Abstract
Exposure of offspring to noninherited maternal antigens (NIMAs) during pregnancy may have an impact on transplantations performed later in life. Using a mouse model, we recently showed that bone marrow transplantation (BMT) from NIMA-exposed offspring to the mother led to a reduction of graft-versus-host disease (GVHD). Since offspring can also be exposed to NIMAs by breastfeeding after birth, we tested whether breast milk could mediate the tolerogenic NIMA effect. We found that oral exposure to NIMAs by breastfeeding alone was sufficient to reduce GVHD, and that in utero exposure to NIMAs is required for maximum reduction of GVHD. The tolerogenic milk effects disappeared when donor mice were injected with CD25 monoclonal antibodies during the lactation period, suggesting a CD4+CD25+ regulatory T cell–dependent mechanism. Our results suggest a previously unknown impact of breastfeeding on the outcome of transplantation.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potential curative treatment for malignant hematologic diseases; however, HSCT from haploidentical-related donors is complicated by a high incidence of severe graft-versus-host disease (GVHD).
The fetus and mother must tolerate each other's alloantigens during pregnancy. Fetal and maternal antigens transmitted through the bidirectional transplacental passage during pregnancy might induce tolerance to noninherited maternal antigens (NIMAs) in the offspring and inherited paternal antigens (IPAs) in the mother.1-4 Exposure of the fetus to allogeneic cells may induce a long-lasting tolerance specific to the alloantigens of the donor cells.5,6 We recently demonstrated using a mouse model that a “child-to-mother” bone marrow transplantation (BMT) from a NIMA-exposed donor reduces the mortality and morbidity of GVHD, but a “mother-to-child” BMT from a mother donor exposed to IPAs from the fetus does not.7 We therefore tested the hypothesis that breastfeeding plays an important role in the buildup of the tolerogenic NIMA effect in a mouse model of a “child-to-mother” BMT because breast milk is rich in maternal major histocompatibility complex (MHC) antigens in both soluble and cellular forms.8-11
Methods
Mice
Female C57BL/6 (B6, H-2b/b) and B6D2F1 (H-2b/d) mice were purchased from Charles River Japan (Yokohama, Japan). B6D1F1 (H-2b/q) mice were produced by mating a DBA/1 (H-2q) female (Japan SLC, Shizuoka, Japan) and a B6 male. NIMA-exposed H-2b mice were produced by mating a B6 (H-2b) male and a B6D2F1 (H-2b/d) female as previously described.7,9,12 Offspring were typed for the H-2 locus by flow cytometry using monoclonal antibodies (mAbs) specific for H-2Kb and H-2Kd (BD Pharmingen, San Diego, CA) as previously described.7 The resultant H-2b/b offspring were nursed by either a B6D2F1 mother (NIMA [in utero + oral]) or a B6 foster mother (NIMA [in utero]). Controls were H-2b mice not exposed to H-2d. To produce NIMA-exposed mice via breastfeeding, B6 neonates were nursed by a B6D2F1 foster mother (NIMA [oral]). Mice were weaned at 3 weeks after birth. These mice were used as BMT donors at 6 to 10 weeks of age. BMT was performed as previously described.7 All animal experiments were performed under the auspices of the Institutional Animal Research Advisory Committee at Okayama University.
BMT
Mice underwent transplantation as previously described.13 In brief, after lethal total body irradiation (TBI; x-ray) was delivered in 2 doses at 3-hour intervals to minimize gastrointestinal toxicity, mice were intravenously injected with 5 × 106 T cell–depleted bone marrow (TCD-BM) cells plus 0.5 to 2 × 106 T cells, CD4+ T cells, or CD25-depleted CD4+ T cells from the donors on day 0. T-cell depletion, CD25 depletion, and splenic CD4+ T-cell isolation were performed using Auto-MACS (Miltenyi Biotec, Tokyo, Japan) as previously described.7,14 Hybridomas secreting anti-CD25 mAbs (clone PC61) were obtained from the ATCC (Manassas, VA). For in vivo depletion of CD25+ cells,14,15 mice were injected subcutaneously with 75 μg/g body weight of anti-CD25 or irrelevant mAbs on days 1 and 8 after birth. They were then housed in sterilized microisolator cages, and given autoclaved hyperchlorinated drinking water for the first 3 weeks after BMT and filtered water thereafter. Survival after BMT was monitored daily and the degree of clinical GVHD was assessed weekly by a scoring system that evaluated changes in 5 clinical parameters: weight loss, posture, activity, fur texture, and skin integrity (maximum index = 10) as previously described.16
Flow cytometry
Flow cytometric analysis was performed as previously described.13 The mAbs used were biotinylated anti–mouse CD25 (clone 7D4); FITC- or PE-conjugated anti–mouse CD4, CD8, H-2Kb, H-2Kd (BD Pharmingen); and Foxp3 (eBioscience, San Diego, CA). For intracellular interferon γ (IFN-γ) staining, splenocytes were incubated for 4 hours with Leukocyte Activation Cocktail and BD GolgiPlug (BD Pharmingen) at 37°C. Then the cells were permeabilized with BD Cytofix/Cytoperm solution (BD Pharmingen) and stained with FITC-conjugated anti–IFN-γ mAbs. Dead cells were identified as 7-amino-actinomycin D (BD Pharmingen)–positive cells. At least 5000 live samples were acquired for analysis.
Cell culture
Cell culture was performed as previously described.17 Splenic CD4+ T cells were cultured at a concentration of 105 cells/well with 4 × 105 irradiated (30 Gy) splenocytes. After culturing for 72 hours, cells were pulsed with 3H-thymidine (1 μCi [0.037 MBq] per well) for further 18 hours. Proliferation was determined using Topcount NXT (Packard Instruments, Meriden, CT).
Statistical analysis
Mann-Whitney U tests were used to analyze cell counts and clinical scores. We used the Kaplan-Meier product limit method to obtain the survival probability and the log-rank test was applied to compare the survival curves. We defined a P value less than .05 as statistically significant.
Results
Breastfeeding is required for the induction of maximum NIMA effects to reduce GVHD
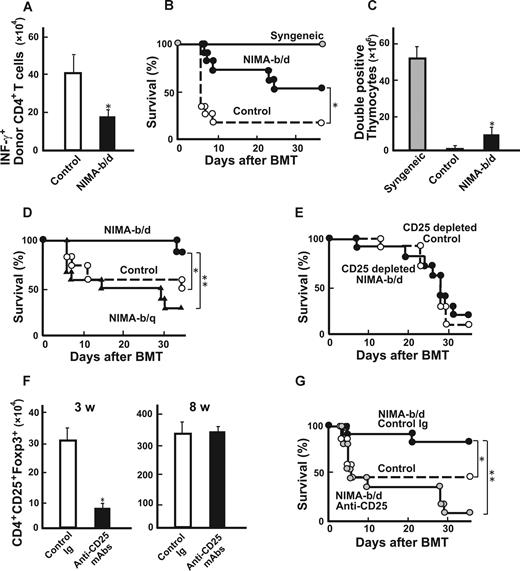
Lethally irradiated B6D2F1 mice received a transplant of 5 × 106 TCD BM from the controls together with 2 × 106 T cells from either NIMA (in utero + oral), NIMA (in utero), NIMA (oral), or control mice. NIMA (in utero + oral) T cells produced less severe GVHD than control T cells, as previously reported7 (Figure 1A). Interestingly, in recipients of NIMA (in utero) and NIMA (oral) T cells, the tolerogenic NIMA effect was partial when considering the mortality and morbidity of GVHD (Figure 1A,B). These results demonstrate that in utero exposure to NIMAs alone could mediate the tolerogenic NIMA effect and that further oral exposure to NIMAs is required for induction of the maximum NIMA effect.
Breastfeeding is required for inducing maximum NIMA effect to reduce GVHD. NIMA-exposed H-2b offspring were produced by mating a B6 male and a B6D2F1 female, and were fed by either a B6D2F1 mother (NIMA [in utero + oral]) or a B6 foster mother (NIMA [in utero]). NIMA-exposed H-2b offspring were produced using a B6D2F1 foster mother to nurse newborn B6 mice (NIMA [oral]). Lethally irradiated B6D2F1 mice received a transplant of 5 × 106 TCD-BM from controls together with 2 × 106 T cells from allogeneic or syngeneic donors. Survival (A) and clinical GVHD scores expressed as mean plus or minus SE (B) after BMT are shown. (A) The results are representative of 3 replicate experiments (n = 17-20/group). (B) Representative data from 1 of the experiments are shown (n = 6/group). *P < .05 compared with controls; **P < .05 compared with NIMA (in utero + oral).
Breastfeeding is required for inducing maximum NIMA effect to reduce GVHD. NIMA-exposed H-2b offspring were produced by mating a B6 male and a B6D2F1 female, and were fed by either a B6D2F1 mother (NIMA [in utero + oral]) or a B6 foster mother (NIMA [in utero]). NIMA-exposed H-2b offspring were produced using a B6D2F1 foster mother to nurse newborn B6 mice (NIMA [oral]). Lethally irradiated B6D2F1 mice received a transplant of 5 × 106 TCD-BM from controls together with 2 × 106 T cells from allogeneic or syngeneic donors. Survival (A) and clinical GVHD scores expressed as mean plus or minus SE (B) after BMT are shown. (A) The results are representative of 3 replicate experiments (n = 17-20/group). (B) Representative data from 1 of the experiments are shown (n = 6/group). *P < .05 compared with controls; **P < .05 compared with NIMA (in utero + oral).
Breastfeeding alone is sufficient to mediate the tolerogenic NIMA effect
We then examined whether NIMA exposure by breastfeeding could induce T-cell hyporesponsiveness to NIMAs. Compared with control T cells, CD4+ T cells isolated from NIMA (oral) mice showed less expansion and IFN-γ production after transfer into irradiated B6D2F1 mice (Figure 2A). Consistent with the reduced donor T-cell responses to NIMAs, NIMA (oral) CD4+ T cells (106) mediated the attenuation of GVHD after BMT, compared with control T cells, as assessed by mortality (Figure 2B) and clinical GVHD scores (data not shown).16 We also observed the tolerogenic NIMA effect in another set of experiments with 2 different T-cell doses (0.25 × 106 and 106; Table 1). The thymus, which is one of the most sensitive organs to GVHD, shows severe atrophy primarily due to a loss of double-positive thymocytes.18 Numbers of double-positive thymocytes markedly decreased in allogeneic controls compared with syngeneic controls (Figure 2C). This thymic atrophy was significantly improved in NIMA (oral) T-cell recipients. We then sought to determine whether GVHD could be further reduced by exposing TCD-BM donors to NIMAs in addition to NIMA exposure of T-cell donors. Lethally irradiated B6D2F1 mice received transplants of 2 × 106 T cells from NIMA (oral) mice and 5 × 106 TCD-BM from NIMA (oral) mice or controls. However, survival (75% vs 70%) and clinical scores (4.0 ± 0.2 vs 3.8 ± 0.4) were not significantly different between the 2 groups. Analysis of donor cell engraftment on day +40 in spleens showed that the recipients of grafts from NIMA-exposed donors had more than 98% donor-derived cells, thus ruling out rejection or mixed chimerism as a potential cause of GVHD suppression.
Breastfeeding-mediated tolerogenic NIMA effect is dependent on CD4+CD25+ cells. A newborn B6 mouse was fed by either a B6 mother (control) or a B6D2F1 foster mother (NIMA-b/d). (A) CD4+ T cells (106) isolated from spleens of NIMA-b/d mice or controls were adoptively transferred into irradiated B6D2F1 mice. Numbers of IFN-γ+ donor CD4+ T cells in spleens 5 days after the transfer are shown as mean plus or minus SD (n = 5/group). (B,C) Lethally irradiated B6D2F1 mice received a transplant of 5 × 106 TCD-BM from control B6 mice together with 2 × 106 T cells from NIMA-b/d, controls, or syngeneic B6D2F1 donors. Survival after BMT (B; n = 11/group) and numbers of double-positive thymocytes at 40 days after BMT (C) are shown. (D) B6D2F1 mice received a transplant as described for panels B and C with T cells from a B6 mouse fed by either a B6D2F1 (NIMA-b/d) or B6D1F1 (NIMA-b/q) foster mother. Survival after BMT is shown (n = 14 /group). (E) BMT was performed as described for panels B and C with 0.5 × 106 CD25-depleted CD4+ T cells from NIMA-b/d or control donors. Survival after BMT is shown (n = 10 /group). (F,G) Newborn B6 mice were nursed by a B6D2F1 mother and subcutaneously injected with 75 μg/g body weight of anti-CD25 or irrelevant mAbs 1 and 8 days after birth. The numbers of Foxp3+CD4+CD25+ cells in spleens at 3 and 8 weeks after birth (n = 3/group, mean ± SD; F) and survival of B6D2F1 mice that received a transplant of CD4+ T cells from anti-CD25–treated or control-treated NIMA-b/d donors or B6 donors (G) are shown (n = 11/group). (A,F) The results are representative of 3 replicate experiments. (B-E,G) Data from 2 similar experiments are combined. *P < .05; **P < .01 versus controls.
Breastfeeding-mediated tolerogenic NIMA effect is dependent on CD4+CD25+ cells. A newborn B6 mouse was fed by either a B6 mother (control) or a B6D2F1 foster mother (NIMA-b/d). (A) CD4+ T cells (106) isolated from spleens of NIMA-b/d mice or controls were adoptively transferred into irradiated B6D2F1 mice. Numbers of IFN-γ+ donor CD4+ T cells in spleens 5 days after the transfer are shown as mean plus or minus SD (n = 5/group). (B,C) Lethally irradiated B6D2F1 mice received a transplant of 5 × 106 TCD-BM from control B6 mice together with 2 × 106 T cells from NIMA-b/d, controls, or syngeneic B6D2F1 donors. Survival after BMT (B; n = 11/group) and numbers of double-positive thymocytes at 40 days after BMT (C) are shown. (D) B6D2F1 mice received a transplant as described for panels B and C with T cells from a B6 mouse fed by either a B6D2F1 (NIMA-b/d) or B6D1F1 (NIMA-b/q) foster mother. Survival after BMT is shown (n = 14 /group). (E) BMT was performed as described for panels B and C with 0.5 × 106 CD25-depleted CD4+ T cells from NIMA-b/d or control donors. Survival after BMT is shown (n = 10 /group). (F,G) Newborn B6 mice were nursed by a B6D2F1 mother and subcutaneously injected with 75 μg/g body weight of anti-CD25 or irrelevant mAbs 1 and 8 days after birth. The numbers of Foxp3+CD4+CD25+ cells in spleens at 3 and 8 weeks after birth (n = 3/group, mean ± SD; F) and survival of B6D2F1 mice that received a transplant of CD4+ T cells from anti-CD25–treated or control-treated NIMA-b/d donors or B6 donors (G) are shown (n = 11/group). (A,F) The results are representative of 3 replicate experiments. (B-E,G) Data from 2 similar experiments are combined. *P < .05; **P < .01 versus controls.
Results of donor T-cell titration experiments
| Donor . | CD4+ T-cell dose, ×106 . | Survival . | Clinical scores at day +14 . |
|---|---|---|---|
| wt | 0 | 3/3 | 0.3 ± 0.2 |
| wt | 0.25 | 5/5 | 3.8 ± 0.1 |
| NIMA (oral) | 0.25 | 5/5 | 2.8 ± 0.1* |
| wt | 1 | 2/5 | 4.7 ± 0.2 |
| NIMA (oral) | 1 | 4/5 | 4.0 ± 0.2* |
| Donor . | CD4+ T-cell dose, ×106 . | Survival . | Clinical scores at day +14 . |
|---|---|---|---|
| wt | 0 | 3/3 | 0.3 ± 0.2 |
| wt | 0.25 | 5/5 | 3.8 ± 0.1 |
| NIMA (oral) | 0.25 | 5/5 | 2.8 ± 0.1* |
| wt | 1 | 2/5 | 4.7 ± 0.2 |
| NIMA (oral) | 1 | 4/5 | 4.0 ± 0.2* |
A newborn B6 mouse was fed by either a B6 mother (control) or a B6D2F1 foster mother (NIMA [oral]). Lethally irradiated B6D2F1 mice received a transplant of 5 × 106 TCD-BM from control B6 mice together with 0.25 × 106 or 106 CD4+ T cells from NIMA (oral) or controls. Survival and clinical scores after BMT are shown.
P < .05.
We then evaluated the antigen specificity of milk-mediated tolerogenic NIMA effects. B6D2F1 mice received transplants with T cells from B6 mice nursed by either a B6D2F1 or B6D1F1 (H-2b/q) foster mother. GVHD was less severe in recipients of grafts from H-2b/d–exposed donors than in those who received grafts from H-2b/q–exposed donors, suggesting an antigen specificity of the NIMA effect (Figure 2D).
Breastfeeding-mediated tolerogenic NIMA effect is dependent on CD4+CD25+ cells
We recently demonstrated that CD4+CD25+ regulatory T cells (TR) play an important role in the tolerogenic NIMA effect.7 We therefore considered whether these TR play a role in the induction of tolerogenic milk effects. When B6D2F1 mice received a transplant of CD25-depleted CD4+ T cells from NIMA (oral) mice, the tolerogenic NIMA effect disappeared (Figure 2E), suggesting that TR in the donor inoculum were involved in the tolerogenic milk effect.
We hypothesized that NIMA-reactive TR are produced during the lactation period. Newborn B6 mice fed by a B6D2F1 foster mother were injected subcutaneously with 75 μg/g body weight of anti-CD25 mAbs on days 1 and 8 after birth to deplete CD25+ TR in vivo.15 Injection of anti-CD25 mAbs significantly reduced the numbers of Foxp3+ TR at 3 weeks of age but this cell population had recovered to normal levels by 8 weeks of age when these mice were used as BMT donors, as reported in previous studies (Figure 2F).15 NIMA effects disappeared in mice that received a transplant of grafts from CD25-treated donors (Figure 2G). These results suggest that development of TR during lactation is critical for the induction of the NIMA effect. We then compared the antigen-specificity of TR isolated from NIMA (oral) mice and TR from control mice. CD4+ CD25+ T cells were isolated from control and NIMA-exposed mice and added to the culture of B6 T cells and B6D2F1 or DBA/1 stimulators in MLR. However, both TR equally suppressed T-cell proliferation in response to NIMAs and third-party alloantigens (data not shown).
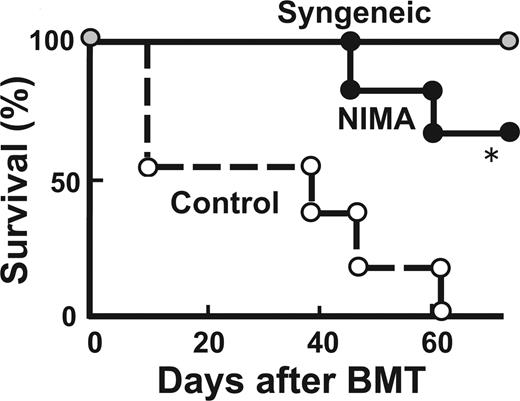
Finally, we evaluated whether the NIMA effect persists when NIMA-exposed donors are older. B6D2F1 mice received a transplant of T cells from 28-week-old NIMA-exposed mice and age-matched control mice. Again, GVHD was reduced in mice that received a transplant from older NIMA mice (Figure 3).
NIMA effects can be mediated by adult donors. Lethally irradiated B6D2F1 mice received a transplant of 5 × 106 TCD-BM from controls together with 2 × 106 T cells from 28-week-old NIMA (in utero + oral) or control donors (n = 5/group). Survival after BMT is shown. *P < .05.
NIMA effects can be mediated by adult donors. Lethally irradiated B6D2F1 mice received a transplant of 5 × 106 TCD-BM from controls together with 2 × 106 T cells from 28-week-old NIMA (in utero + oral) or control donors (n = 5/group). Survival after BMT is shown. *P < .05.
Discussion
We demonstrated that oral exposure to NIMAs by breastfeeding alone could induce a tolerogenic NIMA effect. Donor T cells from NIMA-exposed mice were hyporesponsive to the corresponding antigens, thereby attenuating GVHD and resulting in an improvement in survival after a “child-to-mother” BMT. We also found that both oral and in utero exposures to NIMAs are required for the maximum reduction of GVHD, as with previous observations in experimental skin allografts.9,12 Breastfeeding may thus play an additive role in inducing the tolerogenic NIMA effect. These findings are supported by several clinical studies of renal transplantation, which showed that patients who had been breast-fed survived better after maternal and sibling kidney allografts than those who had not been breast-fed.8,19 These observations may also explain partly why the incidence of GVHD was significantly lower in HSCT from a NIMA-mismatched donor than from an IPA-mismatched donor in mice7 and humans,6,20 and why the survival of sibling kidney allografts expressing noninherited paternal antigens is not as good as the survival of those expressing NIMAs.5 Additional oral exposure to NIMAs by breastfeeding may be required for induction of clinical tolerance.
Breast milk contains maternal MHC antigens in soluble and cellular forms as well as in exosomes.8-11 The components critical in inducing tolerance in our model remain to be identified. Interestingly, BMT from donors continuously fed with proteins extracted from recipient splenocytes reduces experimental GVHD.21 Thus, it is possible that oral exposure to host antigens might improve the outcome of allogeneic HSCT. Oral tolerance is characterized by an inhibition of specific immune responsiveness to subsequent parenteral injections of proteins to which the individual has been previously exposed orally.22 Oral tolerance prevents pathological reactions against environmental and food antigens, and its failure results in an exacerbated inflammation typical of allergies and asthma. Oral tolerance can be mediated by several mechanisms such as deletion, immune deviation, and suppression by TR.23 We recently demonstrated that TR play an important role in the tolerogenic NIMA effect.7 Similarly, in the mouse model of the NIMA cardiac allografts, graft acceptance is associated with a higher frequency of IL-10– and TGF-β–producing CD4+CD25+ cells.24 In this study, we further showed that the tolerogenic NIMA effect mediated by breastfeeding was also dependent upon TR. The tolerogenic NIMA effect was abrogated by depletion of CD25+ T cells of donor innocula, as well as the in vivo depletion of CD25+ cells in neonates on days 1 and 8 after birth, during the breastfeeding period. Injection of anti-CD25 mAbs significantly reduced generation of Foxp3+ TR during this period as shown in Figure 2F. However, this cell population had recovered to normal levels by 8 weeks of age when the mice were used as BMT donors, as shown in previous studies15 ; thus, abrogation of tolerogenic NIMA effects is not due to a decrease in the numbers of Foxp3+ TR. A recent study addressing the kinetics of TR generation during ontogeny demonstrated that the largest single-day gain in numbers of Foxp3+ thymocytes occurred between days 3 and 4, followed by a steady increase in number over the first 3 weeks. Thereafter, TR were continuously generated in the thymus.25 Thus, both de novo generation of TR in the thymus and peripheral expansion of TR appear to be responsible for TR repopulation within 8 weeks. Interestingly, the mechanisms of oral tolerance differ with different antigen doses. A single administration of high-dose antigens causes clonal deletion, whereas repeated administration of low doses of antigens leads to development of TR.26 Thus, repeated oral exposure to NIMAs by breastfeeding might help to produce NIMA-specific TR. However, a single intravenous injection of allogeneic cells can induce T-cell responses against alloantigens in murine neonates.27 This route of antigen exposure may be critical in determining immunity or tolerance.
Our results suggest the antigen specificity of the NIMA effect in vivo. Oral administration of the self-antigen induces antigen-specific TR.28 Breast milk–mediated transfer of an antigen to the neonate from an antigen-exposed mother produces TR that suppress asthma.29 Such an antigen specificity may be mediated by “adaptive” TR that are generated from classical T-cell subsets or “natural” TR under certain conditions of antigenic stimulation, but not “natural” TR that develop in the thymus during the early stages of fetal and neonatal T-cell development.30 In fact, we could not demonstrate antigen-specificity of TR isolated from NIMA-exposed mice in vitro. Antigen-specific expansion of TR in vivo may require a T cell–deficient environment and stimulation with the corresponding antigens in the presence of IL-2.31 Therefore, re-exposure to NIMAs in a lymphopenic environment following pretransplantation conditioning might allow for the generation of “adaptive” TR, thus contributing to antigen specificity in vivo. Further studies are required to evaluate antigen specificity of the NIMA effect. In addition, it should be noted that oral tolerance can be achieved in the absence of Foxp3+CD25+ TR.23,32 Thus, there could be multiple mechanisms underlying the tolerogenic NIMA effect cause by breastfeeding.
In clinical HSCT with grafts from NIMA-exposed donors, GVHD is mild or absent in some, but not in others.20 A history of breastfeeding8,19 as well as the presence of microchimerism33 and anti-HLA antibodies should be considered. MHC allele mismatch combinations between the donor and recipient could matter.24 A recent clinical study identified high- and low-risk HLA allele mismatches for severe GVHD.34 The age of donors may also affect the outcome. In our study, the tolerogenic NIMA effect lasted for at least 28 weeks, suggesting that both child and adult donors can confer the NIMA effect. Lastly, calcineurin inhibitors used for GVHD prevention inhibit activation and expansion of TR, thereby suppressing the NIMA effects.35 The beneficial NIMA effects were observed only if patients were not administered cyclosporine in kidney transplantation.5 Immunosuppressants such as sirolimus and mycophenolate mofetil, which do not interfere with TR, may therefore be of use in NIMA-associated HSCT.35 In conclusion, we demonstrated that breastfeeding plays an important role in the buildup of the tolerogenic NIMA effect, suggesting that novel strategies can be established to induce host-specific tolerance orally in allogeneic HSCT.
Presented as an abstract at the 49th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 10, 2007.36
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by research funds from the Ministry of Education, Culture, Sports, Science and Technology (Tokyo, Japan; no. 20659153; T.T.), Health and Labor Science Research grants (Tokyo, Japan; T.I., T.T.), and Mitsubishi Pharma Research Foundation (Tokyo, Japan; T.T.).
Authorship
Contribution: K. Aoyama conducted the research and wrote the paper; M.K., K.-i.M., D.H., and T.I. conducted the research; K. Akashi, M.H., and M.T. designed the study; and T.T. designed and organized the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takanori Teshima, Center for Cellular and Molecular Medicine, Kyushu University Hospital, 3-1-1 Maidashi, Higashi-ku, Fukuoka, 812-8582 Japan; e-mail: tteshima@cancer.med.kyushu-u.ac.jp.

![Figure 1. Breastfeeding is required for inducing maximum NIMA effect to reduce GVHD. NIMA-exposed H-2b offspring were produced by mating a B6 male and a B6D2F1 female, and were fed by either a B6D2F1 mother (NIMA [in utero + oral]) or a B6 foster mother (NIMA [in utero]). NIMA-exposed H-2b offspring were produced using a B6D2F1 foster mother to nurse newborn B6 mice (NIMA [oral]). Lethally irradiated B6D2F1 mice received a transplant of 5 × 106 TCD-BM from controls together with 2 × 106 T cells from allogeneic or syngeneic donors. Survival (A) and clinical GVHD scores expressed as mean plus or minus SE (B) after BMT are shown. (A) The results are representative of 3 replicate experiments (n = 17-20/group). (B) Representative data from 1 of the experiments are shown (n = 6/group). *P < .05 compared with controls; **P < .05 compared with NIMA (in utero + oral).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/8/10.1182_blood-2008-05-155283/5/m_zh80130932480001.jpeg?Expires=1769327892&Signature=X4zEb6qDG7Me7Gcm~iUVTYCd9nLUJ2b7e89T66R87BOYBvkhBmPBHYg5I3m5C6tlC6Cqzn-hQpX1-VAbsOD2s~OxEotkaE6ANc96D4nfAaK2u0f9JetUy~8v4n2zdnAVnN7y5FjS89n45c08fNh9SpJGuT2Vc8vTRXCsQuPIIj9VDTE3aFGVXMYSSJus0ES7~XpTcxbHzdAqxZk-41raBfAZRq8FH6FYVkyA0TtAbxP~Gp9crQSc~O2bsm7aBQiI-06yi8LNmmAErH~E8F9ELJqoHMPbvXG1JKu3~vaQryEtr~xqCUBvc0Wf9OdqRdG9dvxbkQdFck~7QT0nxWtTPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal