activity in childhood acute leukemia in the present study.
Methods
Cell culture
Leukemia cell lines were cultured as described previously.22 Parental Jurkat cells were obtained from ATCC (Manassas, VA). Jurkat cells transfected with Bcl-2 or control vector were kindly provided by Dr H. Walczak (Heidelberg, Germany)23 and subcloned by serial dilutions for high Bcl-2 expression. Jurkat cells were stably transduced with Smac as previously described using pLXIN empty vector or Smac-containing vector.24 For knockdown of XIAP, Jurkat cells were stably transduced with empty pRETRO-SUPER vector or vector containing shRNA against XIAP (gtggtagtcctgtttcagc) or nonsense control (gatcatgtagatacgctca) as previously described.25 All chemicals were purchased from Sigma (Steinheim, Germany) unless indicated otherwise. XIAP inhibitor 1, XIAP inhibitor 2, and control compound were kindly provided by Idun Pharmaceuticals (now Pfizer, Groton, CT) and correspond to compounds 2, 11, and 15, respectively, described by Oost et al26
Western blot analysis
Western blot analysis was performed as described previously22 using the following antibodies: mouse anti–caspase-8 (1:1000), mouse anti-Omi (1:1000) from ApoTech (Epalinges, Switzerland), rabbit anti–caspase-3 (1:1000; Cell Signaling, Beverly, MA), rabbit anti–caspase-9 (1:1000), mouse anti-XIAP (1:1000), mouse anti-cIAP1 (1:1000), mouse anti-Smac (1:1000) from BD Biosciences (Heidelberg, Germany), rabbit anti-cIAP2 (1:1000; Epitomics, Burlingame, CA), rabbit anti-survivin (1:1000; R&D Systems, Minneapolis, MN), mouse anti-livin (1:1000; Imgenex, San Diego, CA), or mouse anti–β-actin as loading control (1:5000; Sigma) followed by goat-anti–mouse IgG or goat-anti–rabbit IgG conjugated to horseradish peroxidase (1:5000; Santa Cruz Biotechnology, Santa Cruz, CA). Enhanced chemiluminescence was used for detection (Amersham Bioscience, Freiburg, Germany). All Western blots shown are representative of at least 3 independent experiments.
Determination of apoptosis and clonogenic survival
Apoptosis was determined by fluorescence-activated cell sorting analysis (FACScan; BD Biosciences) of DNA fragmentation of propidium iodide–stained nuclei or by forwardside scatter analysis as described previously14,22 after stimulation with TRAIL (from R&D Systems, for cell lines and peripheral blood lymphocytes [PBLs]; from PeproTech [Rocky Hill, NJ] for primary leukemia cells) or agonistic anti-CD95 (APO-1) antibody.27 For clonogenic assay, Jurkat cells were treated with XIAP inhibitors and TRAIL for 12 hours and then seeded in a 1% methylcellulose medium (StemCell Technologies, Vancouver, BC) consisting of a 2.6% solution of methylcellulose in Iscove MDM (40%), RPMI1640 medium with 1% penicillin/streptomycin (49%), 1% l-glutamine, and 10% FCS at 0.05 to 0.1 × 104 cells/mL. After 8 to 10 days, colonies with more than 40 cells per colony were counted. For assessment of apoptosis in primary leukemia samples, leukemia blasts were derived from children treated for ALL at the Ludwig Maximilians University Children's Hospital (Munich, Germany) from 2006 to 2008 after informed consent was obtained in accordance with the Declaration of Helsinki. The study was approved by the Ethical Committee at the University Children's Hospital. Samples were obtained by bone marrow puncture at initial diagnosis before the onset of therapy, isolated using Ficoll Isopaque (Amersham Bioscience), and stimulated directly after isolation. Characteristics of patients are summarized in Table 1. For colony assay of primary cells, 8 × 104 cells/mL were seeded in RPMI medium containing 20% FCS, penicillin 100 U/mL, streptomycin 100 μg/mL, gentamycin 0.1 mg/mL, l-glutamine 2 mM, insulin 6 μg/mL, transferrin 3 μg/mL, selenium 4 ng/mL, 1% HEPES buffer, and 0.5% methylcellulose (CellSystems, St Katherinen, Germany). Pictures were taken 72 hours after stimulation using the cell screen machine from Innovatis (Bielefeld, Germany) and analyzed by Scientific Counter Programm software from DatInf (Tübingen, Germany) using a threshold resembling 5 cells/colony.
Patient characteristics
| Patient no. . | Age, y . | Sex . | Type of leukemia . | Origin of sample . |
|---|---|---|---|---|
| 1 | 11 | Male | Pre-B ALL | Bone marrow |
| 2 | 10 | Female | Pre-B ALL | Bone marrow |
| 3 | 1 | Male | Pre-B ALL | Bone marrow |
| 4 | 3 | Male | Pre-B ALL | Bone marrow |
| 5 | 15 | Male | Pre-B ALL | Bone marrow |
| 6 | 10 | Male | Pre-B ALL | Bone marrow |
| 7 | 8 | Female | cALL | Bone marrow |
| 8 | 18 | Male | cALL | Bone marrow |
| 9 | 5 | Female | cALL | Peripheral blood |
| 10 | 16 | Female | Pre-B ALL | Peripheral blood |
| 11 | 0,7 | Male | Pre-B ALL | Bone marrow |
| 12 | 8 | Female | T-ALL | Peripheral blood |
| 13 | 7 | Female | Pre-B ALL | Bone marrow |
| 14 | 12 | Male | T-ALL | Pleural effusion |
| Patient no. . | Age, y . | Sex . | Type of leukemia . | Origin of sample . |
|---|---|---|---|---|
| 1 | 11 | Male | Pre-B ALL | Bone marrow |
| 2 | 10 | Female | Pre-B ALL | Bone marrow |
| 3 | 1 | Male | Pre-B ALL | Bone marrow |
| 4 | 3 | Male | Pre-B ALL | Bone marrow |
| 5 | 15 | Male | Pre-B ALL | Bone marrow |
| 6 | 10 | Male | Pre-B ALL | Bone marrow |
| 7 | 8 | Female | cALL | Bone marrow |
| 8 | 18 | Male | cALL | Bone marrow |
| 9 | 5 | Female | cALL | Peripheral blood |
| 10 | 16 | Female | Pre-B ALL | Peripheral blood |
| 11 | 0,7 | Male | Pre-B ALL | Bone marrow |
| 12 | 8 | Female | T-ALL | Peripheral blood |
| 13 | 7 | Female | Pre-B ALL | Bone marrow |
| 14 | 12 | Male | T-ALL | Pleural effusion |
The status of the disease was primary for all patients.
y indicates year; and pre-B ALL, B-cell precursor.
Microscopy of apoptotic cells
Cells were collected by centrifugation (5 minutes at 200 g [600 rpm]) and stained with 0.02% 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI; Roche Diagnostics, Mannheim, Germany) in methanol for 5 minutes to stain nuclear DNA. Pictures were taken using an Olympus AX70 “Provis” microscope (Hamburg, Germany).
Determination of mitochondrial membrane potential and cytochrome c release
Caspase activity
Caspase activity was determined as previously described.29 Caspase inhibitors N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (zVAD.fmk) and N-benzyloxycarbonyl-Asp-Glu-Val-Asp-fluoromethylketone (zDEVD.fmk) were obtained from Bachem (Heidelberg, Germany).
Cell surface and cytoplasmic staining
Surface expression of TRAIL receptors or CD95 was assessed as previously described.25 For cytoplasmic staining of Bcl-2, cells were permeabilized with 0.3% saponin/PBS, stained with mouse anti–Bcl-2 monoclonal antibody (Dako, Hamburg, Germany) for 30 minutes at 4°C, washed with 0.1% saponin/PBS, incubated with FITC-labeled anti–mouse secondary antibody for 30 minutes at 4°C in the dark, washed with 0.1% saponin/PBS, and analyzed by flow cytometry. To assess Bak conformation change, 2 × 106 cells were fixed in 2% PFA for 15 minutes, incubated with mouse anti-Bak (Calbiochem, Darmstadt, Germany) in permeabilization buffer (PBS with 0.5% BSA and 0.05% saponin) for 1 hour at 4°C, followed by rabbit anti–mouse-F(ab′)2IgG/biotin (Dako) for 1 hour at 4°C and streptavidin-PE (BD Biosciences) for 1 hour at 4°C, and analyzed by flow cytometry.
NOD/SCID mouse model of pediatric leukemia
To establish an in vivo mouse model of childhood leukemia, fresh leukemic blasts derived from a patient with T-cell ALL were injected into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice via the tail vein. Mouse peripheral blood was regularly monitored for engraftment of human leukemia cells by staining with anti–human CD45 antibody (BD Biosciences). Continuous xenografts were established by harvesting human leukemia cells from the spleen of engrafted mice and inoculating recipient mice with 107 spleen cells intravenously. Treatment started on day 3 with 40 mg/kg XIAP inhibitor 2 and/or 50 μg TRAIL/mouse. Drugs were administered intraperitoneally 5 days/week for 4 weeks and mice were killed on day 34 at the onset of clinical symptoms of leukemia in the first mice. Body weight was monitored twice weekly; blood cell counts and the percentage of human CD45 positive cells were assessed on day 20 and day 34. Animal experiments were performed in accordance with relevant institutional and national regulations; research protocols were approved by Ludwig-Maximilians-University (Munich, Germany).
Statistical analysis
Statistical significance was assessed by Student t test or Mann-Whitney U test, where appropriate, using Winstat (R. Fitch Software, Bad Krozingen, Germany) or SPSS (SPSS GmbH Software, Munich, Germany) software.
Results
Expression of IAPs and IAP antagonists in acute leukemia cells
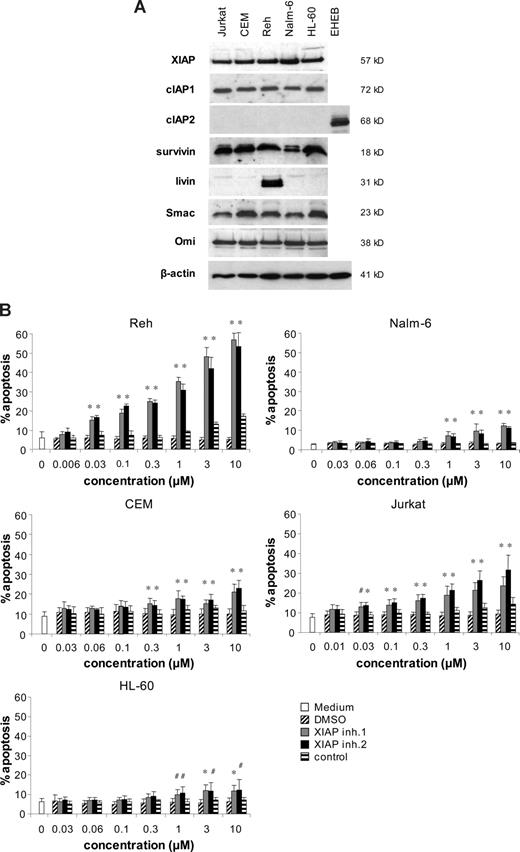
To investigate the role of XIAP in regulating apoptosis in acute leukemia cells, we selected 2 T-cell ALL cell lines (Jurkat, CEM), 2 B-cell precursor ALL cell lines (Reh, Nalm-6), and 1 acute myeloid leukemia (AML) cell line (HL-60). First, we analyzed expression of IAPs and IAP antagonists by Western blot analysis. XIAP, cIAP1, and survivin were expressed at high levels in all leukemia cell lines, whereas livin expression was detected only in Reh cells (Figure 1A). cIAP2 was barely detectable in acute leukemia cell lines compared with EHEB chronic lymphocytic leukemia (CLL) cells used as positive control for cIAP2 expression (Figure 1A). In addition to IAPs, endogenous IAP antagonists Smac/DIABLO and Omi/HtrA2 were expressed in all acute leukemia cell lines tested (Figure 1A).
XIAP inhibitors trigger apoptosis in acute leukemia cells. (A) Protein expression of XIAP, cIAP1, cIAP2, survivin, livin, Smac, Omi, and β-actin was assessed in acute leukemia cell lines by Western blotting. EHEB CLL cells were used as positive control for cIAP2 expression. (B) Acute leukemia cell lines were left untreated (□) or were treated for 48 hours with indicated concentrations of XIAP inhibitors, control compound, or DMSO. Apoptosis was determined by forwardside scatter analysis and flow cytometry. Mean and SD of 3 experiments each performed in triplicate are shown. For statistical analysis, t test was performed comparing XIAP inhibitors to control compound. #P < .05; *P < .01.
XIAP inhibitors trigger apoptosis in acute leukemia cells. (A) Protein expression of XIAP, cIAP1, cIAP2, survivin, livin, Smac, Omi, and β-actin was assessed in acute leukemia cell lines by Western blotting. EHEB CLL cells were used as positive control for cIAP2 expression. (B) Acute leukemia cell lines were left untreated (□) or were treated for 48 hours with indicated concentrations of XIAP inhibitors, control compound, or DMSO. Apoptosis was determined by forwardside scatter analysis and flow cytometry. Mean and SD of 3 experiments each performed in triplicate are shown. For statistical analysis, t test was performed comparing XIAP inhibitors to control compound. #P < .05; *P < .01.
XIAP inhibitors as single agents trigger apoptosis in acute leukemia cells
Next, we examined the effect of neutralizing XIAP on apoptosis of acute leukemia cells. To this end, we used 2 distinct small molecule XIAP inhibitors that bind to the BIR3 domain of XIAP with single-digit nanomolar affinity.26 A close structural analog that only weakly binds to the BIR3 domain of XIAP served as control.26 Interestingly, treatment with XIAP inhibitors as single agents resulted in a marked increase in apoptosis in Jurkat and Reh cells and—to a lesser extent—in CEM, Nalm-6, and HL-60 cells in a dose-dependent manner (Figure 1B). The control compound was nontoxic compared with solvent (DMSO) except at high concentrations in Reh cells, which were most susceptible to XIAP inhibitors among the cell lines studied (Figure 1B), indicating that the cytotoxicity of XIAP inhibitors is mechanism specific. These findings demonstrate that small molecule XIAP inhibitors as single agents induce apoptosis at nanomolar to micromolar concentrations in acute leukemia cells.
XIAP inhibitors cooperate with TRAIL to induce apoptosis in acute leukemia cells
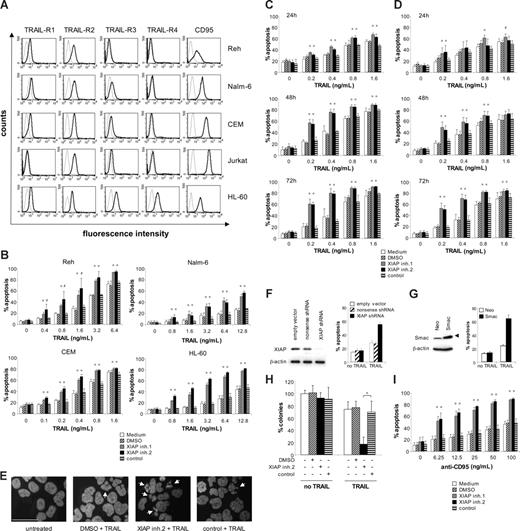
Many cancers including acute leukemia present with primary or acquired resistance to the death receptor ligand TRAIL, calling for strategies to enhance the efficacy of TRAIL in acute leukemia. Therefore, we asked whether XIAP inhibitors may lower the threshold for TRAIL-induced apoptosis in acute leukemia cells. To address this question, we first examined cell surface expression of TRAIL receptors in acute leukemia cells. All acute leukemia cell lines exhibit surface expression of at least one of the 2 agonistic TRAIL receptors TRAIL-R1 and -R2, and most also express one or both of the antagonistic TRAIL receptors TRAIL-R3 and -R4 (Figure 2A). In addition, all acute leukemia cell lines show high or moderate surface expression of CD95, another death receptor of the TNF superfamily (Figure 2A). Importantly, addition of nontoxic concentrations of XIAP inhibitors significantly enhanced TRAIL-induced apoptosis in a dose-dependent manner in all acute leukemia cell lines (Figure 2B,C). In contrast, the control compound had no effect on TRAIL-induced apoptosis compared with solvent, demonstrating the specificity of the sensitizing effect (Figure 2B,C).
XIAP inhibitors sensitize acute leukemia cells for TRAIL- or CD95-induced apoptosis. (A) Surface expression of TRAIL receptors TRAIL-R1 to -R4 and CD95 on acute leukemia cell lines was determined by fluorescence-conjugated antibodies and flow cytometry (thin line indicates cells stained with isotype control; thick line, cells stained with anti–TRAIL-R1 to -R4 or anti-CD95 antibodies). Fluorescence intensity (x-axis) is blotted against counts (y-axis). A representative experiment of 3 independent experiments is shown. (B-D) Acute leukemia cell lines were treated for 72 hours with indicated concentrations of TRAIL in the absence (□) or presence of XIAP inhibitors, control compound, or DMSO (Reh: 6 nM; Nalm-6: 60 nM; CEM: 60 nM; HL-60: 30 nM, Jurkat: 10 nM). (B,C) Apoptosis was determined by forwardside scatter analysis and flow cytometry in Reh, Nalm-6, CEM, HL-60 cells (B), and Jurkat (C) cells. (D) Apoptosis was determined in Jurkat cells by fluorescence-activated cell sorting (FACS) analysis of DNA fragmentation of propidium iodide–stained nuclei. (E) Morphologic features of apoptosis were determined by DAPI staining in Jurkat cells that were left untreated or were treated for 48 hours with 0.4 ng/mL TRAIL and 10 nM XIAP inhibitor 2, control compound, or DMSO. Arrows indicate apoptotic cells; scale bar represents 200 μm. (F) Jurkat cells transduced with XIAP or control shRNA or empty vector were analyzed for XIAP expression by Western blotting (left panel) and for apoptosis after treatment with 0.4 ng/mL TRAIL for 72 hours by forwardside scatter analysis and flow cytometry (right panel). (G) Jurkat cells transduced with empty vector or a vector containing Smac cDNA were analyzed for Smac expression by Western blotting (left panel) and for apoptosis after treatment with 0.4 ng/mL TRAIL for 72 hours by forwardside scatter analysis and flow cytometry (right panel); arrowhead indicates Flag-tagged Smac. (H) Clonogenic survival was assessed by colony assay as described in “Methods” in Jurkat cells that were left untreated or were treated with 0.4 ng/mL TRAIL in the presence or absence of 10 nM XIAP inhibitor 2, control compound, or DMSO. The percentage of colonies relative to untreated cells are shown. (I) Jurkat cells were treated for 72 hours with indicated concentrations of agonistic anti-CD95 antibodies in the absence (□) or presence of 10 nM XIAP inhibitors, control compound, or DMSO. Apoptosis was determined by forwardside scatter analysis and flow cytometry. (B-D,F-I) Mean and SD of 3 experiments each performed in triplicate (all except H) or duplicate (H) are shown. For statistical analysis, t test was performed comparing XIAP inhibitors to control compound (B-D,H-I), XIAP shRNA to control shRNA (F), or Smac to empty vector (G). #P < .05; *P < .01; **P < .001.
XIAP inhibitors sensitize acute leukemia cells for TRAIL- or CD95-induced apoptosis. (A) Surface expression of TRAIL receptors TRAIL-R1 to -R4 and CD95 on acute leukemia cell lines was determined by fluorescence-conjugated antibodies and flow cytometry (thin line indicates cells stained with isotype control; thick line, cells stained with anti–TRAIL-R1 to -R4 or anti-CD95 antibodies). Fluorescence intensity (x-axis) is blotted against counts (y-axis). A representative experiment of 3 independent experiments is shown. (B-D) Acute leukemia cell lines were treated for 72 hours with indicated concentrations of TRAIL in the absence (□) or presence of XIAP inhibitors, control compound, or DMSO (Reh: 6 nM; Nalm-6: 60 nM; CEM: 60 nM; HL-60: 30 nM, Jurkat: 10 nM). (B,C) Apoptosis was determined by forwardside scatter analysis and flow cytometry in Reh, Nalm-6, CEM, HL-60 cells (B), and Jurkat (C) cells. (D) Apoptosis was determined in Jurkat cells by fluorescence-activated cell sorting (FACS) analysis of DNA fragmentation of propidium iodide–stained nuclei. (E) Morphologic features of apoptosis were determined by DAPI staining in Jurkat cells that were left untreated or were treated for 48 hours with 0.4 ng/mL TRAIL and 10 nM XIAP inhibitor 2, control compound, or DMSO. Arrows indicate apoptotic cells; scale bar represents 200 μm. (F) Jurkat cells transduced with XIAP or control shRNA or empty vector were analyzed for XIAP expression by Western blotting (left panel) and for apoptosis after treatment with 0.4 ng/mL TRAIL for 72 hours by forwardside scatter analysis and flow cytometry (right panel). (G) Jurkat cells transduced with empty vector or a vector containing Smac cDNA were analyzed for Smac expression by Western blotting (left panel) and for apoptosis after treatment with 0.4 ng/mL TRAIL for 72 hours by forwardside scatter analysis and flow cytometry (right panel); arrowhead indicates Flag-tagged Smac. (H) Clonogenic survival was assessed by colony assay as described in “Methods” in Jurkat cells that were left untreated or were treated with 0.4 ng/mL TRAIL in the presence or absence of 10 nM XIAP inhibitor 2, control compound, or DMSO. The percentage of colonies relative to untreated cells are shown. (I) Jurkat cells were treated for 72 hours with indicated concentrations of agonistic anti-CD95 antibodies in the absence (□) or presence of 10 nM XIAP inhibitors, control compound, or DMSO. Apoptosis was determined by forwardside scatter analysis and flow cytometry. (B-D,F-I) Mean and SD of 3 experiments each performed in triplicate (all except H) or duplicate (H) are shown. For statistical analysis, t test was performed comparing XIAP inhibitors to control compound (B-D,H-I), XIAP shRNA to control shRNA (F), or Smac to empty vector (G). #P < .05; *P < .01; **P < .001.
To further investigate the role of XIAP in the regulation of apoptosis in acute leukemia cells, we selected Jurkat T-cell ALL cells as a model system, since they respond to the combination of XIAP inhibitors and TRAIL (Figure 2C) and also undergo apoptosis following treatment with XIAP inhibitors as single agents (Figure 1B). Kinetic analysis revealed that XIAP inhibitors enhanced TRAIL-induced apoptosis in a time-dependent manner in Jurkat cells (Figure 2C). To further explore whether cells die via apoptotic cell death, we also examined DNA fragmentation, one of the biochemical hallmarks of apoptosis, as well as some morphologic features of apoptosis. Cells exposed to the combination of XIAP inhibitors and TRAIL displayed enhanced DNA fragmentation (Figure 2D) as well as typical signs of nuclear apoptosis (ie, nuclear shrinkage), chromatin condensation, and nuclear fragmentation (Figure 2E), confirming the apoptotic nature of cell death. Furthermore, we antagonized XIAP by genetic approaches, using RNAi-mediated knockdown of XIAP as well as overexpression of the endogenous antagonist Smac. Similarly, XIAP knockdown or Smac overexpression significantly increased TRAIL-mediated apoptosis (Figure 2F,G). Further, ectopic expression of XIAP inhibited the cooperative interaction of XIAP inhibitors and TRAIL (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). To explore the possibility that TRAIL causes a decrease in XIAP levels that might increase the susceptibility of cells to XIAP inhibitors, we analyzed the effect of TRAIL on protein levels of XIAP. No changes in XIAP protein expression were detected upon treatment with TRAIL at a concentration that cooperated with XIAP inhibitors to trigger apoptosis (Figure S1B). This indicates that the cooperative interaction of TRAIL and XIAP inhibitors is not mediated by TRAIL-induced down-regulation of XIAP. Together, this set of experiments demonstrates that inhibition of XIAP acts in concert with TRAIL to induce apoptosis in acute leukemia cells.
XIAP inhibition cooperates with TRAIL to inhibit clonogenic survival of acute leukemia cells
In addition to short-term apoptosis assays, we also assessed the effect of XIAP inhibition on long-term clonogenic survival of leukemia cells. Importantly, XIAP inhibitor 2 cooperated with TRAIL to reduce clonogenic growth of Jurkat cells compared with cells treated with the control compound and TRAIL (Figure 2F). By comparison, treatment with XIAP inhibitor 2 or TRAIL alone had no or only a minor effect on colony formation (Figure 2F). These findings demonstrate that XIAP inhibition cooperates with TRAIL to inhibit clonogenicity and long-term survival of acute leukemia cells.
XIAP inhibitors enhance CD95-induced apoptosis in acute leukemia cells
To exclude that the apoptosis-sensitizing effect of XIAP inhibitors was restricted to the death-inducing ligand TRAIL, we extended our studies to CD95 as another death receptor system. Notably, XIAP inhibitors also significantly enhanced CD95-induced apoptosis in Jurkat cells (Figure 2G). These findings indicate that XIAP inhibitors lower the threshold for apoptosis after death receptor ligation in acute leukemia cells.
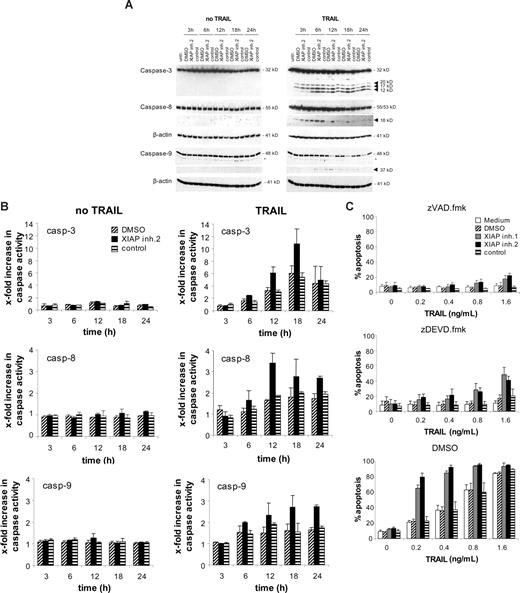
XIAP inhibition cooperates with TRAIL to induce activation of caspases
To systematically gain insight into the molecular mechanisms of the cooperative action of XIAP inhibitors and TRAIL in leukemia cells, we first explored whether inhibition of XIAP has an effect on TRAIL-induced activation of the caspase cascade. Monitoring cleavage of caspases by Western blot analysis revealed that addition of XIAP inhibitor 2 enhanced processing of caspase-3 as indicated by increased levels of the active caspase-3 fragments (Figure 3A). In addition, the caspase-8 active fragment p18 was detectable for a prolonged time after TRAIL stimulation in the presence of the XIAP inhibitor 2 compared with cells treated with the control compound or solvent indicating that XIAP inhibitor 2 enhances caspase-8 activation (Figure 3A). Inhibition of caspase-3 by zDEVD.fmk reduced the enhanced cleavage of caspase-8 in cells treated with XIAP inhibitor and TRAIL (Figure S3), indicating that the increased caspase-3 activity in these cells contributes to the enhanced caspase-8 cleavage. Furthermore, we directly assessed caspase activity by enzymatic caspase assays. XIAP inhibitor 2 increased TRAIL-induced activity of caspases-3, -8, and -9 compared with the control compound (Figure 3B). To examine whether caspases were required for apoptosis induction, we tested the effect of the broad range caspase inhibitor zVAD.fmk and of zDEVD.fmk, a relatively selective inhibitor of effector caspase-3/7. Apoptosis induced by TRAIL and XIAP inhibitors was almost completely blocked by zVAD.fmk and substantially reduced by zDEVD.fmk (Figure 3C). These data show that inhibition of XIAP enhances TRAIL-induced apoptosis in a caspase-dependent manner in acute leukemia cells.
XIAP inhibition enhances TRAIL-induced activation of caspases. Jurkat leukemia cells were left untreated or were treated with 0.4 ng/mL TRAIL and/or 10 nM XIAP inhibitor 2, control compound, or DMSO for indicated times. (A) Caspase activation was analyzed by Western blotting. Arrowheads indicate caspase cleavage fragments; the asterisks mark unspecific bands. (B) Caspase activity was determined by FACS analysis as described in “Methods,” and x-fold increase in caspase activity is shown. (C) Jurkat leukemia cells were left untreated (□) or were treated for 72 hours with indicated concentrations of TRAIL and/or 10 nM XIAP inhibitors, control compound, or DMSO and 20 μM zVAD.fmk, 50 μM zDEVD.fmk, or DMSO. Apoptosis was determined by forwardside scatter analysis and flow cytometry. Mean and SD of 3 experiments each performed in triplicate are shown.
XIAP inhibition enhances TRAIL-induced activation of caspases. Jurkat leukemia cells were left untreated or were treated with 0.4 ng/mL TRAIL and/or 10 nM XIAP inhibitor 2, control compound, or DMSO for indicated times. (A) Caspase activation was analyzed by Western blotting. Arrowheads indicate caspase cleavage fragments; the asterisks mark unspecific bands. (B) Caspase activity was determined by FACS analysis as described in “Methods,” and x-fold increase in caspase activity is shown. (C) Jurkat leukemia cells were left untreated (□) or were treated for 72 hours with indicated concentrations of TRAIL and/or 10 nM XIAP inhibitors, control compound, or DMSO and 20 μM zVAD.fmk, 50 μM zDEVD.fmk, or DMSO. Apoptosis was determined by forwardside scatter analysis and flow cytometry. Mean and SD of 3 experiments each performed in triplicate are shown.
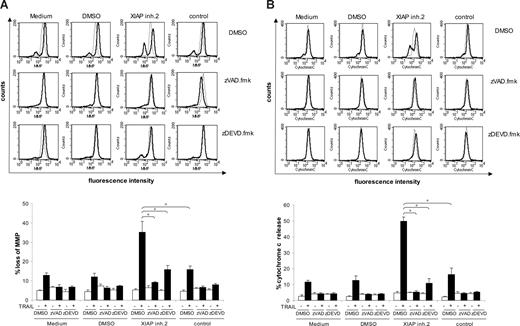
XIAP inhibition cooperates with TRAIL to induce mitochondrial perturbations in a caspase-dependent manner
We next asked whether XIAP inhibition has an effect on signaling via the mitochondrial pathway during TRAIL-induced apoptosis, since Jurkat leukemia cells are type II cells that depend on the mitochondrial contribution to death receptor–induced apoptosis.6 Although treatment with TRAIL alone caused only a slight drop of mitochondrial membrane potential, addition of XIAP inhibitor 2, but not of the control compound, significantly enhanced this TRAIL-induced loss of mitochondrial membrane potential (Figure 4A). In addition, XIAP inhibitor 2, but not the control compound, significantly increased the release of cytochrome c from mitochondria upon treatment with TRAIL (Figure 4B). These findings demonstrate that XIAP inhibition markedly enhances TRAIL-induced loss of mitochondrial membrane potential and cytochrome c release, suggesting that inhibition of XIAP promotes mitochondrial perturbations.
XIAP inhibition enhances TRAIL-induced mitochondrial perturbations in a caspase-dependent manner. Jurkat leukemia cells were treated for 24 hours with 0.4 ng/mL TRAIL and/or 10 nM XIAP inhibitor 2, control compound, or DMSO and 20 μM zVAD.fmk, 50 μM zDEVD.fmk, or DMSO. Mitochondrial transmembrane potential (A) and cytochrome c release (B) were assessed by FACS analysis. Representative experiments of at least 3 experiments are shown in top panels (dotted line indicates no TRAIL; solid line, TRAIL-treated cells); mean and SD of 3 experiments each performed in duplicate are shown in the bottom panels. For statistical analysis, t test was performed. *P < .01.
XIAP inhibition enhances TRAIL-induced mitochondrial perturbations in a caspase-dependent manner. Jurkat leukemia cells were treated for 24 hours with 0.4 ng/mL TRAIL and/or 10 nM XIAP inhibitor 2, control compound, or DMSO and 20 μM zVAD.fmk, 50 μM zDEVD.fmk, or DMSO. Mitochondrial transmembrane potential (A) and cytochrome c release (B) were assessed by FACS analysis. Representative experiments of at least 3 experiments are shown in top panels (dotted line indicates no TRAIL; solid line, TRAIL-treated cells); mean and SD of 3 experiments each performed in duplicate are shown in the bottom panels. For statistical analysis, t test was performed. *P < .01.
Therefore, to explore the possibility of a cross talk between XIAP-regulated caspase activation and mitochondrial injury, we next examined whether caspase activity was required for mitochondrial perturbations during apoptosis induced by XIAP inhibitors and TRAIL. Notably, addition of zVAD.fmk almost completely blocked loss of mitochondrial membrane potential and cytochrome c release in cells treated with the combination of XIAP inhibitor 2 and TRAIL compared with cells that were exposed to XIAP inhibitor 2 and TRAIL in the absence of zVAD.fmk (Figure 4A,B). Interestingly, also the caspase-3/7 inhibitor zDEVD.fmk strongly reduced drop of mitochondrial membrane potential and almost completely prevented cytochrome c release following treatment with XIAP inhibitor 2 and TRAIL (Figure 4A,B). Although chemical inhibitors of caspases lack absolute specificity,30 these findings indicate that the enhanced loss of mitochondrial membrane potential and cytochrome c release, which is caused by the combination of XIAP inhibition and TRAIL, is largely mediated by effector caspases (ie, caspase-3/7). Thus, XIAP inhibition may promote a caspase-dependent feedback mitochondrial amplification loop during TRAIL-induced apoptosis in acute leukemia cells.
XIAP inhibitors overcome Bcl-2–mediated resistance to TRAIL-induced apoptosis
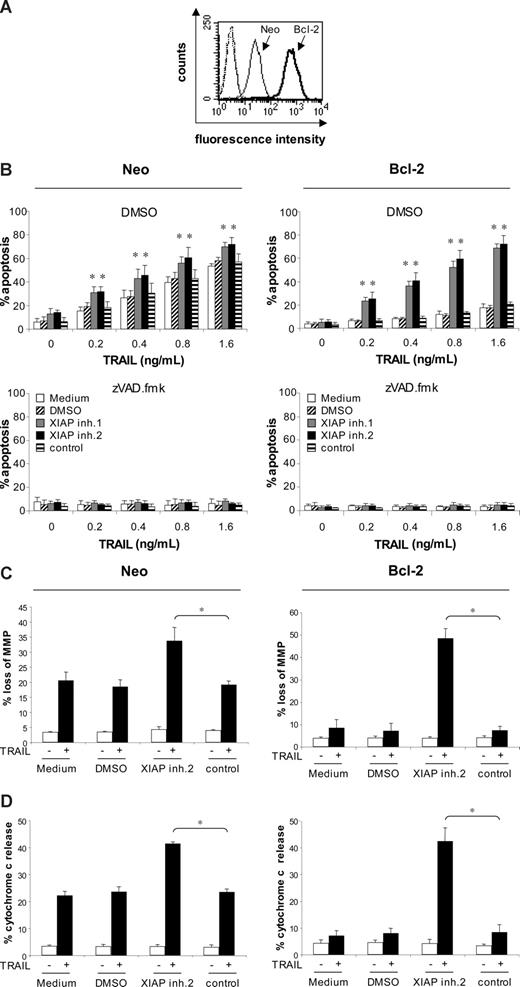
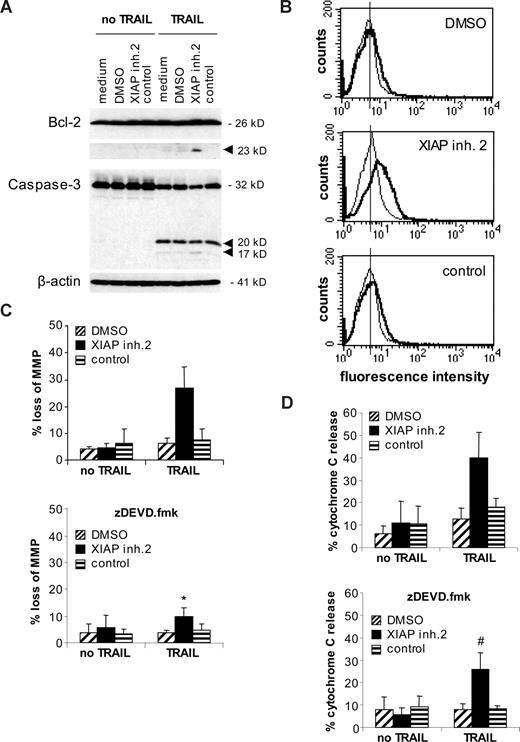
Next, we asked whether the combined use of XIAP inhibitors and TRAIL is also effective in acute leukemia cells with a dysfunctional mitochondrial apoptosis pathway (eg, because of increased expression of Bcl-2). High Bcl-2 levels and a decrease of the Bax/Bcl-2 ratio have been associated with poor prognosis in childhood ALL in some studies.31,32 We therefore investigated the effect of XIAP inhibitors on TRAIL-induced apoptosis in Jurkat leukemia cells with ectopic expression of Bcl-2 (Figure 5A). Bcl-2 overexpression almost completely inhibited TRAIL-induced apoptosis (Figure 5B), consistent with the idea that type II Jurkat cells depend on the mitochondrial pathway for TRAIL-induced apoptosis.6,7,33 Interestingly, XIAP inhibitors similarly cooperated with TRAIL to induce apoptosis in Bcl-2–overexpressing and vector control cells (Figure 5B top panel), showing that XIAP inhibitors are able to overcome Bcl-2–mediated resistance toward TRAIL. Apoptosis induced by XIAP inhibitors and TRAIL was inhibited by the broad range caspase inhibitor zVAD.fmk in both Bcl-2–overexpressing and vector control cells (Figure 5B bottom panel), demonstrating that XIAP inhibitors potentiate TRAIL-induced apoptosis in a caspase-dependent manner. In addition, Bcl-2 overexpression inhibited apoptosis that was induced by toxic concentrations of XIAP inhibitors (Figure S3). To explore whether the combination of XIAP inhibitors and TRAIL triggers apoptosis in Bcl-2–overexpressing cells by bypassing the requirement of the mitochondrial pathway for TRAIL-induced apoptosis or by breaking mitochondrial resistance, we examined parameters of mitochondrial apoptosis that have been reported to be under the control of Bcl-2.34 XIAP inhibitor 2 and TRAIL similarly triggered loss of mitochondrial potential and cytochrome c release in Bcl-2–overexpressing and vector control cells, whereas overexpression of Bcl-2 inhibited loss of mitochondrial membrane potential and cytochrome c release induced by treatment with TRAIL alone (Figure 5C,D). In Bcl-2–overexpressing cells, cleavage of Bcl-2 protein was substantially increased in cells treated with the combination of XIAP inhibitor and TRAIL, which was associated with enhanced cleavage of caspase-3 into the active p17 fragment (Figure 6A). Further, the XIAP inhibitor combined with TRAIL caused increased Bak conformational change compared with Bcl-2–overexpressing cells that were treated with either agent alone (Figure 6B). Bax conformational change could not be assessed in Jurkat cells, since they lack Bax expression due to a mutation.35 Moreover, the enhanced mitochondrial damage that was induced by the XIAP inhibitor and TRAIL (ie, loss of mitochondrial membrane potential and cytochrome c release) was significantly reduced in the presence of the caspase-3 inhibitor zDEVD.fmk (Figure 6C,D). Together, these data suggest that XIAP inhibitors overcome Bcl-2–mediated resistance to TRAIL, at least in part, by enhancing caspase-3 activation, which in turn acts back on the mitochondria by promoting cleavage of Bcl-2, Bak conformational change, and mitochondrial perturbations.
XIAP inhibitors overcome Bcl-2–mediated resistance to TRAIL-induced apoptosis. (A) Bcl-2 expression was determined in Jurkat leukemia cells transfected with empty vector (Neo) or Bcl-2 by cytoplasmic staining and flow cytometry (dotted thin and thick lines indicate vector control and Bcl-2–overexpressing cells stained with isotype control; thin line, vector control cells stained with Bcl-2 antibody; thick line, Bcl-2–overexpressing cells stained with Bcl-2 antibody). Fluorescence intensity (x-axis) is blotted against counts (y-axis). (B) Jurkat leukemia cells transfected with empty vector (Neo) or Bcl-2 were treated for 72 hours with indicated concentrations of TRAIL in the absence (□) or presence of 10 nM (top and middle panels) or indicated concentrations (bottom panels) of XIAP inhibitors, control compound, or DMSO and 20 μM zVAD.fmk or DMSO. Apoptosis was determined by forwardside scatter analysis and flow cytometry. (C,D) Jurkat leukemia cells transfected with empty vector (Neo) or Bcl-2 were treated for 48 hours with 0.4 ng/mL TRAIL and/or 10 nM XIAP inhibitor 2, control compound, or DMSO. Mitochondrial transmembrane potential (C) and cytochrome c release (D) were assessed by FACS analysis. Mean and SD of 3 experiments each performed in triplicate are shown. For statistical analysis, t test was performed comparing XIAP inhibitor 2 to control compound, *P < .01.
XIAP inhibitors overcome Bcl-2–mediated resistance to TRAIL-induced apoptosis. (A) Bcl-2 expression was determined in Jurkat leukemia cells transfected with empty vector (Neo) or Bcl-2 by cytoplasmic staining and flow cytometry (dotted thin and thick lines indicate vector control and Bcl-2–overexpressing cells stained with isotype control; thin line, vector control cells stained with Bcl-2 antibody; thick line, Bcl-2–overexpressing cells stained with Bcl-2 antibody). Fluorescence intensity (x-axis) is blotted against counts (y-axis). (B) Jurkat leukemia cells transfected with empty vector (Neo) or Bcl-2 were treated for 72 hours with indicated concentrations of TRAIL in the absence (□) or presence of 10 nM (top and middle panels) or indicated concentrations (bottom panels) of XIAP inhibitors, control compound, or DMSO and 20 μM zVAD.fmk or DMSO. Apoptosis was determined by forwardside scatter analysis and flow cytometry. (C,D) Jurkat leukemia cells transfected with empty vector (Neo) or Bcl-2 were treated for 48 hours with 0.4 ng/mL TRAIL and/or 10 nM XIAP inhibitor 2, control compound, or DMSO. Mitochondrial transmembrane potential (C) and cytochrome c release (D) were assessed by FACS analysis. Mean and SD of 3 experiments each performed in triplicate are shown. For statistical analysis, t test was performed comparing XIAP inhibitor 2 to control compound, *P < .01.
XIAP inhibition promotes Bcl-2 cleavage and Bak conformational change in Bcl-2–overexpressing Jurkat cells. (A) Jurkat leukemia cells transfected with Bcl-2 were treated for 12 hours with 1.6 ng/mL TRAIL and/or 10 nM XIAP inhibitor 2, control compound, or DMSO. Cleavage of Bcl-2 and caspase-3 was analyzed by Western blotting. Arrowheads indicate cleavage fragments. (B) Jurkat leukemia cells transfected with Bcl-2 were treated for 12 hours with 0.4 ng/mL TRAIL and/or 10 nM XIAP inhibitor 2, control compound, or DMSO. Bak conformational change was assessed by FACS analysis. Fluorescence intensity (x-axis) is plotted against cell counts (y-axis). A representative experiment of 3 experiments is shown (thin line indicates untreated cells; solid line, TRAIL-treated cells). (C,D) Jurkat leukemia cells transfected with Bcl-2 were treated for 48 hours with 0.4 ng/mL TRAIL and/or 10 nM XIAP inhibitor 2, control compound, or DMSO in the absence (top panels) or presence (bottom panels) of 20 μM zDEVD.fmk. Mitochondrial transmembrane potential (C) and cytochrome c release (D) were assessed by FACS analysis. Mean and SD of 3 experiments each performed in triplicate are shown. For statistical analysis, t test was performed comparing samples treated with XIAP inhibitor 2 and TRAIL in the absence (top panels of C and D) and presence (bottom panels of C and D) of zDEVD.fmk. #P < .05; *P < .01.
XIAP inhibition promotes Bcl-2 cleavage and Bak conformational change in Bcl-2–overexpressing Jurkat cells. (A) Jurkat leukemia cells transfected with Bcl-2 were treated for 12 hours with 1.6 ng/mL TRAIL and/or 10 nM XIAP inhibitor 2, control compound, or DMSO. Cleavage of Bcl-2 and caspase-3 was analyzed by Western blotting. Arrowheads indicate cleavage fragments. (B) Jurkat leukemia cells transfected with Bcl-2 were treated for 12 hours with 0.4 ng/mL TRAIL and/or 10 nM XIAP inhibitor 2, control compound, or DMSO. Bak conformational change was assessed by FACS analysis. Fluorescence intensity (x-axis) is plotted against cell counts (y-axis). A representative experiment of 3 experiments is shown (thin line indicates untreated cells; solid line, TRAIL-treated cells). (C,D) Jurkat leukemia cells transfected with Bcl-2 were treated for 48 hours with 0.4 ng/mL TRAIL and/or 10 nM XIAP inhibitor 2, control compound, or DMSO in the absence (top panels) or presence (bottom panels) of 20 μM zDEVD.fmk. Mitochondrial transmembrane potential (C) and cytochrome c release (D) were assessed by FACS analysis. Mean and SD of 3 experiments each performed in triplicate are shown. For statistical analysis, t test was performed comparing samples treated with XIAP inhibitor 2 and TRAIL in the absence (top panels of C and D) and presence (bottom panels of C and D) of zDEVD.fmk. #P < .05; *P < .01.
XIAP inhibitors do not reverse the lack of toxicity of TRAIL on PBLs
To investigate whether XIAP inhibitors alone or in combination with TRAIL are cytotoxic to nonmalignant cells, we extended our studies to normal PBLs that were isolated from healthy donors. We assayed both unstimulated PBLs as well as activated PBLs 6 days after stimulation with PHA, since sensitivity of peripheral lymphocytes to CD95-mediated apoptosis has been reported to increase upon mitogen stimulation.36 Dose-response experiments revealed that unstimulated as well as stimulated PBLs were almost completely refractory toward treatment with XIAP inhibitors alone (Figure S4A,B). Importantly, XIAP inhibitors also had not effect on apoptosis sensitivity of unstimulated or stimulated PBLs toward TRAIL even at TRAIL concentrations 500 times higher than those required to induce apoptosis in Jurkat leukemia cells (Figure S4C,D, compare Figure 2C). Similarly, XIAP inhibitors did not reverse the resistance of unstimulated PBLs to CD95-mediated apoptosis even at high concentrations of agonistic anti-CD95 antibodies (Figure S4E). XIAP inhibitors also had no additional effect on CD95-induced apoptosis of stimulated PBLs (Figure S4F). The observation that PBLs acquire sensitivity toward CD95-induced apoptosis upon mitogen stimulation (Figure S4F), which was accompanied by an increase in CD95 surface expression (Figure S4G), is in line with previous reports.36,37 The lack of responsiveness of PBLs to TRAIL was not due to absent expression of agonistic TRAIL receptors, since unstimulated and stimulated PBLs express agonistic TRAIL-R2 on their surface (Figure S4G). In addition, there was no consistent increase in XIAP expression in PBLs compared with Jurkat cells, although XIAP expression in stimulated PBLs varied among healthy donors (Figure S4H). IAP antagonists Smac/DIABLO and Omi/HtrA2 were expressed in both PBLs and Jurkat cells, whereas expression of cIAP1, cIAP2, and survivin was absent or very weak in unstimulated PBLs compared with Jurkat cells (Figure S4H). Therefore, it appears unlikely that the resistance of unstimulated and stimulated PBLs to XIAP inhibitors and TRAIL is simply due to high XIAP expression or to the absence of agonistic TRAIL receptors in these cells. However, the underlying mechanism(s) for the differential sensitivity of normal peripheral blood versus leukemia cells toward XIAP inhibitors and TRAIL is subject to further investigation. Together, this set of data demonstrates that XIAP inhibitors are nontoxic to normal PBLs and also do not enhance TRAIL- or CD95-induced apoptosis at concentrations that are sufficient to sensitize acute leukemia cells for TRAIL- or CD95-triggered apoptosis.
XIAP inhibitors act in concert with TRAIL to trigger apoptosis in primary childhood ALL cells and also induce apoptosis as single agents
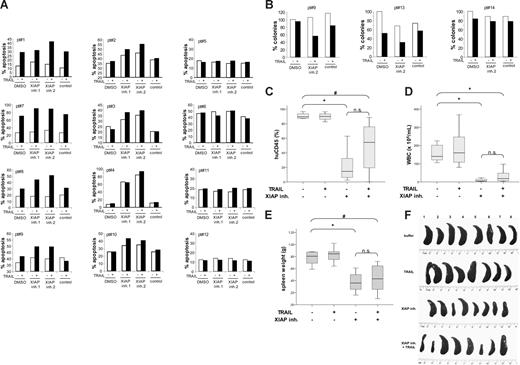
To validate the results obtained in cell lines, we extended our studies to freshly isolated, primary leukemic blasts derived from samples of children with common B-cell precursor ALL before the onset of chemotherapy. Patients' characteristics are summarized in Table 1. Importantly, XIAP inhibitors acted in concert with TRAIL to trigger apoptosis in leukemic blasts in 8 of 12 primary samples (Figure 7A patient nos. 1-4,7-10). In addition, XIAP inhibitors as single agents induced apoptosis in 4 of 12 primary samples (Figure 7A patient nos. 2-4,10). Treatment with TRAIL alone caused apoptosis in 4 of 12 samples (Figure 7A patient nos. 1,7-9), whereas 4 of 12 samples were refractory toward treatment with TRAIL and/or XIAP inhibitors (Figure 7A patient nos. 5,6,11,12). In addition, XIAP inhibitor 2 cooperated with TRAIL to reduce colony formation of primary ALL blasts in 2 of 3 samples, and treatment with either the XIAP inhibitor or TRAIL alone reduced colony formation in 1 of 3 samples (Figure 7B). Although there was some variability among the samples, reflecting the heterogeneity of primary samples, these experiments provide proof-of-principle that XIAP inhibitors as single agents elicit apoptosis in primary pediatric ALL cells and cooperate with TRAIL to induce apoptosis.
Effect of XIAP inhibitors and TRAIL on primary childhood ALL cells ex vivo and in a NOD/SCID mouse model in vivo. Primary leukemic blasts from children with ALL before the onset of chemotherapy were left untreated (□) or were treated for 24 hours with 1 μg/mL TRAIL (■) in the presence of 10 nM XIAP inhibitors, control compound, or DMSO. Apoptosis was determined by forwardside scatter analysis and flow cytometry (A) and colony formation was assessed as described in “Methods” (B). (C-F) NOD/SCID mice were inoculated with 107 human leukemic cells. Treatment started on day 3 with 40 mg/kg XIAP inhibitor 2 and/or 50 μg TRAIL/mouse. Drugs were administered intraperitoneally 5 days/week for 4 weeks and mice were killed on day 34. The percentage of human CD45+ cells on day 34 (C), the number of white blood cells on day 34 (D), and spleen weight on day 34 (E) are depicted by box plots; the line inside each box denotes median; boxes, 25th and 75th percentiles; error bars, minimum and maximum; #P < .05; *P < .01; and n.s., not significant. (F) Spleens are depicted at autopsy on day 34.
Effect of XIAP inhibitors and TRAIL on primary childhood ALL cells ex vivo and in a NOD/SCID mouse model in vivo. Primary leukemic blasts from children with ALL before the onset of chemotherapy were left untreated (□) or were treated for 24 hours with 1 μg/mL TRAIL (■) in the presence of 10 nM XIAP inhibitors, control compound, or DMSO. Apoptosis was determined by forwardside scatter analysis and flow cytometry (A) and colony formation was assessed as described in “Methods” (B). (C-F) NOD/SCID mice were inoculated with 107 human leukemic cells. Treatment started on day 3 with 40 mg/kg XIAP inhibitor 2 and/or 50 μg TRAIL/mouse. Drugs were administered intraperitoneally 5 days/week for 4 weeks and mice were killed on day 34. The percentage of human CD45+ cells on day 34 (C), the number of white blood cells on day 34 (D), and spleen weight on day 34 (E) are depicted by box plots; the line inside each box denotes median; boxes, 25th and 75th percentiles; error bars, minimum and maximum; #P < .05; *P < .01; and n.s., not significant. (F) Spleens are depicted at autopsy on day 34.
XIAP inhibitor exerts antileukemic activity in vivo in a mouse model of childhood ALL
Finally, we evaluated the in vivo efficacy of XIAP inhibitor 2 alone and in combination with TRAIL in a NOD/SCID mouse model of childhood ALL. Xenografts established from pediatric ALL samples have been reported to provide a clinically relevant experimental in vivo model of childhood leukemia.38 Strikingly, in vivo treatment with the XIAP inhibitor caused a profound reduction of leukemia burden compared with the control group as determined by the percentage of leukemic blasts, white blood cell count, and spleen weight (Figure 7C-F). The addition of TRAIL did not further enhance the antileukemic activity of the XIAP inhibitor compared with treatment with XIAP inhibitor as single agent (Figure 7C-F), possibly because of the profound effect of the XIAP inhibitor alone at the concentration used. Parallel analysis of body weight revealed no signs of treatment-related toxicity (Table 2). These findings demonstrate that the XIAP inhibitor exerts in vivo antileukemic activity against pediatric ALL without detectable toxicity.
Discussion
XIAP inhibitors alone and in combination with TRAIL trigger apoptosis in childhood acute leukemia
Here, we provide for the first time evidence that small molecule XIAP inhibitors trigger apoptosis in childhood acute leukemia cells and cooperate with TRAIL in apoptosis induction. This conclusion is supported by several independent pieces of evidence. First, XIAP inhibitors at subtoxic concentrations, but not a structurally related control compound, act in concert with TRAIL to elicit apoptosis in acute leukemia cells. Second, XIAP inhibitors cooperate with TRAIL to reduce clonogenic survival of acute leukemia cells, demonstrating that they also suppress long-term survival. Third, XIAP inhibitors break resistance to TRAIL that is imposed by Bcl-2 overexpression, indicating that they may overcome a defect in the apoptotic pathway that is common in acute leukemia and associated with poor prognosis. Fourth, XIAP inhibitors at equimolar concentrations are nonlethal to normal PBLs, alone or in combination with TRAIL or anti-CD95 agonistic antibodies, pointing to some tumor selectivity. Fifth, XIAP inhibitors, but not the control compound, act in concert with TRAIL to trigger apoptosis in primary leukemic blasts from children with ALL and also kill ALL cells as single agents. Sixth, the XIAP inhibitor exhibits in vivo antileukemic activity in a mouse model of pediatric ALL. Thus, small molecule XIAP inhibitors present a promising novel approach for apoptosis-based therapy of childhood acute leukemia.
There is accumulating evidence that XIAP plays an important role in regulating apoptosis sensitivity of cancer cells and also bears prognostic impact.19,41 For example, in childhood AML, high expression of XIAP in leukemic blasts has been described to correlate with poor survival.42 We previously demonstrated proof-of-concept in a glioblastoma mouse model that Smac peptides that neutralize XIAP enhance TRAIL-induced killing in vivo.21 Small molecule inhibitors targeting the BIR2 domain of XIAP were subsequently reported to induce apoptosis when administered as single agents in Jurkat T-cell leukemia, AML, and diffuse large B-cell lymphoma.41,43,44 However, the important question whether XIAP antagonists can be used as a sensitizer to restore or enhance the responsiveness of childhood acute leukemia cells toward TRAIL has not yet been addressed. Thus, our study is the first to demonstrate that XIAP inhibitors act in concert with TRAIL to induce apoptosis in childhood acute leukemia. Studies evaluating XIAP in a specific type of cancer, for example in pediatric acute leukemia, are relevant for the rational use of XIAP antagonists as molecular cancer therapeutics in human cancers, since it is increasingly becoming clear that XIAP regulates apoptosis in a context-dependent (eg, cell type–dependent) and stimulus-dependent manner. For example, XIAP inhibitors directed against the BIR2 domain of XIAP failed to enhance AraC-mediated cell death in Jurkat T-cell leukemia and AML cells.41,43 It was also reported that knockdown of XIAP by RNA interference had no effect on etoposide-induced apoptosis of Jurkat leukemia cells.45 Thus, by demonstrating that XIAP inhibitors cooperate with TRAIL to induce apoptosis in childhood acute leukemia cell lines and also primary leukemic blasts, our study has important implications for the design of effective combination therapies with TRAIL for children with acute leukemia (eg, together with small molecule XIAP inhibitors). It will be subject to our future investigations to also test subtoxic concentrations of the XIAP inhibitor in combination with TRAIL in vivo, as the XIAP inhibitor at the concentration used already profoundly reduced leukemia burden in NOD/SCID mice.
XIAP inhibitors overcome Bcl-2–mediated resistance to TRAIL
Another conceptual advance of our study is that we demonstrate for the first time that XIAP inhibitors overcome Bcl-2–mediated resistance to TRAIL in leukemia cells. XIAP inhibitors may break Bcl-2–imposed resistance to TRAIL by promoting caspase-3 activation, which in turn triggers additional mitochondrial damage in response to TRAIL (eg, via Bcl-2 cleavage and Bak conformational change). By comparison, DNA-damaging anticancer drugs or ionizing irradiation have been reported to sensitize cancer cells for TRAIL by bypassing the requirement of mitochondria-dependent events for effector caspase activation without overruling the Bcl-2–mediated inhibition of the mitochondrial pathway.46,47
Combination strategy for apoptosis-based cancer therapy of childhood leukemia
Our study also highlights the significance of a combination approach for apoptosis-based cancer therapy in leukemia. The idea to directly trigger apoptosis in cancer cells by stimulating death receptors is attractive, since these receptors have a direct link to the cell's intrinsic death machinery.3 Recombinant soluble TRAIL or antibodies that engage agonistic TRAIL receptors were shown to induce apoptosis in numerous cancer cell lines and preclinical cancer models and are currently evaluated in early clinical trials alone or in combination with conventional chemotherapeutics.10,11 However, many human cancers including leukemia may present with intrinsic or acquired resistance to TRAIL.11 We previously reported that 50% of primary samples obtained from children with acute leukemia before the onset of chemotherapy were refractory to TRAIL-mediated apoptosis.14 In some of these resistant samples, TRAIL even attenuated spontaneous apoptosis and stimulated proliferation,14 pointing to a prosurvival function of TRAIL under certain conditions. In addition, results from early clinical trials indicate that mechanisms of resistance to TRAIL receptor agonists may have to be taken into consideration.48,49 The failure of acute leukemia cells to undergo apoptosis upon exposure to TRAIL may be caused by defects in the apoptotic machinery (eg, by high levels of IAPs such as XIAP).18 This highlights the demand to pursue rational TRAIL-based combination protocols that counter resistance mechanisms in leukemia cells to ensure the success of TRAIL receptor agonists in the clinic. Numerous studies have shown that the combined use of conventional chemotherapeutic drugs or irradiation can augment TRAIL-induced apoptosis in cancer cells.11 Ideally, however, agents used in combination with TRAIL should be nontoxic and should not undermine the tumor-specific proapoptotic effects of TRAIL. Since small molecule IAP inhibitors that mimic the N-terminal part of Smac have recently entered phase 1 clinical trials,50 it is of great interest to explore their potential also in pediatric cancers, for example in childhood ALL. By demonstrating that XIAP inhibitors cooperate with the death ligand TRAIL to trigger apoptosis in acute leukemia cells but not in normal lymphocytes, our data have important clinical implications for the design of TRAIL-based protocols. It is important to note that the cooperative interaction between XIAP inhibitors and TRAIL to induce apoptosis occurred in a dose-dependent manner, especially at subtoxic concentrations. This suggests that XIAP inhibitors may be used in TRAIL-based protocols to reduce the dose of TRAIL receptor agonists that are required for antileukemic activity, thereby potentially reducing the risk of toxic side effects and increasing the therapeutic index of TRAIL-containing regimens. In addition, combination therapies using relatively low doses of each agent may offer advantages, since drug concentrations that are found to be active in vitro may not be achieved in vivo. Further, the combination of XIAP inhibitors and TRAIL may be of special relevance in leukemias, which poorly respond to treatment with TRAIL alone (eg, because of Bcl-2 overexpression). In conclusion, XIAP inhibitors in combination with TRAIL and also as single agents present a promising novel approach for apoptosis-targeted therapies of childhood acute leukemia, which warrants further exploitation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank C. Hulford and M. Luzzio (Pfizer) for providing XIAP inhibitors, H. Walczak (Heidelberg, Germany) for providing Bcl-2–transfected Jurkat cells, J. Moyer and K. Coleman (Pfizer) for helpful discussions, and I. Fichtner (Berlin, Germany) for help with animal experiments.
This work has been partially supported by grants from the Deutsche Forschungsgemeinschaft (Bonn, Germany), the Deutsche Krebshilfe (Bonn, Germany), the Kind-Philipp Stiftung (Bonn, Germany), EU (Apoptrain APO-SYS, Brussels, Belgium), and IAP6/18 (Brussels, Belgium, S.F., and M.F.).
Authorship
Contribution: M.F. and S.L. performed research and statistical analysis and analyzed and interpreted data; M.V. analyzed and interpreted data; K.S. performed research and analyzed data; I.J. performed research and analyzed and interpreted data; K.-M.D. interpreted data; S.F. designed research, analyzed and interpreted data, and wrote the paper; and all authors approved the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Simone Fulda, University Children's Hospital, Eythstr 24, D-89075 Ulm, Germany; e-mail: simone.fulda@uniklinik-ulm.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal