Dendritic cells (DCs) can be classified into 2 distinct subsets: conventional DCs (cDCs) and plasmacytoid DCs (pDCs). cDCs can prime antigen-specific T-cell immunity, whereas in vivo function of pDCs as antigen-presenting cells remains controversial. We evaluated the contribution of pDCs to allogeneic T-cell responses in vivo in mouse models of graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation by an add-back study of MHC-expressing pDCs into major histocompatibility complex-deficient mice that were resistant to GVHD. Alloantigen expression on pDCs alone was sufficient to prime alloreactive T cells and cause GVHD. An inflammatory environment created by host irradiation has the decisive role in maturing pDCs for T-cell priming but this process does not require Toll-like receptor signaling. Thus, functional outcomes of pDC–T-cell interactions depend on the immunologic context of encounter. To our knowledge, these results are the first to directly demonstrate an in vivo pathogenic role of pDCs as antigen-presenting cells in an antigen-specific T cell–mediated disease in the absence of other DC subsets and to provide important insight into developing strategies for tolerance induction in transplantation.
Introduction
The interaction of naive T cells and dendritic cells (DCs) is essential for initiating primary immune responses. DCs can be divided into 2 distinct subsets: conventional DCs (cDCs) and plasmacytoid DCs (pDCs) according to their immunophenotype and functional properties.1,–3 pDCs represent a CD11cint B220+ DC subset that differs from the CD11chigh B220− major histocompatibility complex (MHC) class IIhigh cDCs, commonly viewed as the classic stimulators of naive T cells. One distinctive feature of pDCs is their capacity to rapidly produce high levels of type I interferon (IFN) in response to viral and bacterial stimuli, highlighting the importance of pDCs in innate immune responses.2,,,,,–8 pDCs express low levels of surface MHC and classical costimulatory molecules; therefore, they are poor T-cell stimulators.5,,,,,–11 In contrast, pDCs matured with CD40 ligands or Toll-like receptor (TLR) ligands are potent antigen-presenting cells (APCs), capable of stimulating naive T-cell proliferation and differentiation to helper, killer, memory, and regulatory T cells in vitro.7,12,13 In vivo, injection of pDCs activated by synthetic oligodeoxynucleotides containing unmethylated cytosine-guanine motifs (CpG), but not immature pDCs, is capable of eliciting antigen-specific CD8+ T-cell responses.10,14 On the other hand, OVA-pulsed pDCs protected mice against OVA-induced asthma development.15 pDCs in the tumor-draining lymph nodes express indole 2, 3-dioxygenase, and suppress antitumor T-cell responses.16 In patients with ovarian cancer, large numbers of pDCs, which induced interleukin 10 (IL10)–producing regulatory T cells, were found in ascites.17 pDCs mediate tolerance and prolong survival of cardiac allografts.11,18,19 Several recent clinical observations also suggest that pDCs play important regulatory roles in transplant outcome. An increased ratio of pDCs/cDCs is associated with the successful withdrawal of immunosuppressants after liver transplants.20 In allogeneic hematopoietic stem cell transplantation (HSCT), low pDC count in the peripheral blood is a risk for graft-versus-host disease (GVHD),21 while larger numbers of pDCs in donor bone marrow (BM) are associated with increased relapse.22 Collectively, accumulating data suggest that pDCs are mostly tolerogenic in vivo. However, it remains unclear whether pDCs as APCs have a causative role in antigen-specific T cell–mediated diseases in vivo, although pDCs are involved in the pathogenesis of systemic lupus erythematosus (SLE) and psoriasis through IFN-α production.23,24
GVHD, the major obstacle to successful outcome after allogeneic HSCT, is mediated by donor T cells stimulated by recipient DCs.25,–27 MHC class I– or II–deficient (H2-Ab1−/−) mice are resistant to CD8- and CD4-dependent GVHD, respectively.26,–28 When H2-Ab1−/− mice are repopulated with syngeneic MHC class II–expressing DCs, these mice succumb to acute GVHD.26,28 Thus, recognition of MHC class II alloantigens on host-derived DCs, alone, is sufficient to prime donor CD4+ T cells and cause lethal acute GVHD. CD4-mediated GVHD can develop even in the absence of MHC class II alloantigen expression on GVHD target cells, such as epithelium, endothelium, and parenchyma.26,28 Thus, this GVHD model system using H2-Ab1−/− mice presents a stringent test of the allostimulatory capacity of a DC subset when the donor and recipient differ at only MHC class II loci. Using this model system with modification, we addressed whether pDCs as APCs have a causative role in antigen-specific T cell–mediated diseases, such as GVHD, or induce tolerance.
Methods
Mice
Female C57BL/6 (B6: H-2b, CD45.2+), B6D2F1 (H-2b/d), and BALB/c (H-2d) mice were purchased from Charles River Japan (Yokohama, Japan). C3H/HeJ (C3H: H-2k) and AKR/J (AKR: H-2k) mice were purchased from Japan SLC (Shizuoka, Japan). B6-Ly5a (H-2b, CD45.1+), and C3H-background β2m-deficient (β2m−/−: C3.129P2(B6)-B2mtm1Unc/Dcr) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6-background MHC class II−/− mice (H2-Ab1−/−: B6.129-Abbtm1 N12) were from Taconic (Germantown, NY). B6-background Toll–IL-1 receptor domain-containing adaptor inducing IFN-β/myeloid differentiation factor 88 double-deficient (TRIF/MyD88 DKO) mice29 were kindly provided by Dr Kiyoshi Takeda at Kyushu University (Fukuoka, Japan). The age of the mice ranged between 9 and 16 weeks. Mice were maintained in a specific pathogen-free condition and received normal chow and hyperchlorinated drinking water for the first 3 weeks after transplantation. All animal experiments were performed under the auspices of the Institutional Animal Care and Research Advisory Committee at the Department of Animal Resources at Kyushu University.
Cell isolation
To expand DCs, we injected mice subcutaneously once daily with 10 μg recombinant human fms-like tyrosine kinase 3 ligand (FL; Amgen, Seattle, WA) for 10 consecutive days, and cDCs, pDCs, and B cells were isolated, as previously described, with a modification.28,30 Briefly, cDCs were enriched from splenocytes using CD11c microbeads and the AutoMACS system (Miltenyi Biotec, Bergisch Gladbach, Germany), followed by cell sorting of CD11chigh B220− cells using a BD FACSAria (BD Biosciences, San Jose, CA). B cells were enriched from splenocytes with B220 microbeads and the AutoMACS, and sorted as CD11c− B220+ cells. pDCs were enriched from BM by depleting CD3+, CD19+, CD11b+, CD49b+, and Ly-76+ cells using a cocktail of biotin-conjugated mAbs, streptavidin-microbeads, and the AutoMACS system, followed by a FACS sorting of CD11cint B220+ cells. CD4+ T cells were negatively selected from splenocytes by depleting CD8+, CD49b+, CD11b+, Ly-76+, and B220+ cells. CD8+ T cells were negatively selected from splenocytes by depleting CD4+, CD49b+, CD11b+, Ly-76+, and B220+ cells, using the AutoMACS system, followed by a FACS sorting of CD4−CD8+ cells. T-cell depletion (TCD) of donor BM cells was performed using CD90 microbeads and the AutoMACS system.
Induction and assessment of GVHD
GVHD was induced as previously described.28 In brief, mice received 11 Gy total body irradiation (TBI), split into 2 doses separated by 4 hours to minimize gastrointestinal toxicity, and injected intravenously with 2 × 106 each APC subset on day −1. On day 0, mice were injected intravenously with 2 × 106 CD4+ or CD8+ T cells with or without 5 × 106 TCD-BM. Survival after BMT was monitored daily, and the degree of clinical GVHD was assessed weekly using a scoring system that evaluated changes in 5 clinical parameters: weight loss, posture, activity, fur texture, and skin integrity (maximum index = 10).31 Acute GVHD was also assessed by detailed histopathologic analysis of the liver and intestine. Slides stained with hematoxylin and eosin were examined systematically using a semiquantitative scoring system.32 Pictures from tissue sections were taken at room temperature using an ProgRes 3012 mF digital camera (Jenoptik Laser Optik Systeme, Jena, Germany) mounted on an Olympus BX51 microscope (Olympus, Tokyo, Japan) and analyzed using a ProgRes PlugIn for PCI software version 5.0 (Jenoptik Laser Optik Systeme). Images were acquired using an UPlan Apochromat 10×/0.40 numeric aperture (NA) or a Plan Apochromat 40×/0.90 NA WLSM objective, depending on the desired magnification.
Cell culture and enzyme-linked immunosorbent assay
All culture media and incubation conditions have been described previously.33 CD4+ T cells were cultured at a concentration of 2 × 105 cells/well with 104 irradiated (20 Gy) APCs. After culturing for 3 days, supernatants were harvested for cytokine measurements, and cells were pulsed with 3H-thymidine (1 μCi per well) for further 16 hours. Proliferation was determined using a Topcount NXT (Packard Instruments, Meriden, CT). In some experiments, 1 μM CpG 1668 (Sigma-Aldrich Japan, Ishikari, Japan) or 100 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich Japan) was added to the culture. To determine secretion of IFN-α and IL-12 p70 from APCs, 105 APCs were incubated with 1 μM CpG 2216 (Sigma-Aldrich Japan) for 16 hours. Enzyme-linked immunosorbent assay (ELISA) for IFN-γ (BD Biosciences), IFN-α (PBL Biomedical Laboratories, Piscataway, NJ), and IL-12 p70 (R&D Systems, Minneapolis, MN) was performed according to the manufacturer's protocols with the sensitivity of 31.3 pg/mL, 12.5 pg/mL, and 2.5 pg/mL, respectively.
Flow cytometric analysis
The mAbs used were fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, or allophycocyanin-conjugated anti–mouse TCRβ, CD4, CD8α, CD11c, CD11b, CD19, CD49b, CD45.1, CD45.2, CD90.2, CD86, B220 (CD45R), H-2Kb, H-2Kd, H-2Kk, I-Ab, Ly6C (BD Biosciences), mPDCA-1 (Miltenyi Biotec), and Foxp3 (eBioscience, San Diego, CA). Cells were stained as previously described.33 Irrelevant IgG2a/b mAbs were used as a negative control. For intracellular IFN-γ staining, splenocytes were incubated for 4 hours with leukocyte activation cocktail and BD GolgiPlug (BD Biosciences) at 37°C. Then, the cells underwent permeabilization with BD Cytofix/Cytoperm solution (BD Biosciences) and were stained with FITC-conjugated anti–IFN-γ mAbs. For carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling, CD4+ T cells were stained in phosphate-buffered saline (PBS) with 1 μM CFSE (Molecular Probes, Invitrogen, Eugene, OR), as described earlier.34 At least 5000 live samples were acquired for analysis. Dead cells were identified as 7-amino-actinomycin D (BD Biosciences) or 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes) positive cells. The cells were analyzed using a FACSCalibur or a FACSAria flow cytometer (BD Biosciences), and the data were analyzed using FlowJo software (TreeStar, San Carlos, CA).
Statistical analysis
Mann-Whitney U tests were used to analyze cell counts and clinical scores. We used the Kaplan-Meier product limit method to obtain the survival probability and the log-rank test was applied to compare the survival curves. We defined P values of less than .05 as statistically significant.
Results
Isolation and characterization of pDCs
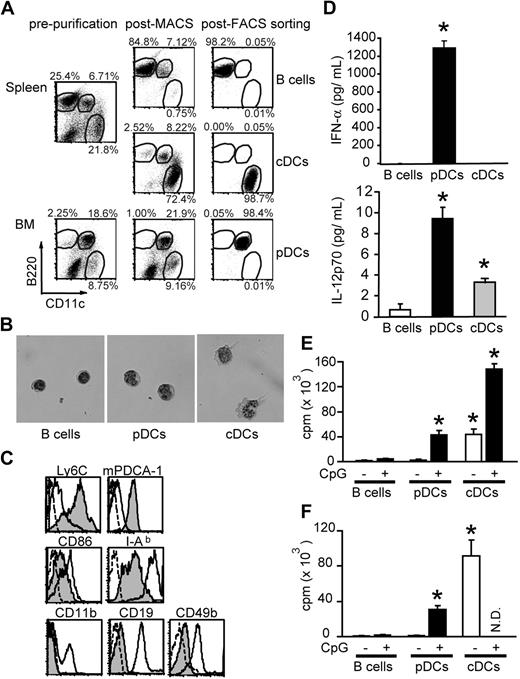
Low frequency of pDCs in vivo has hampered the study of this cell population. To expand DCs in vivo, mice were injected with 10 μg FL for 10 days.6,28,30 As previously reported,35,36 FL treatment resulted in an increase in frequency of CD11c+B220+ pDCs to 6.7% in spleen and 18.6% in BM (Figure 1A). pDCs, cDCs, and B cells were then isolated as described in the methods section. The purity of each population was greater than 98% with less than 0.1% contamination by the others (Figure 1A). pDCs appeared plasmacytoid round shaped with excentered nuclei and basophilic cytoplasm (Figure 1B). They expressed low levels of CD86 and MHC class II but high levels of Ly6C and mPDCA-1 (Figure 1C). These cells did not express CD19, CD49b, or CD11b, thus ruling out contamination of B cells, natural killer (NK) cells, and IFN-producing killer DCs37,38 in the pDC fraction. After stimulation with CpG 2216, pDCs secreted high levels of IFN-α and IL-12 p70, whereas cDCs secreted moderate levels of IL-12 p70, but not IFN-α, as has been described39 (Figure 1D).
Isolation of pDCs and cDCs. B cells (CD11c− B220+), pDCs (CD11cint B220+), and cDCs (CD11chigh B220−) were isolated from the spleen or BM of FL-treated mice. (A) Two-dimensional counter plots of B220 and CD11c staining. Percentages of cells are shown enclosed in circles. (B) Cell morphology stained with May-Giemsa (magnification ×400). (C) Immunophenotyping. Filled histograms; pDCs, broken-lined open histograms; pDCs stained with isotype controls, solid-lined open histograms for Ly6C, mPDCA-1, CD86, I-Ab, and CD11b; cDCs, those for CD19 and CD49b; B cells and enriched CD49b+ cells as positive controls, respectively. (D) Production of IFN-α (top) and IL-12p70 (bottom) after incubation of cells with CpG 2216 for 16 hours (mean ± SD). (E,F) Aliquots of 2 × 105 BALB/c CD4+ T cells were cultured with 104 cells from each APC subset isolated from FL-treated (E) and FL-untreated mice (F), with or without 1 μM CpG 1668, and their proliferation 3 days later was shown as mean plus or minus SD. N.D. indicates not done. Data are representative of at least 2 similar experiments. *P < .05 compared with B cells.
Isolation of pDCs and cDCs. B cells (CD11c− B220+), pDCs (CD11cint B220+), and cDCs (CD11chigh B220−) were isolated from the spleen or BM of FL-treated mice. (A) Two-dimensional counter plots of B220 and CD11c staining. Percentages of cells are shown enclosed in circles. (B) Cell morphology stained with May-Giemsa (magnification ×400). (C) Immunophenotyping. Filled histograms; pDCs, broken-lined open histograms; pDCs stained with isotype controls, solid-lined open histograms for Ly6C, mPDCA-1, CD86, I-Ab, and CD11b; cDCs, those for CD19 and CD49b; B cells and enriched CD49b+ cells as positive controls, respectively. (D) Production of IFN-α (top) and IL-12p70 (bottom) after incubation of cells with CpG 2216 for 16 hours (mean ± SD). (E,F) Aliquots of 2 × 105 BALB/c CD4+ T cells were cultured with 104 cells from each APC subset isolated from FL-treated (E) and FL-untreated mice (F), with or without 1 μM CpG 1668, and their proliferation 3 days later was shown as mean plus or minus SD. N.D. indicates not done. Data are representative of at least 2 similar experiments. *P < .05 compared with B cells.
Next, we examined the allostimulatory capacity of pDCs in mixed lymphocyte reaction. Freshly isolated pDCs were poor inducers of allogeneic CD4+ T-cell proliferation as previously reported3 (Figure 1E). However, pDCs matured with CpG 1668 were capable of priming T cells nearly as effectively as cDCs (Figure 1E). Similar results were obtained when pDCs were isolated from mice without treatment with FL (Figure 1F).
MHC class II–expressing pDCs alone sufficiently stimulate donor CD4+ T cells to cause GVHD in MHC class II–deficient mice
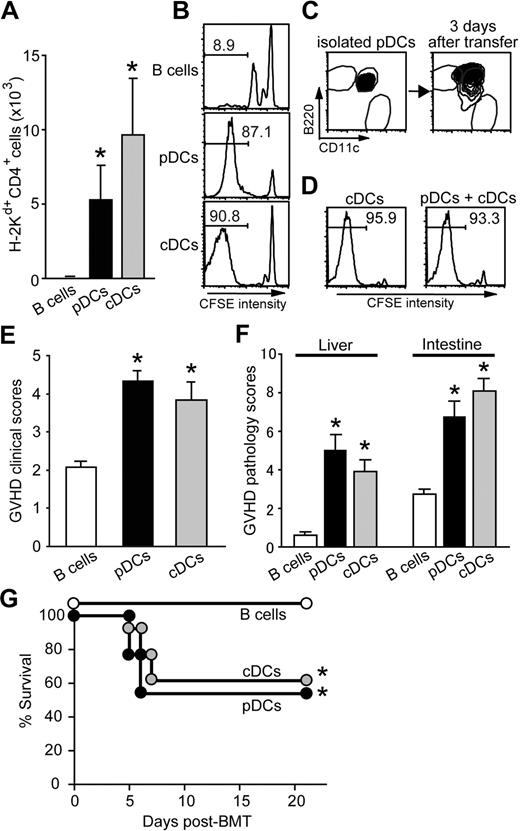
H2-Ab1−/− B6 mice are resistant to CD4-dependent GVHD.26,28 We studied whether the add-back of MHC class II+/+ pDCs could prime alloreactive T cells using this system with modification. In our previous studies, we used a bm12 → B6 model across MHC class II disparity alone. In contrast, the B6 and BALB/c strain combination used in the current study differs at both MHC class I and class II loci. To avoid the confounding effects of MHC class I and CD8+ T cells, therefore, purified CD4+ T cells were used to induce GVHD, while whole T cells were used in the previous studies. Contamination of CD8+ T cells in the CD4+ cell fraction was < 0.1%. H2-Ab1−/− B6 (H-2b) mice were irradiated with 11 Gy TBI and injected with 2 × 106 pDCs, cDCs, or B cells isolated from wild-type (WT) B6 mice on day −1. Mice were then injected with 2 × 106 CD4+ T cells from BALB/c (H-2d) donors that differ at MHC and multiple minor histocompatibility antigens (miHAs) from B6 mice on day 0. On day 0, we confirmed homing of the injected cDCs and pDCs to spleen and lymph nodes (LNs), where mature DCs form long-lived contacts with T cells (data not shown).40 Flow cytometric analysis of the mesenteric LNs (mLNs) and spleen on day +6 demonstrated the significant expansion of donor CD4+ T cells (H-2Kd+CD4+) in animals that had been repopulated with cDCs or pDCs compared with those with B cells (Figure 2A). No CD8+ T-cell expansion was observed (< 0.1%), confirming the stimulation of only CD4+ donor T cells in this system. Similarly, CFSE-labeled donor CD4+ T cells showed robust cell division in animals preinjected with cDCs or pDCs, while those underwent some homeostatic divisions in animals preinjected with B cells (Figure 2B). Donor CD4 expansion in these recipients was associated with increased expression of IFN-γ (data not shown). Evaluation of Foxp3 expression on CD4+ T cells ruled out the possibility of expansion of Foxp3+ regulatory T cells in response to pDCs (data not shown). We then investigated the effects of delayed add-back of pDCs on GVHD. H2-Ab1−/− mice were irradiated on day −4 and injected with pDCs on day −1, followed by the injection of CFSE-labeled BALB/c CD4+ T cells on day 0. Flow cytometric analysis of the spleen showed robust division of donor CD4+ T cells, thus suggesting that TBI-mediated inflammation persist at least for 3-4 days after TBI (data not shown). In these experiments, we did not have syngeneic controls, since it was apparent in a previous study of DC add-back28 that there was no expansion of donor T cells in syngeneic controls, where H2-Ab1−/− B6 recipients that had been repopulated with WT B6 DCs were injected with CD4+ T cells from congeneic B6-Ly5a mice.
Host pDCs or cDCs alone are sufficient to stimulate alloreactive CD4+ T cells and induce GVHD. (A,B) Totals of 2 × 106 BALB/c (H-2Kd+) CD4+ T cells were transferred to irradiated H2-Ab1−/− mice preinjected with 2 × 106 WT B cells, pDCs, or cDCs. Expansion of H-2Kd+ donor CD4+ T cells in the mLNs (mean ± SD;A) and cell division of CFSE-labeled donor CD4+ T cells in the spleens (B). (C) pDCs were injected to irradiated H2-Ab1−/− mice. Expression levels of B220 and CD11c on I-Ab+ cells were analyzed 3 days after transfer and compared with those before transfer. (D) Aliquots of 2 × 106 CFSE-labeled BALB/c CD4+ T cells were transferred to irradiated H2-Ab1−/− mice preinjected with 106 pDCs plus 106 cDCs or 2 × 106 cDCs from WT B6 mice. Cell divisions of donor CD4+ T cells in the spleens on day 7 are shown. (E,F) BALB/c CD4+ T cells were transferred as above and GVHD clinical scores (E), and pathology scores in the liver and intestine (F) on day 6 are shown as mean plus or minus SEM. (G) Lethally irradiated H2-Ab1−/− mice preinjected with pDCs, cDCs, or B cells were injected with 2 × 106 CD4+ T cells and 5 × 106 TCD-BM from BALB/c mice. Survival after BMT is shown. Results from 2 similar experiments were combined. *P < .05 compared with B cells.
Host pDCs or cDCs alone are sufficient to stimulate alloreactive CD4+ T cells and induce GVHD. (A,B) Totals of 2 × 106 BALB/c (H-2Kd+) CD4+ T cells were transferred to irradiated H2-Ab1−/− mice preinjected with 2 × 106 WT B cells, pDCs, or cDCs. Expansion of H-2Kd+ donor CD4+ T cells in the mLNs (mean ± SD;A) and cell division of CFSE-labeled donor CD4+ T cells in the spleens (B). (C) pDCs were injected to irradiated H2-Ab1−/− mice. Expression levels of B220 and CD11c on I-Ab+ cells were analyzed 3 days after transfer and compared with those before transfer. (D) Aliquots of 2 × 106 CFSE-labeled BALB/c CD4+ T cells were transferred to irradiated H2-Ab1−/− mice preinjected with 106 pDCs plus 106 cDCs or 2 × 106 cDCs from WT B6 mice. Cell divisions of donor CD4+ T cells in the spleens on day 7 are shown. (E,F) BALB/c CD4+ T cells were transferred as above and GVHD clinical scores (E), and pathology scores in the liver and intestine (F) on day 6 are shown as mean plus or minus SEM. (G) Lethally irradiated H2-Ab1−/− mice preinjected with pDCs, cDCs, or B cells were injected with 2 × 106 CD4+ T cells and 5 × 106 TCD-BM from BALB/c mice. Survival after BMT is shown. Results from 2 similar experiments were combined. *P < .05 compared with B cells.
To rule out the possibility that allostimulatory capacity of pDCs is due to FL treatment, pDCs were isolated from mice without treatment with FL, and their allostimulatory capacity was evaluated similarly. Again, CD4+ T cells primed by pDCs underwent robust cell divisions (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) and differentiated into effectors secreting high levels of IFN-γ (Figure S1B) in H2-Ab1−/− mice 7 days after transfer, indicating that the ability of pDCs to prime T cells is not due to FL treatment. Therefore, we restricted our subsequent analysis to FL-expanded pDCs.
There was still a possibility that pDCs differentiate into cDCs fully endowed with APC function after transfer to H2-Ab1−/− mice.41 To examine this possibility, we isolated and injected pDCs into irradiated H2-Ab1−/− mice. Flow cytometric analysis of the spleens, 3 days later, showed that pDCs still maintained the phenotype of pDCs (Figure 2C), thus ruling out this possibility. Next, we evaluated whether pDCs were capable of down-regulating T-cell activation induced by cDCs. To investigate this, CFSE-labeled donor CD4+ T cells were transferred to H2-Ab1−/− mice preinjected with cDCs alone or with cDCs and pDCs. However, flow cytometric analysis of the spleen 7 days after transfer showed that the addition of pDCs did not suppress donor CD4 expansion mediated by cDCs (Figure 2D).
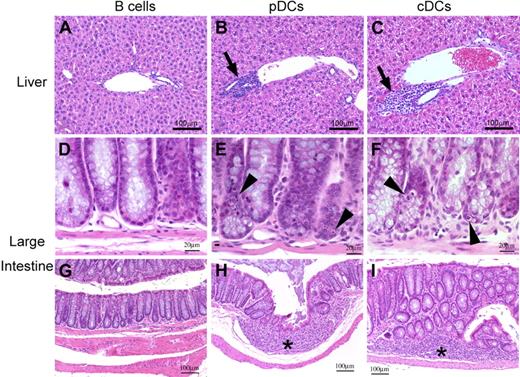
We then tested the hypothesis that alloantigen expression on pDCs alone is sufficient for the induction of GVHD target organ damage. H2-Ab1−/− mice were irradiated and injected with 2 × 106 WT pDCs, cDCs, or B cells on day −1. Mice were then injected with 2 × 106 BALB/c CD4+ T cells. Mice preinjected with pDCs or cDCs developed significant GVHD, as assessed by the clinical scores31 (Figure 2E) and pathology scores on day +632 (Figure 2F), compared with those with B cells. Liver histology of mice preinjected with pDCs showed standard histologic features of acute GVHD, including mononuclear cell infiltration in bile ducts and portal triads, and hepatocellular damage with acidophilic bodies (Figure 3B). Histopathology of the small and large intestine also showed significant changes in these mice, including villous atrophy with epithelial apoptosis (Figure 3E), as well as lymphocytic infiltration and granulation tissue formation (Figure 3H). To note, these pathologic features were similar to those observed in recipients preinjected with cDCs (Figure 3C, F, and I), whereas animals preinjected with B cells showed no significant pathologic signs of GVHD, as previously described28 (Figure 3A,D,G). These results demonstrated that pDCs alone are sufficient to activate donor CD4+ T cells to trigger GVHD as effectively as cDCs. Finally, we evaluated whether pDC-mediated T-cell activation induces GVHD mortality. Lethally irradiated H2-Ab1−/− mice preinjected with 2 × 106 WT pDCs, cDCs, or B cells on day −1 were injected with 2 × 106 CD4+ T cells and 5 × 106 TCD-BM from BALB/c donors on day 0. Mice preinjected with pDCs or cDCs developed lethal GVHD (Figure 2G).
pDCs or cDCs alone mediate standard acute GVHD. Histologic findings of the liver (A-C) and large intestine (D-I). Periportal mononuclear infiltrates in the liver (panels B,C), and crypt cell apoptosis (panels E,F) and granulation tissue (* in panels H,I) in the large intestine are shown.
pDCs or cDCs alone mediate standard acute GVHD. Histologic findings of the liver (A-C) and large intestine (D-I). Periportal mononuclear infiltrates in the liver (panels B,C), and crypt cell apoptosis (panels E,F) and granulation tissue (* in panels H,I) in the large intestine are shown.
pDCs are solely sufficient to activate alloreactive CD8+ T cells in β2m-deficient mice
We examined whether presence of allogeneic pDCs could also stimulate donor CD8+ T cells in β2m−/− mice with impaired cellular expression of functional MHC class I. Irradiated β2m−/− C3H mice (CD90.2+) were injected with WT pDCs, cDCs, or B cells on day −1, followed by the injection with 2 × 106 CD8+ T cells from MHC-matched, miHA-mismatched AKR mice (CD90.1+) on day 0. Flow cytometric analysis of the mLNs and spleen on day +6 showed significantly greater expansion (Figure 4A) and IFN-γ production (Figure 4B) of donor CD8+ T cells (CD90.2− CD8+) in mice preinjected with pDCs or cDCs than in those with B cells. Thus, alloantigen expression on pDCs can solely prime alloreactive CD8+ T cells in vivo as potently as cDCs, although we were unable to examine whether these CD8+ T cells could cause GVHD target organ injury because miHA expression on target epithelium was required to induce GVHD in MHC-matched, miHA mismatched HSCT.27,42
Alloantigen expression on pDCs alone is sufficient to stimulate alloreactive CD8+ T cells. CD8+ T cells from AKR (CD90.2−) mice were transferred to irradiated β2m−/− C3H (CD90.2+) mice preinjected with WT B cells, pDCs, or cDCs. Expansion of CD90.2− donor CD8+ T cells in mLNs (A) and IFN-γ expression on donor CD8+ T cells in spleens (B) 6 days after transfer are shown as means plus or minus SEM. Data are representative of 2 similar experiments. *P < .05.
Alloantigen expression on pDCs alone is sufficient to stimulate alloreactive CD8+ T cells. CD8+ T cells from AKR (CD90.2−) mice were transferred to irradiated β2m−/− C3H (CD90.2+) mice preinjected with WT B cells, pDCs, or cDCs. Expansion of CD90.2− donor CD8+ T cells in mLNs (A) and IFN-γ expression on donor CD8+ T cells in spleens (B) 6 days after transfer are shown as means plus or minus SEM. Data are representative of 2 similar experiments. *P < .05.
Cognate interaction between pDCs and T cells is required for pDCs to prime alloreactive T cells
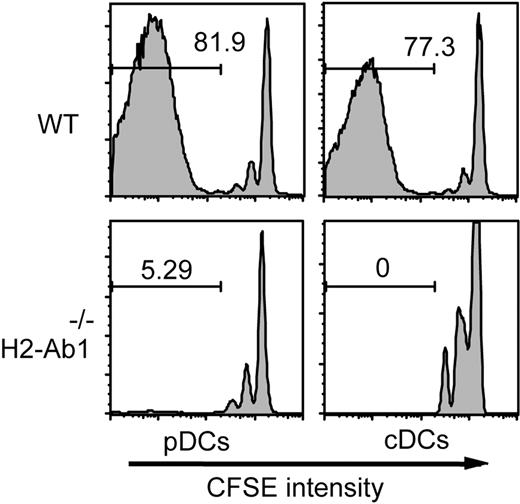
pDCs have the ability to produce large amounts of cytokines; therefore, they can be pathogenic through cytokine production as in SLE.23 To confirm the pathogenic role of pDCs functioning as APCs in GVHD, CFSE-labeled BALB/c CD4+ T cells were adoptively transferred to irradiated H2-Ab1−/− mice preinjected with H2-Ab1−/− pDCs. Flow cytometric analysis of the spleens 6 days after transfer showed that almost 90% of CFSE-labeled T cells had progressed through at least 3 cell divisions in animals preinjected with WT pDCs or cDCs, whereas only a small population did so with some homeostatic divisions in mice with H2-Ab1−/− pDCs or cDCs (Figure 5). These results demonstrate that cognate interaction between pDCs and T cells is required for pDCs to prime alloreactive T cells.
Cognate interaction between pDCs and T cells is required for T-cell activation. CFSE-labeled BALB/c CD4+ T cells were transferred to irradiated H2-Ab1−/− mice preinjected with 2 × 106 pDCs and cDCs isolated from WT or H2-Ab1−/− B6 mice. Cell divisions of H-2Kd+ donor CD4+ T cells in the spleens 6 days after transfer are shown. Data are representative of 2 similar experiments.
Cognate interaction between pDCs and T cells is required for T-cell activation. CFSE-labeled BALB/c CD4+ T cells were transferred to irradiated H2-Ab1−/− mice preinjected with 2 × 106 pDCs and cDCs isolated from WT or H2-Ab1−/− B6 mice. Cell divisions of H-2Kd+ donor CD4+ T cells in the spleens 6 days after transfer are shown. Data are representative of 2 similar experiments.
Irradiation is critical for pDCs to prime alloreactive T cells
Activation of pDCs is critical to prime alloreactive T cells as shown in Figure 1. Since it has been shown that irradiation induces maturation of cDCs in vivo,27 we hypothesized that pretransplantation TBI also plays an important role in activating pDCs. To test this hypothesis, we irradiated mice with 11Gy TBI and performed a flow cytometric analysis of the spleens and mLNs 10 hours after TBI. Expression of CD86 and MHC class II was up-regulated on both pDCs and cDCs isolated from irradiated mice, compared with those from unirradiated mice (Figure 6A). Next, we investigated whether maturation of pDCs are mediated by direct effects of irradiation on pDCs or by effects of irradiation on host tissues. To examine this, 80 × 106 BM cells and splenocytes collected from B6 (CD45.2+) mice were transferred to congeneic B6-Ly5a (CD45.1+) mice that had been irradiated 1 hour before transfer. Flow cytometric analysis of the mLNs isolated 10 hours later showed up-regulated MHC class II expression on CD45.2+ pDCs in irradiated mice compared with those isolated from unirradiated mice (Figure 6B). Collectively, irradiation, likely from the inflammation, is responsible for maturation of pDCs.
Host irradiation is prerequisite for pDC maturation to prime T cells. (A) Expression of CD86 and I-Ab expression on B cells, pDCs, and cDCs in the spleen and mLNs isolated from irradiated mice 10 hours after irradiation (filled histograms) and from unirradiated control mice (broken-lined open histograms). (B) Unirradiated (top) or irradiated (bottom) B6-Ly5a (CD45.1+) mice were injected with 80 × 106 BM cells and splenocytes isolated from FL-treated B6 (CD45.2+) mice. Expression of I-Ab on CD45.2+ B cells and pDCs in mLNs 10 hours after injection is shown. (C) Aliquots of 20 × 106 CFSE-labeled BALB/c (H-2Kd+) CD4+ T cells were transferred to unirradiated H2-Ab1−/− mice preinjected with 6 × 106 WT pDCs or cDCs. Cell division of donor CD4+ T cells in the spleens on day +6 is shown. (D) Similarly, CFSE-labeled BALB/c CD4+ T cells were transferred to unirradiated H2-Ab1−/− mice preinjected with freshly isolated pDCs or pDCs stimulated with CpG 1668 1 μM for 24 hours in vitro. Cell division of donor CD4+ T cells in the spleens on day +6 is shown. Data are representative of 2 similar experiments.
Host irradiation is prerequisite for pDC maturation to prime T cells. (A) Expression of CD86 and I-Ab expression on B cells, pDCs, and cDCs in the spleen and mLNs isolated from irradiated mice 10 hours after irradiation (filled histograms) and from unirradiated control mice (broken-lined open histograms). (B) Unirradiated (top) or irradiated (bottom) B6-Ly5a (CD45.1+) mice were injected with 80 × 106 BM cells and splenocytes isolated from FL-treated B6 (CD45.2+) mice. Expression of I-Ab on CD45.2+ B cells and pDCs in mLNs 10 hours after injection is shown. (C) Aliquots of 20 × 106 CFSE-labeled BALB/c (H-2Kd+) CD4+ T cells were transferred to unirradiated H2-Ab1−/− mice preinjected with 6 × 106 WT pDCs or cDCs. Cell division of donor CD4+ T cells in the spleens on day +6 is shown. (D) Similarly, CFSE-labeled BALB/c CD4+ T cells were transferred to unirradiated H2-Ab1−/− mice preinjected with freshly isolated pDCs or pDCs stimulated with CpG 1668 1 μM for 24 hours in vitro. Cell division of donor CD4+ T cells in the spleens on day +6 is shown. Data are representative of 2 similar experiments.
These results suggest that pDCs are incapable of priming alloreactive T cells in unirradiated mice as effectively as in irradiated mice. To test this hypothesis, we transferred CFSE-labeled BALB/c CD4+ T cells to unirradiated H2-Ab1−/− mice preinjected with WT B cells, pDCs, or cDCs. Donor CD4+ T cells were significantly proliferated in mice preinjected with cDCs 6 days after transfer (Figure 6C), although this cell division appeared to be less potent compared with that in irradiated animals. In contrast, few cell divisions were observed in mice preinjected with B cells or pDCs. We next evaluated whether maturation of pDCs was critical for T-cell activation. Isolated pDCs were cultured with CpG 1668 for 24 hours and injected into unirradiated H2-Ab1−/− mice, followed by the transfer of CFSE-labeled BALB/c CD4+ T cells. CpG-stimulated pDCs stimulate proliferation of donor T cells even in unirradiated mice (Figure 6D).
TLR signaling is not required for pDCs to prime alloreactive T cells
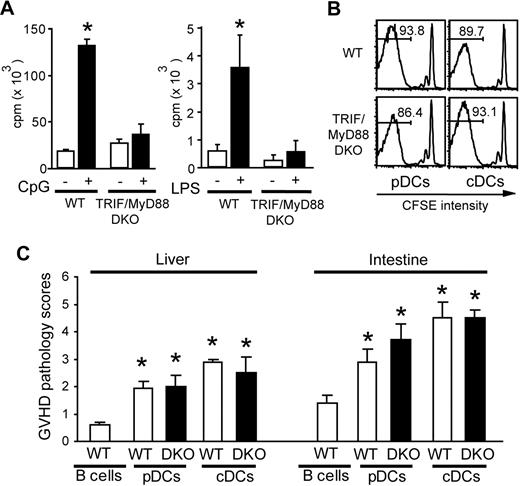
Stimulation with TLR ligands is crucial for the maturation and activation of pDCs.7,14 We, therefore, hypothesized that maturation of pDCs after TBI is mediated by TLR engagement. To test this hypothesis, we used TRIF/MyD88 DKO mice, where the TLR-dependent signaling pathway was critically abolished.29 pDCs isolated from TRIF/MyD88 DKO mice were phenotypically identical to WT pDCs (data not shown) but did not respond to CpG or LPS stimulation (Figure 7A), as has been shown.29 However, TRIF/MyD88 DKO cDCs and pDCs were capable of stimulating donor CD4+ T-cell division in irradiated H2-Ab1−/− mice 6 days after transfer (Figure 7B) and induced significant pathologic GVHD (Figure 7C) as effectively as WT cDCs and pDCs. Thus, TLR signaling is not required for pDCs and cDCs to prime alloreactive T cells.
TLR signaling is not required for pDCs to prime alloreactive T cells. (A) A total of 2 × 105 BALB/c CD4+ T cells were cultured with 104 WT or TRIF/MyD88 DKO (DKO) pDCs with or without CpG 1668 1 μM (left) or LPS 10 μg/mL (right) to determine cell proliferation. Data are shown as mean (± SD). (B,C) CFSE-labeled BALB/c CD4+ T cells (H-2Kd) were transferred to irradiated H2-Ab1−/− mice preinjected with pDCs or cDCs isolated from WT or DKO B6 mice. Cell divisions of H-2Kd+ donor CD4+ cells in the spleens (B) and GVHD pathology scores in the liver and intestine (C) are shown. Data from 3 similar experiments are combined and shown as means plus or minus SEM (n = 7). *P < .05.
TLR signaling is not required for pDCs to prime alloreactive T cells. (A) A total of 2 × 105 BALB/c CD4+ T cells were cultured with 104 WT or TRIF/MyD88 DKO (DKO) pDCs with or without CpG 1668 1 μM (left) or LPS 10 μg/mL (right) to determine cell proliferation. Data are shown as mean (± SD). (B,C) CFSE-labeled BALB/c CD4+ T cells (H-2Kd) were transferred to irradiated H2-Ab1−/− mice preinjected with pDCs or cDCs isolated from WT or DKO B6 mice. Cell divisions of H-2Kd+ donor CD4+ cells in the spleens (B) and GVHD pathology scores in the liver and intestine (C) are shown. Data from 3 similar experiments are combined and shown as means plus or minus SEM (n = 7). *P < .05.
Discussion
GVHD is initiated by the interaction of alloreactive donor T cells and host DCs.25,28 Our GVHD model system using MHC-deficient mice presents a stringent test on the allostimulatory functions of a subpopulation of APCs.28 Using this model system with modification, we addressed whether pDCs are pathogenic in GVHD. We found that alloantigen expression on pDCs alone sufficiently stimulates donor T cells to trigger GVHD in the absence of other APC subsets. Induction of GVHD required cognate interaction of pDCs and T cells, since MHC class II expression on pDCs was absolutely required for stimulating donor CD4+ T cells. It has been suggested that pDCs are involved in the pathogenesis of SLE and psoriasis through IFN-α production23,24 ; however, to our knowledge, our study is the first to directly demonstrate in vivo pathogenic role of pDCs as APCs in an antigen-specific T cell–mediated disease in the absence of other DC subsets.
Our results are in sharp contrast to previous reports suggesting that pDCs mediate tolerance in vivo. In cancer patients, pDCs are incapable of inducing antitumor immune responses but, instead, may induce regulatory T cells that inhibit immunity.16,17 In animal model of asthma, pDCs in the lung prevent asthmatic reactions to harmless inhaled antigens.15 In experimental cardiac transplantation, pDCs mediate tolerance and prolong survival of allografts.11,18,19 It has been suggested that pDCs functioning as APCs generate suppressive or regulatory T cells that mediate tolerance.12,13,16,–18 In contrast, our study showed that alloantigen-presenting pDCs were incapable of suppressing activation of alloreactive T cells but, instead, induced immunity even in the absence of other APC subsets. Differences between previous studies and our study may be attributed to pretransplantation irradiation essential for performing HSCT, which was used in our study.
We have shown that TBI is critical for pDC maturation to prime T cells; TBI up-regulates expression of MHC and costimulatory molecules on pDCs. It has been shown that TBI induces phenotypic and functional maturation of cDCs.27 Our results confirm and extend these findings. TBI also maturates pDCs, and this process is dependent on a TBI-mediated inflammatory environment. Although pDCs need to be in a certain state of maturation to prime naive T cells, cDCs can stimulate donor T cells even in unirradiated mice. Thus, the capacity of pDCs to prime naive T cells is far less efficient than cDCs in an uninflamed environment. Host pDCs may not be responsible for the induction of transfusion-associated GVHD in humans.43,44
It has been shown that TBI plays an important role in the pathogenesis of GVHD; GVHD is less severe in recipients that have had gentle rather than intensive preconditioning treatments.45,46 TBI activates and damages host tissue to secrete endogenous factors, such as heat-shock proteins and proinflammatory cytokines like tumor necrosis factor-α and IL-1. TBI-mediated gut injury allows the translocation of exogenous microbial products, such as LPS.27,45,47,48 DC maturation can be triggered by such endogenous and exogenous “danger signals” as well as by activated T cells, NK cells, and NKT cells.1,3,4,49 Since many of these stimuli bind to TLRs, we hypothesized that TLR signaling is required for pDC maturation. However, pDCs from TRIF/MyD88 DKO mice with defective TLR signaling29 were activated by TBI and activate donor T cells to cause GVHD as potently as WT pDCs. Thus, pDCs can be matured by pathways other than TLR signaling, such as nucleotide-binding oligomerization domain-like receptors, retinoic acid–inducible gene I, melanoma differentiation-associated gene 5, DNA-dependent activator of interferon-regulatory factors, C-type lectin receptors, CD40 ligands, and cytokine and chemokine receptors.49,50
Immature cDCs also play a role in maintaining tolerance, and thus, irrespective of the subset of DCs, DC maturation may be a control checkpoint in the initiation of immunity.3,51 Although this simple concept has been revised by several reports showing that mature cDCs and pDCs can induce regulatory T-cell responses,12,13 our results indicate that pDCs mature and acquire APC function to induce antigen-specific immunity in the inflamed tissue. Thus, functional outcomes of pDC–T-cell interactions depend on the immunologic context of encounter.
It is important to note that pDCs mediate clinically and histologically standard GVHD that was not indistinguishable from cDC-mediated GVHD. Thus, our results further extend the current paradigm that host DCs play a critical role in initiating GVHD.25,28 Inactivation of host DCs can be a novel strategy to prevent GVHD.25 Our results suggest that immunologic elimination of cDCs may not be sufficient for complete prevention of GVHD, thus providing important information for developing strategies aimed at inactivating host DCs to prevent GVHD.
The online version of this article contains a data supplement.
Presented in abstract form at the 49th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 10, 2007.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was supported by research funds from the Ministry of Education, Culture, Sports, Science, and Technology grant 2659153 (T.T.), Health and Labor Science Research grants (T.T.), and the Mitsubishi Pharma Research Foundation (Tokyo, Japan; T.T.).
Authorship
Contribution: M.K. conducted research and wrote the paper; D.H., K.A., K.M., K.K., and H.N. conducted research; M.H., M.T., and K.A. designed the study; and T.T. designed the study and organized the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takanori Teshima, Center for Cellular and Molecular Medicine, Kyushu University Hospital, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan; e-mail: tteshima@cancer.med.kyushu-u.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal