To the editor:
Glucose 6-phosphate dehydrogenase (G6PD) deficiency is the world's most common enzymopathy1 and caused by mutations in the X-chromosomal G6PD gene. The enzyme catalyzes the first reaction of the hexose monophosphate shunt, which results in 2 main products: pentose phosphate sugars and the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH). The latter is involved in reductive processes. G6PD deficiency is most pronounced in erythrocytes, compared with other cells, because red blood cells lack biosynthetic capacity. Therefore, G6PD-deficient erythrocytes are less able to withstand oxidative stress, leading to (acute) hemolytic anemia.1
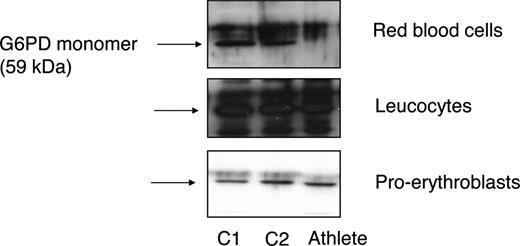
We recently studied a 37-year-old white man who is an elite long distance runner and has attained high class rankings in European, world, and Olympic competitions in the last 15 years while being severely G6PD-deficient. He never showed any clinical signs of hemolysis, and the G6PD deficiency was detected by chance. Routine laboratory investigations showed no abnormalities, except for low haptoglobin and slightly elevated unconjugated bilirubin levels over the years, suggesting the presence of mild chronic hemolysis (Table 1). The athlete's erythrocytes were found to exhibit strongly reduced G6PD activity. G6PD activity in leukocytes was also decreased, though less pronounced. These findings were confirmed by Western blot analysis (Figure 1). DNA analysis revealed a missense mutation in exon 8 (c.844 G>C: Asp282His) of G6PD. This mutation has been previously found to underlie several biochemically unique G6PD variants.2 To further characterize the G6PD variant, kinetic measurements were performed using leukocyte-derived G6PD (Table 1). These results led us to conclude that the kinetic properties of the mutant enzyme were only slightly altered. Hence, G6PD deficiency in this athlete appears to be mainly due to the mutant protein's instability, in particular in erythrocytes (Figure 1).
Representative results of routine and specialized laboratory investigations
| Laboratory investigation . | Patient . | Reference . |
|---|---|---|
| Routine | ||
| Hemoglobin, mmol/L | 9.4 | 8.6-10.7 |
| Erythrocytes, ×1012/L | 4.8 | 4.2-5.5 |
| MCV, fL | 87 | 80-97 |
| Reticulocytes, ×109/L | 25 | 25-120 |
| Creatinine, μmol/L | 84 | 74-120 |
| Creatinine kinase, U/L | 114 | 15-175 |
| γ-glutamyl transferase, U/L | 17 | 15-70 |
| Aspartate aminotransferase, U/L | 29 | 15-45 |
| Alanine aminotransferase, U/L | 21 | 10-50 |
| Lactate dehydrogenase, U/L | 494 | 300-620 |
| Unconjugated bilirubin, μmol/L | 24 | 3-21 |
| Haptoglobin, g/L | < 0.1 | 0.3-2.0 |
| Specialized laboratory investigation | ||
| G6PD activity (U/g Hb) in erythrocytes | 0.8 | 7.1-11.5 |
| PK activity (U/g Hb) in erythrocytes | 7.2 | 6.9-14.5 |
| HK activity (U/g Hb) in erythrocytes | 1.12 | 1.0-1.6 |
| G6PD activity (U/g protein) in leukocytes | 530 | 815-877 |
| Calculated G6PD activity (U/g protein) in muscle cells* | 0.5 | 3-4.7 |
| Km for G6P (μmol/L) in leukocytes | 40 | 55-71 |
| Km for NADP (μmol/L) in leukocytes | 45 | 66-76 |
| Ki value for NADPH (μmol/L) in leukocytes | 153 | 142-159 |
| G6PD activity at pH (range, 6.5-9.0) and elevated temperature (39°C, mimicking increased body temperature) | Normal | Normal |
| Oxidative hemolysis | 1.5% | < 2% |
| Laboratory investigation . | Patient . | Reference . |
|---|---|---|
| Routine | ||
| Hemoglobin, mmol/L | 9.4 | 8.6-10.7 |
| Erythrocytes, ×1012/L | 4.8 | 4.2-5.5 |
| MCV, fL | 87 | 80-97 |
| Reticulocytes, ×109/L | 25 | 25-120 |
| Creatinine, μmol/L | 84 | 74-120 |
| Creatinine kinase, U/L | 114 | 15-175 |
| γ-glutamyl transferase, U/L | 17 | 15-70 |
| Aspartate aminotransferase, U/L | 29 | 15-45 |
| Alanine aminotransferase, U/L | 21 | 10-50 |
| Lactate dehydrogenase, U/L | 494 | 300-620 |
| Unconjugated bilirubin, μmol/L | 24 | 3-21 |
| Haptoglobin, g/L | < 0.1 | 0.3-2.0 |
| Specialized laboratory investigation | ||
| G6PD activity (U/g Hb) in erythrocytes | 0.8 | 7.1-11.5 |
| PK activity (U/g Hb) in erythrocytes | 7.2 | 6.9-14.5 |
| HK activity (U/g Hb) in erythrocytes | 1.12 | 1.0-1.6 |
| G6PD activity (U/g protein) in leukocytes | 530 | 815-877 |
| Calculated G6PD activity (U/g protein) in muscle cells* | 0.5 | 3-4.7 |
| Km for G6P (μmol/L) in leukocytes | 40 | 55-71 |
| Km for NADP (μmol/L) in leukocytes | 45 | 66-76 |
| Ki value for NADPH (μmol/L) in leukocytes | 153 | 142-159 |
| G6PD activity at pH (range, 6.5-9.0) and elevated temperature (39°C, mimicking increased body temperature) | Normal | Normal |
| Oxidative hemolysis | 1.5% | < 2% |
(y = 0.39x + 0.198).9
Instability of mutant G6PD. Western blot analysis was performed on red blood cells, leukocytes, and ex vivo–cultured proerythroblasts10 from 2 controls (C1 and C2) and the athlete, using a polyclonal antibody against G6PD (kindly provided by Robin van Bruggen, Sanquin Research, Amsterdam, The Netherlands). In contrast to leukocytes and ex vivo–cultured proerythroblasts, mutant G6PD was not detectable in the athlete's erythrocytes.
Instability of mutant G6PD. Western blot analysis was performed on red blood cells, leukocytes, and ex vivo–cultured proerythroblasts10 from 2 controls (C1 and C2) and the athlete, using a polyclonal antibody against G6PD (kindly provided by Robin van Bruggen, Sanquin Research, Amsterdam, The Netherlands). In contrast to leukocytes and ex vivo–cultured proerythroblasts, mutant G6PD was not detectable in the athlete's erythrocytes.
Heavy exercise can accelerate the generation of reactive oxygen species (ROS), exceeding the capacity of antioxidant defenses.3 Because this may result in hemolysis and muscle degeneration, it has been postulated that G6PD-deficient persons with red blood cell enzyme activities less than 15% of normal should avoid prolonged high-intensity physical exercise.4 Two recent studies concluded that G6PD-deficient persons are able to perform mild to moderate exercise without higher oxidative stress than G6PD-nondeficients.5,6 Of particular interest are 2 case reports describing adverse effects of exercise on G6PD-deficient persons. The first describes a 20-year-old G6PD-deficient man who was hospitalized for myalgia and myoglobinuria after intense exercise; he carried the same G6PD variant as our case.7 The second is a 30-year-old pentathlon-trained athlete with G6PD deficiency who suffered from loss of consciousness and pigmenturia during the last meters of a 12-km competitive run.8 Contrary to both these reports, our athlete showed no more severe signs of myalgia, myoglobinuria, or hemoglobinuria than other elite long-distance runners, although residual activity in erythrocytes was approximately 9%, and the calculated activity9 in muscle cells was 13% of normal. We postulate that the athlete described here has developed a unique balance between his G6PD deficiency and exercise-induced disturbances of blood glutathione and lipid peroxidation that allows him to perform strenuous exercise.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard van Wijk, PhD, University Medical Center Utrecht, Laboratory for Red Blood Cell Research, Department of Clinical Chemistry and Haematology, Room G.03.550, PO Box 85500, 3508 GA Utrecht, The Netherlands; e-mail: R.vanWijk@umcutrecht.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal