Abstract
Increased fetal hemoglobin expression in adulthood is associated with acute stress erythropoiesis. However, the mechanisms underlying γ-globin induction during the rapid expansion of adult erythroid progenitor cells have not been fully elucidated. Here, we examined COUP-TFII as a potential repressor of γ-globin gene after stem cell factor (SCF) stimulation in cultured human adult erythroid progenitor cells. We found that COUP-TFII expression is suppressed by SCF through phosphorylation of serine/threonine phosphatase (PP2A) and correlated well with fetal hemoglobin induction. Furthermore, down-regulation of COUP-TFII expression with small interfering RNA (siRNA) significantly increases the γ-globin expression during the erythroid maturation. Moreover, SCF-increased expression of NF-YA associated with redox regulator Ref-1 and cellular reducing condition enhances the effect of SCF on γ-globin expression. Activation of Erk1/2 plays a critical role in SCF modulation of downstream transcriptional factor COUP-TFII, which is involved in the regulation of γ-globin gene induction. Our data show that SCF stimulates Erk1/2 MAPK signaling pathway, which regulates the downstream repressor COUP-TFII by inhibiting serine/threonine phosphatase 2A activity, and that decreased COUP-TFII expression resulted in γ-globin reactivation in adult erythropoiesis. These observations provide insight into the molecular pathways that regulate γ-globin augmentation during stress erythropoiesis.
Introduction
In humans, hemoglobin production switching from fetal hemoglobin (HbF; α2γ2) to adult hemoglobin (α2β2) occurs on birth as a result of γ- to β-globin gene switching. This switching probably requires developmental stage–specific changes in transcription factor or chromatin remodeling activities or both that lead to either repression of γ-globin gene expression or activation of β-globin genes (or both).1-3 HbF production is normally reduced to very low levels (<1%) of the total hemoglobin in adults.4 However, various physiologic and pathologic conditions that are associated with acute erythroid stress increase HbF expression in adulthood and appear to involve rapid expansion of erythroid progenitors, which activates their inherent ability to synthesize HbF.5-8 Several investigators have attempted to reproduce experimentally the elevation of HbF in response to acute stress in vitro using growth-related cytokines, but the results have been challenged by others.9,10 Stem cell factor (SCF) has been shown to greatly increase HbF production and is thought to influence HbF production through signaling pathways in erythropoiesis,11-13 providing an important model for investigation of the molecular mechanisms underlying this reactivation.
SCF initiates its effects by binding to the c-kit receptor, which results in receptor dimerization and activation of multiple signaling pathways, including the Erk1/2 and p38 mitogen-activated protein kinase (MAPK) pathways, among others.14,15 It has been shown that HbF induced by SCF is mediated by the Erk1/2 MAPK pathway.16
Although much is known already about cis-acting DNA binding and transcription factors in the regulation of γ-globin gene expression, the role of downstream transcription regulators involved in SCF/c-kit signaling-enhanced γ-globin expression remains largely unknown. Among the large number of proteins binding to the γ-globin promoter region, we focused our study on 2 proteins, the stage-specifically expressed nuclear receptor chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) and the direct repeat erythroid definitive binding protein TR2/TR4, because both bind to the direct repeat (DR) elements in the ϵ- and γ-globin promoters in vitro and have been implicated in repression of ϵ- or γ-globin expression.17-19 Several other ubiquitously expressed trans-activators, including CP1/NF-Y, the CCAAT displacement protein, and the CCAAT enhancer-binding protein, have also been shown to bind this region to silence γ-globin gene expression in vitro.20 Mutation of the DR elements that inhibit protein binding result in ϵ- or γ-globin gene expression in adult mice, indicating that the DR elements participate in ϵ- or γ-globin silencing.17,21,22 These observations suggest that proteins binding this region play a central role in governing transcriptional regulation of the ϵ- and γ-globin genes.
COUP-TFII binding to the distal CCAAT box region leads to γ-globin inhibition through its repressor domains and prevents NF-Y binding to this overlapping region, suggesting that the loss of COUP-TFII binding might contribute to the hereditary persistence of fetal hemoglobin phenotype at least in part by favoring transcriptional activators binding to the γ-globin promoter.23 COUP-TFII has been shown to be regulated by various signaling pathways, including the MAPK and Shh signaling pathways.24,25 In this study, we explored the hypothesis that SCF signaling may occur by inhibition or removal of the repressor COUP-TFII that binds to the DR site of the γ-globin promoter, and which in turn could subsequently result in increased γ-globin expression in adult erythropoiesis.
Methods
Cell culture and reagents
Human peripheral blood CD34+ cells from healthy donors were isolated and cultured for 14 days in a-MEME medium (Sigma) supplemented with 4 U/mL EPO (Amgen) for differentiation of the erythroid lineage. CD34+ cells used in all experiments were obtained with the use of protocols approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), and donor informed consent was obtained in accordance with the Declaration of Helsinki. For Western blot experiments, cells that had been cultured with EPO for 6 days were stimulated with 50 ng/mL SCF (R&D Systems Inc) for the indicated time periods, with or without indicated inhibitors added 30 minutes before SCF stimulation. For analysis of the expression of γ- or β-globin, COUP-TFII, NF-YA mRNA, cells grown in EPO for 6 days were treated with SCF from day 6 to day 14 with or without the following inhibitors: the Erk1/2 inhibitor PD98059 (20 μM), the p38 MAPK inhibitor SB202109 (5 μM); the thiol-containing antioxidant N-acetylcysteine (NAC; 1-100 μM), and the reducing agent 2-mercaptoethanol (2-ME; 5-500 μM). NAC and 2-ME were purchased from Sigma. All other inhibitors were purchased from Calbiochem.
RNA preparation and quantitative polymerase chain reaction analysis
Total RNA was extracted with the use of the RNeasy Mini kit (QIAGEN) on days 6, 8, 10, 12, and 14 after SCF was added on day 6 cultured CD34+ cells. For reverse transcription, 1 μg total RNA per sample was used as a template for cDNA synthesis with the use of Superscript III (Invitrogen) following the manufacturer's guidelines. Quantitative real-time polymerase chain reaction (PCR) was carried out with 50 ng of reverse-transcribed total RNA as the template in each PCR reaction as described previously,26 and all samples were run in triplicate. The following primer and probe sequences were used: γ-globin forward primer, 5′-GGCAACCTGTCCTCTGCCTC-3′; γ-globin reverse primer, 5′-GAAATGGATTGCCAAAACGG-3′; γ-globin probe, 5′-FAM-CAAGCTCCTGGGAAATGTGCTGGTG-TAMRA-3′; β-globin forward primer, 5′-CTCATGGCAAGAAAGTGCTCG-3′; β-globin reverse primer, 5′-AATTCTTTGCCAAAGTGATGGG-3′; β-globin probe, 5′-FAM-CGTGGATCCTGAGAACTTCAGGCTCCT-TAMRA-3′. TaqMan primers and probes for COUP-TFII, TR2, TR4, NF-YA, and control gene β-actin were purchased as Assays-on-Demand Gene Expression Products (PE Applied Biosystems). A plasmid DNA encoding γ- and β-globin, COUP-TFII, NF-YA, and control β-actin template were used to generate a standard curve (20-2 000 000 copies) for determination of copy number. Statistical significance for all experiments was determined by Student paired t test analyses.
Immunoblotting analysis
Extracted total protein from CD34+ cells was prepared with the use of the Protein Extraction Reagent (Pierce Biotechnology) as recommended by the manufacturer. Proteins (30 μg/lane) were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and then analyzed with antibodies to the following proteins: phospho-serine/threonine phosphatase 2A (pY307; Epitomics); anti–human COUP-TFII/NR2F2 (R&D Systems Inc); γ-hemoglobin, thioredoxin (Trx), Ref-1, NF-YA (Santa Cruz Biotechnology Inc); phospho-p38 MAPK (Thr180/Tyr182), total p38, phospho-Erk1/2 (Thr202/Tyr204), total Erk2 (Cell Signaling Inc); and β-actin (Sigma). Horseradish peroxidase–conjugated secondary antibodies (Zymed) were used for chemiluminescent detection of protein (enhanced chemiluminescence kit; Amersham Biosciences).
Immunofluorescence
Cytospin preparations were made with CD34+ cells obtained after 6 days in culture. Cell slides were fixed in chilled acetone/methanol at a 1:1 volume for 10 minutes, then air dried. After blocking in 2% bovine serum albumin–PBS for 1 hour at room temperature, the slides were incubated with rabbit anti–human Trx or Ref-1 at a 1:100 dilution overnight at 4°C. After washing, the slides were immunostained with FITC-conjugated goat anti–rabbit IgG secondary antibodies. Fluorescence staining was observed with a Zeiss laser-scanning microscope 310 with a 63× water immersion lens. Cytospins were performed on day 12 cells after siCOUP-TFII and cell types were identified by morphology after Giemsa staining. Images were acquired using an Olympus BX51 microscope (Olympus America) with an UPlan FLN 20×/0.50 lens. A PAXcam EDU camera (Midwest Information Systems) and PAX-it software (Midwest Information Systems) were used to capture images.
Chromatin immunoprecipitation assay
After CD34+ cells were stimulated by SCF for 14 days, they were used for chromatin immunoprecipitation (ChIP) assays according the manufacturer's instructions (Upstate Biotechnology). Briefly, unstimulated cells were used as a control. Cells cultured for 14 days were fixed with 1% formaldehyde for 10 minutes at 37°C. After cross-linking, the chromatin complex was isolated and fragmented. Chromatin fragments were precipitated with anti–COUP-TFII or anti-RNA polymerase II (all from Santa Cruz Biotechnology Inc). For chromatin quantification, standard curves were generated with 1:10 serial dilutions of input DNA. Primers used for amplifying the γ-globin gene promoter were designed to the paxtil region between −1350 and −1100, 5′-AAGCCTTACACAGGATTATGAAGTCTG-3′ (forward) and 5′-ACATGGCAGGAAGTATTCATGCTG-3′ (reverse), and the detax region between −150 and −20, 5′-CTGTCTGAAACGGTCCCTGG-3′ (forward) and 5′-CTGGCCTCACTGGATACTCT-3′ (reverse).
siRNA transfection
COUP-TFII siRNA (Silencer Pre-designed) and a control non–targeting siRNA (negative siRNA no. 2) were purchased from Ambion. CD34+ cells were cultured in EPO-containing medium. On day 6, cells (2 × 106 cells/100 μL in nucleofector solution V) were transiently transfected with mock, control non–targeting siRNA or different dose (1.0, 2.5, 5.0 μg) COUP-TFII siRNA by electroporation with the use of an Amaxa Nucleofector I device (Human CD34+ Nucleofector Solution, program U-08; Amaxa Biosystems). After 48 hours, the cells were harvested and used for testing silencing efficiency, and subsequent RNA was extracted on day 12–cultured cells. Quantitative reverse transcription (RT)–PCR was performed to determine the expression of COUP-TFII, γ-, and β-globin gene. Fluorescence analyses were performed with a LSM 310 Zeiss confocal microscope (Carl Zeiss Inc) using a 40×/0.75 water immersion lense. FITC was excited at 488 nm argon laser and the Emission was selected with a 505-550 nm band-pass filter. Images were collected and confocal z-sections were acquired at 0.5-mm intervals. Images were analyzed using ImageJ software (NIH) and further processed using Adobe Photoshop software 7.0 (Adobe Systems).
Results
SCF-suppressed COUP-TFII expression by inactivation of protein phosphatase 2A inversely correlates with γ-globin induction during erythroid differentiation
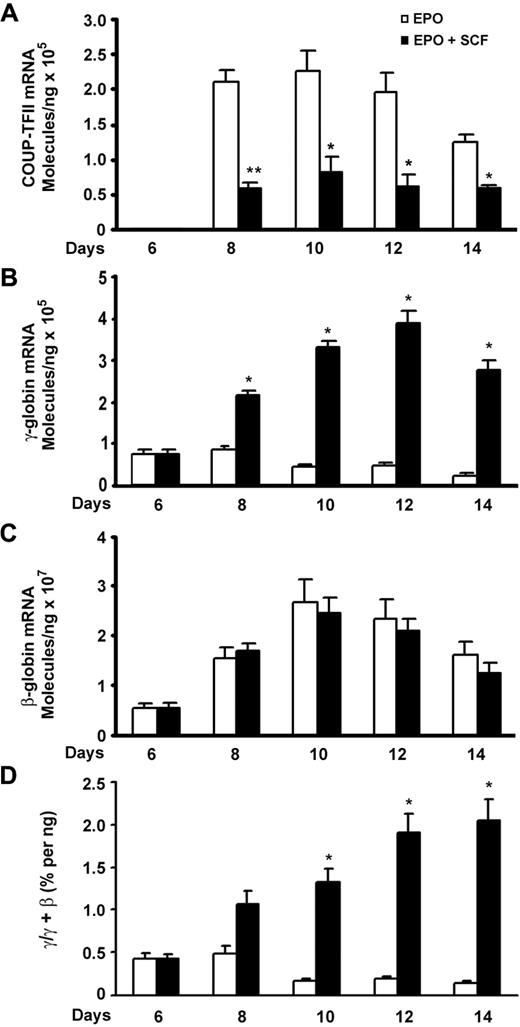
We attempted to identify the possible downstream transcription factors that are involved in SCF-induced γ-globin expression. Given that COUP-TFII and TR2/TR4 are known to silence ϵ- or γ-globin transcription in adult erythropoiesis,17-19 we used quantitative RT-PCR to quantitatively evaluate the expression pattern of COUP-TFII and TR2/TR4 during the erythroid differentiation in response to SCF stimulation. For this purpose, primary human CD34+ cells were cultured in the presence of EPO over a 2-week period, and erythroid progenitors showed increased growth during the first week, followed by reduced proliferation and terminal differentiation during the second week.26 This culture system enabled us to compare the expression pattern of individual genes during erythroid differentiation.16,27 On day 6, SCF was added to the culture medium, and mRNA was collected on subsequent days and compared with control cells grown in the absence of SCF. In EPO-alone cells, the expression of COUP-TFII mRNA was undetectable on day 6 but rose on day 8 and peaked on day 10, then decreased slightly again on day 14 (Figure 1A). When SCF was added on day 6, we detected a decrease in the level of COUP-TFII mRNA in SCF-stimulated cells compared with that of unstimulated control cells during erythroid maturation, with most profound suppressed effect occurring on day 8 (P < .01). No changes were observed in the expression of TR2/TR4 mRNA (data not shown). Interestingly, the peak in COUP-TFII expression coincides with a modest rise in β-globin expression during terminal erythroid differentiation, suggesting that COUP-TFII may trigger the repression of γ-globin gene expression during terminal differentiation. Indeed, our quantitative RT-PCR results showed that SCF-reduced expression of COUP-TFII mRNA was inversely correlated with up-regulation of γ-globin mRNA during erythroid maturation (Figure 1A-B).
SCF-suppressed COUP-TFII expression inversely correlates with increase in γ-globin during erythroid differentiation. CD34+ cells cultured in EPO-containing medium for 6 days were stimulated with SCF (50 ng/mL) from day 6 to day 14, and RNA extracted from cells harvested on days 6, 8, 10, 12, and 14 was analyzed by quantitative RT-PCR. (A) Expression levels of COUP-TFII mRNA in EPO versus EPO plus SCF. (B) Expression levels of γ-globin mRNA in EPO versus EPO plus SCF. (C) Expression levels of β-globin mRNA in EPO versus EPO plus SCF. (D) The ratio of γ/(γ + β) globin percentages are shown in EPO versus EPO plus SCF. Results are shown as mean ± SD from 3 independent donors that were analyzed in separate experiments. *P < .05 versus SCF-stimulated cells. **P < .01 versus SCF-stimulated cells.
SCF-suppressed COUP-TFII expression inversely correlates with increase in γ-globin during erythroid differentiation. CD34+ cells cultured in EPO-containing medium for 6 days were stimulated with SCF (50 ng/mL) from day 6 to day 14, and RNA extracted from cells harvested on days 6, 8, 10, 12, and 14 was analyzed by quantitative RT-PCR. (A) Expression levels of COUP-TFII mRNA in EPO versus EPO plus SCF. (B) Expression levels of γ-globin mRNA in EPO versus EPO plus SCF. (C) Expression levels of β-globin mRNA in EPO versus EPO plus SCF. (D) The ratio of γ/(γ + β) globin percentages are shown in EPO versus EPO plus SCF. Results are shown as mean ± SD from 3 independent donors that were analyzed in separate experiments. *P < .05 versus SCF-stimulated cells. **P < .01 versus SCF-stimulated cells.
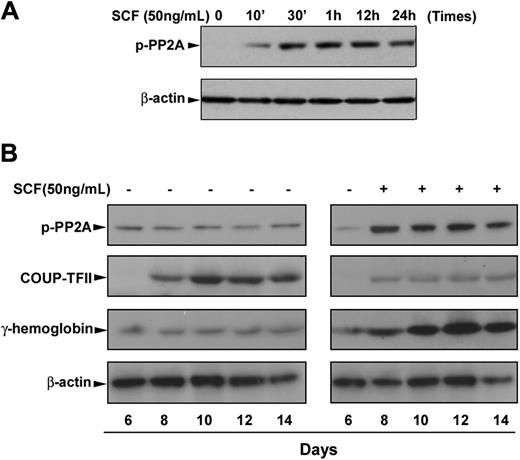
We next sought to gain insight into the possible mechanism for SCF suppression of COUP-TFII expression. The serine/threonine PP2A has been reported to be required for sonic hedgehog–induced COUP-TFII expression,25 and its activity is functionally inactivated when it is phosphorylated at residue Thr307,28 raising the possibility that SCF suppression of COUP-TFII may be mediated by inactivation of PP2A in erythroblasts, resulting in the γ-globin induction. For this purpose, we performed time-course studies of the phosphorylation of PP2A during erythroid differentiation. As expected, immunoblot analysis showed that SCF stimulation of day 6 cells rapidly induced phosphorylation of PP2A, and this induction was sustained over 24 hours (Figure 2A). Consistent with our hypothesis, time course experiments showed that SCF-induced phosphorylation of PP2A constitutively appears through erythroid differentiation compared with very low levels of phosphorylated PP2A in EPO control (Figure 2B). In contrast, the expression of COUP-TFII protein was sharply suppressed by SCF treatment, which inversely correlated with the increased expression of fetal hemoglobin during erythroid maturation (Figure 2B). The reduction in COUP-TFII protein levels seen after SCF treatment is consistent with its mRNA expression pattern, showing decreased expression levels in response to SCF (Figure 1A). These findings suggest that the SCF signaling pathway may depend on phosphorylation of PP2A to inhibit the COUP-TFII expression, resulting in γ-globin induction in adult erythropoiesis.
SCF-induced phosphorylation of PP2A correlates with increased fetal hemoglobin during erythroid differentiation. CD34+ cells were cultured in EPO-containing medium for 6 days before the addition of SCF. (A) Cells were incubated with SCF (50 ng/mL) for the indicated times, and whole cell lysates (30 μg) were analyzed by immunoblot for phosphorylation of PP2A. (B) Cells were incubated with SCF (50 ng/mL) from day 6 to day 14, and total cell lysates (30 μg) harvested on the indicated culture days were analyzed by immunoblot for phosphorylation of PP2A, COUP-TFII, or fetal hemoglobin expression. The lowest panel shows the same blot stripped and reprobed with total β-actin antibody to confirm that similar amounts of protein extracts were analyzed in each lane. Representative immunoblots from 3 independent experiments are shown.
SCF-induced phosphorylation of PP2A correlates with increased fetal hemoglobin during erythroid differentiation. CD34+ cells were cultured in EPO-containing medium for 6 days before the addition of SCF. (A) Cells were incubated with SCF (50 ng/mL) for the indicated times, and whole cell lysates (30 μg) were analyzed by immunoblot for phosphorylation of PP2A. (B) Cells were incubated with SCF (50 ng/mL) from day 6 to day 14, and total cell lysates (30 μg) harvested on the indicated culture days were analyzed by immunoblot for phosphorylation of PP2A, COUP-TFII, or fetal hemoglobin expression. The lowest panel shows the same blot stripped and reprobed with total β-actin antibody to confirm that similar amounts of protein extracts were analyzed in each lane. Representative immunoblots from 3 independent experiments are shown.
SCF stimulation decreases COUP-TFII binding to the γ-globin promoter
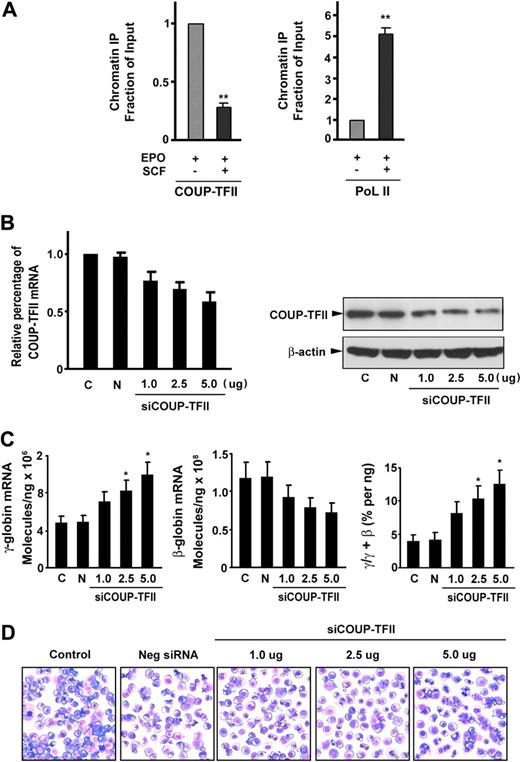
To investigate whether SCF decreases COUP-TFII binding to the γ-globin gene promoter, we performed a ChIP assay in CD34+ cells stimulated with or without SCF (using antibodies to COUP-TFII or RNA polymerase II). ChIP assays of the γ-globin gene promoter showed that COUP-TFII occupancy at the γ-globin promoter in SCF-stimulated cells was significantly reduced to 28.4% compared with its occupancy in unstimulated cells (Figure 3A left). In addition, SCF stimulation resulted in a 5.1-fold increase in RNA polymerase II binding compared with that observed in unstimulated cells (Figure 3A right), suggesting a reciprocal and proportional increased recruitment of RNA polymerase II to the promoter in response to decreased COUP-TFII binding. These findings indicate that SCF promotes displacement of the COUP-TFII repressor complex, followed by a simultaneous and proportional recruitment of the basal transcription machinery RNA polymerase II to the γ-globin promotor, resulting in γ-globin gene reactivation.
SCF suppresses COUP-TFII binding to the γ-globin promoter and knockdown of endogenous COUP-TFII induces γ-globin expression in erythroblasts. (A) Antibodies against COUP-TFII and RNA polymerase II (PoL II) were used to immunoprecipitate chromatin. Precipitated DNA was amplified and quantitated by real-time PCR with primers flanking the γ-globin gene promoter. Results are expressed as relative proportions of immunoprecipitated DNA (ratios of immunoprecipitated versus input DNA) normalized to the ratio obtained for the γ-globin promoter in unstimulated cells (arbitrarily set at 1). (B) Effect of different doses of silencing COUP-TFII mRNA or protein levels was evaluated by quantitative RT-PCR or immunoblot analysis on day 12 cultured cells after transiently transfected with either COUP-TFII or control non–targeting siRNA by Amaxa electroporation. (C) Quantitative RT-PCR analysis of γ-, β-globin mRNA levels, and the ratio of γ/(γ + β) globin percentages in negative control or knockdown COUP-TFII (different doses) cells. (D) Representative fields of Giemsa-stained erythroid cell at day 12 after knockdown of COUP-TFII at different doses. Results are shown as mean ± SD from 3 independent donors that were analyzed in separate experiments. *P < .05 versus negative control siRNA.
SCF suppresses COUP-TFII binding to the γ-globin promoter and knockdown of endogenous COUP-TFII induces γ-globin expression in erythroblasts. (A) Antibodies against COUP-TFII and RNA polymerase II (PoL II) were used to immunoprecipitate chromatin. Precipitated DNA was amplified and quantitated by real-time PCR with primers flanking the γ-globin gene promoter. Results are expressed as relative proportions of immunoprecipitated DNA (ratios of immunoprecipitated versus input DNA) normalized to the ratio obtained for the γ-globin promoter in unstimulated cells (arbitrarily set at 1). (B) Effect of different doses of silencing COUP-TFII mRNA or protein levels was evaluated by quantitative RT-PCR or immunoblot analysis on day 12 cultured cells after transiently transfected with either COUP-TFII or control non–targeting siRNA by Amaxa electroporation. (C) Quantitative RT-PCR analysis of γ-, β-globin mRNA levels, and the ratio of γ/(γ + β) globin percentages in negative control or knockdown COUP-TFII (different doses) cells. (D) Representative fields of Giemsa-stained erythroid cell at day 12 after knockdown of COUP-TFII at different doses. Results are shown as mean ± SD from 3 independent donors that were analyzed in separate experiments. *P < .05 versus negative control siRNA.
COUP-TFII is a potent suppressor of γ-globin gene
To further investigate the functional role of COUP-TFII in mediating γ-globin gene induction, we examined whether down-regulation of endogenous COUP-TFII expression in erythroid progenitor cells with a specific COUP-TFII siRNA would lead to an increase in γ-globin expression in erythroblasts. We tested the effects of COUP-TFII suppression on day 12 cells after transfection of specific COUP-TFII siRNA (1.0 to approximately 5.0 μg) on day 6 cells with the use of Amaxa. We confirmed that the expression of COUP-TFII mRNA was depleted by 42% (knockdown) in a dose-dependent manner compared with control non–targeting siRNA-transfected cells (Figure 3B left). The identical reductions in COUP-TFII protein were observed after transfection of COUP-TFII siRNA (Figure 3B right). With this knockdown of COUP-TFII (5.0 μg siRNA), we observed a 2.1-fold increase in the level of γ-globin mRNA and a corresponding decrease in β-globin expression (compared with control non–targeting siRNA-transfected cells) on day 12 of differentiation (Figure 3C). The divergent changes in the expression levels of γ- and β-globin mRNA on day 12 by knockdown of COUP-TFII cells resulted in a significant increase in the ratio of γ/(γ + β) globin (3.2- ± 0.8-fold; P < .05; Figure 3C). In addition, silencing of COUP-TFII in different doses resulted in the pattern of differentiation nearly identical to that seen in EPO alone control (Figure 3D), suggesting that COUP-TFII knockdown did not perturb overall erythroid differentiation. Together, these results suggest that knockdown of COUP-TFII is sufficient to promote the induction of γ-globin expression in adult erythropoiesis.
SCF increases NF-YA associated with Ref-1
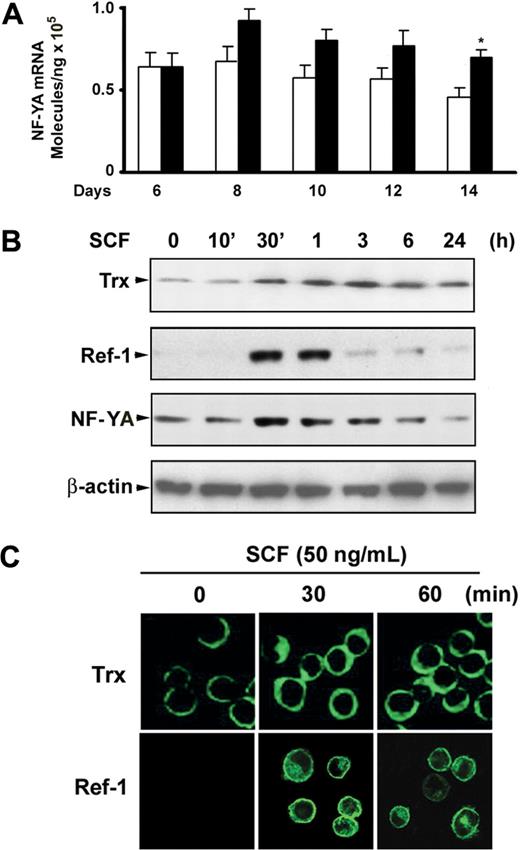
Given that both COUP-TFII and NF-Y bind to the ϵ- and γ-globin promoter in the CCAAT box region and that COUP-TFII directly competes with NF-Y for binding to the CCAAT region of γ-globin promoter resulted in regulation of γ-globin expression,23 we speculated that suppression of COUP-TFII may be associated with up-regulation of NF-Y. Functional NF-Y levels are regulated by alterations in the cellular concentration of its NF-YA subunit, because expression of the NF-YA subunit has been shown to be regulated, whereas NF-YB and NF-YC subunits are constitutively expressed.29,30 With the use of quantitative real-time PCR, we detected increases in the level of NF-YA mRNA in SCF-treated cells compared with those of untreated control cells during erythroid maturation (Figure 4A). It has been reported that NF-YA is the DNA-binding and regulatory subunit of NF-Y trimer,29,30 and its transcriptional level and DNA binding activity are regulated by cellular redox.31 Meanwhile, intracellular redox status is maintained by redox-regulator molecules such as Trx or APE/Ref-1 (Ref-1). We therefore examined whether SCF modulates Trx or Ref-1 expression in erythroblast. As expected, immunoblot analysis showed that SCF stimulation of day 6 cells slightly elevated the expression level of Trx protein at 30 minutes, and expression was sustained for 24 hours (Figure 4B). Interestingly, strong induction of Ref-1 by SCF appeared at 30 to 60 minutes, and this effect disappeared by 3 hours (Figure 4B). This expression pattern differed from that of Trx, but it correlated with the NF-YA protein expression pattern that occurred in response to SCF. To determine whether these transcription factors accumulated in the nucleus in response to SCF, we investigated the subcellular localization of Trx and Ref-1 after SCF stimulation. Immunofluorescence microscopy analysis showed a perinuclear cytoplasmic localization of Trx in unstimulated erythroblasts, and no clear translocation to the nucleus was observed after SCF stimulation. However, Ref-1 appeared to be localized both to the nucleus and to a perinuclear cytoplasmic region after SCF stimulation (Figure 4D). These results imply that SCF-induced NF-YA accumulation might occur by modulation of the cellular redox status by Ref-1, but not Trx, and this might activate the DNA binding of NF-YA to the γ-globin promoter.
Expression of NF-YA in response to SCF during erythroid differentiation. (A) Quantitative real-time PCR was performed to determine NF-YA expression levels for the indicated days after CD34+ cells were added with SCF (50 ng/mL) from day 6 to day 14. Results are shown as mean ± SD from 3 different donors. *P < .05 versus unstimulated cells. (B) CD34+ cells grown in EPO-containing medium for 6 days were incubated with SCF (50 ng/mL) for the indicated times, and whole cell lysates (30 μg) were analyzed by immunoblot with thioredoxin (Trx), Ref-1, or NF-YA antibodies. The lowest panel shows the same blot stripped and reprobed with anti–β-actin antibody to confirm that similar amounts of protein extracts were analyzed in each lane. (C) Immunofluorescence analysis of Trx and Ref-1 localization after cells grown in EPO-containing medium for 6 days were stimulated with SCF (50 ng/mL) for the indicated times. Representative immunoblots from 3 independent experiments are shown.
Expression of NF-YA in response to SCF during erythroid differentiation. (A) Quantitative real-time PCR was performed to determine NF-YA expression levels for the indicated days after CD34+ cells were added with SCF (50 ng/mL) from day 6 to day 14. Results are shown as mean ± SD from 3 different donors. *P < .05 versus unstimulated cells. (B) CD34+ cells grown in EPO-containing medium for 6 days were incubated with SCF (50 ng/mL) for the indicated times, and whole cell lysates (30 μg) were analyzed by immunoblot with thioredoxin (Trx), Ref-1, or NF-YA antibodies. The lowest panel shows the same blot stripped and reprobed with anti–β-actin antibody to confirm that similar amounts of protein extracts were analyzed in each lane. (C) Immunofluorescence analysis of Trx and Ref-1 localization after cells grown in EPO-containing medium for 6 days were stimulated with SCF (50 ng/mL) for the indicated times. Representative immunoblots from 3 independent experiments are shown.
Cellular reducing condition enhances the effect of SCF
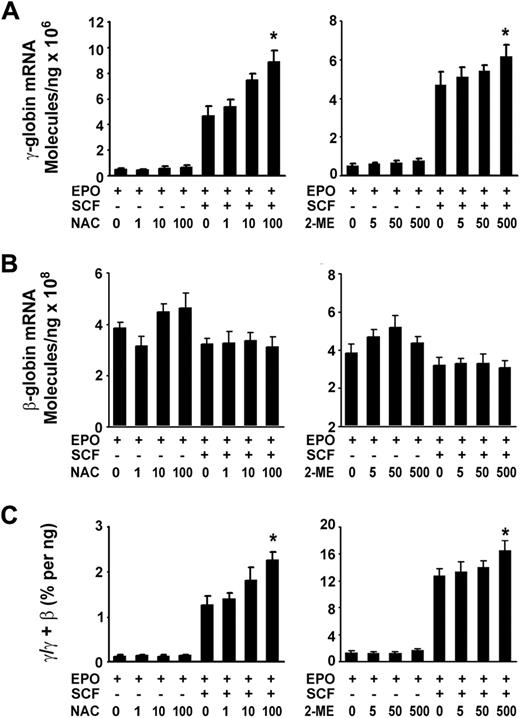
Given that SCF transient induction of the cellular redox proteins in erythroblasts led us to test whether the cellular redox states also mediates the induction of γ-globin. We then examined the expression of γ-globin stimulated by SCF under reducing conditions with the application of NAC, a thiol-protecting antioxidant precursor of glutathione, or 2-ME, a well-known reducing compound. Real-time quantitative PCR results showed that both NAC and 2-ME had enhanced effects on SCF-induced γ-globin expression in a concentration-dependent manner (Figure 5A) and increased the γ/(γ + β) total globin ratio (Figure 5C). However, these reducing components had no significant effects on β-globin mRNA expression compared with those in untreated cells (EPO) or those in treated cells (EPO plus SCF; Figure 5B). These results suggest that a reducing environment favored γ-globin induction in response to SCF. However, the sole addition of NAC or 2-ME to the culture did not significantly increase the expression of γ-globin mRNA and γ/(γ + β) ratio.
Cellular reducing condition enhances the effect of SCF. CD34+ cells cultured with or without SCF (50 ng/mL) in the presence or absence of the indicated concentration of NAC or 2-ME from day 6 to day 14. Total RNA was isolated, and quantitative real-time PCR was performed to analyze the expression of γ-globin mRNA under the reducing conditions (A), the expression of β-globin mRNA under the reducing conditions (B), the ratios of γ/(γ + β) globin percentages under the reducing conditions (C). Results are shown as mean ± SD from 3 independent donors that were analyzed in separate experiments. *P < .05 versus unstimulated cells.
Cellular reducing condition enhances the effect of SCF. CD34+ cells cultured with or without SCF (50 ng/mL) in the presence or absence of the indicated concentration of NAC or 2-ME from day 6 to day 14. Total RNA was isolated, and quantitative real-time PCR was performed to analyze the expression of γ-globin mRNA under the reducing conditions (A), the expression of β-globin mRNA under the reducing conditions (B), the ratios of γ/(γ + β) globin percentages under the reducing conditions (C). Results are shown as mean ± SD from 3 independent donors that were analyzed in separate experiments. *P < .05 versus unstimulated cells.
SCF-induced γ-globin reactivation is mediated by activation of Erk1/2 through down-regulation of COUP-TFII
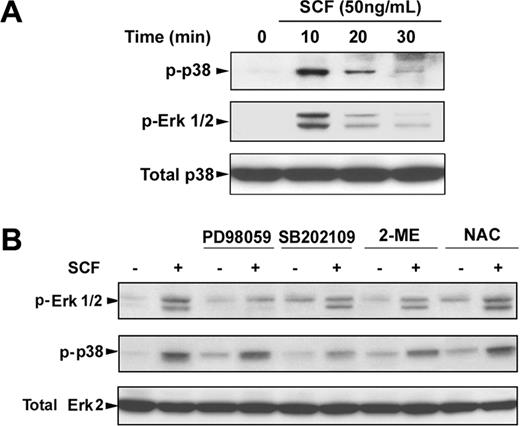
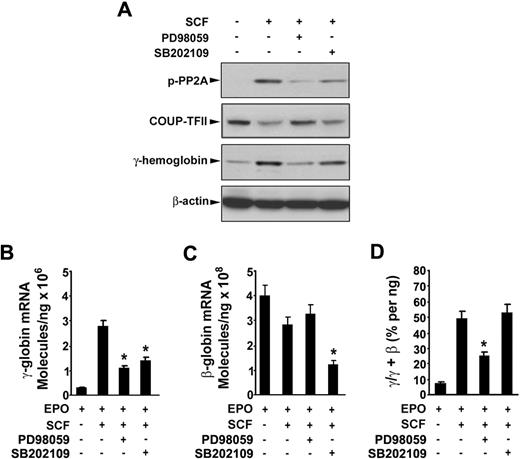
To determine the possible signaling cascade involved in SCF-induced γ-globin gene expression, we first investigated the Erk1/2 or p38 MAPK signaling pathway or both pathways in erythroblasts. Incubation of day 6 cells with SCF rapidly induced phosphorylation of Erk1/2 and p38 within 10 minutes, and the phosphorylation disappeared by 30 minutes (Figure 6A). Pretreatment of day 6 cells with the Erk1/2 inhibitor PD98059 (20 μM) or p38 MAPK inhibitor SB202109 (5 μM) significantly suppressed the phosphorylation of Erk1/2 and p38 stimulated by SCF (Figure 6B). Interestingly, treatment of the cells with SCF in the presence of NAC enhanced the SCF-induced phosphorylation of Erk1/2 and p38, suggesting the NAC with SCF had the synergistic effect on phosphorylation of Erk1/2 and p38, which is consistent with our above results (Figure 5). Furthermore, to determine whether the Erk1/2 or p38 MAPK pathways mediated SCF-suppressed COUP-TFII expression leading to an increase in γ-globin expression, we treated the day 6 cells with SCF in the presence or absence of PD98059 (20 μM) or SB202109 (5 μM) for 8 days (from day 6 to day 14), and proteins were collected on day 14 for analysis of the PP2A phosphorylation, COUP-TFII, and γ-hemoglobin status. Immunoblot analysis showed that inhibitors PD98059 and SB202109 markedly prevented the SCF-induced phosphorylation of PP2A, down-regulated COUP TFII, and increased γ-hemoglobin (Figure 7A), indicating that COUP-TFII lies downstream of Erk1/2 and p38 MAPK. We next examined the effects of inhibitors PD98059 and SB203580 on SCF-induced γ-globin gene expression with quantitative real-time PCR. Treatment of day 6 cells with SCF in the presence or absence of PD98059 or SB203580 from day 6 to day 14 showed that PD98059 significantly inhibited SCF induction of γ-globin mRNA levels on day 14 (Figure 7B; EPO alone, 0.42 ± 0.04 × 106 molecules/ng; SCF, 2.79 ± 0.27 × 106 molecules/ng; after PD98059, 0.98 ± 0.09 × 106 molecules/ng; P < .05), further supporting our above-mentioned results (Figure 7A). In contrast, this inhibitor did not significantly decrease β-globin mRNA levels on day 14 compared with that in unstimulated cells (EPO; Figure 7C). Moreover, PD98059 significantly decreased the γ/(γ + β) total globin ratio from 49.57% (± 3.48%) in cells treated with SCF to 23.04% (± 1.55%; P < .05) in SCF plus PD98059 (Figure 7D). These results are consistent with the previous studies in showing that the Erk1/2 MAPK signaling involves the γ-globin gene and HbF induction.16 However, we found the p38 MAPK inhibitor SB202190 had no significant effect on the γ/(γ + β) total globin ratio, because SB202190 inhibited both γ- and β-globin expression. These results suggest that SCF activation of Erk1/2 induced phosphorylation of PP2A, which led to down-regulation of COUP-TFII, and resulted in an increase in γ-globin expression by inhibition of the repressor effect on γ-globin.
SCF-induced activation of Erk1/2 and p38 MAPK. CD34+ cells were cultured in EPO-containing medium for 6 days before the addition of SCF. (A) Cells were incubated with SCF (50 ng/mL) for the indicated times, and whole cell lysates (30 μg) were analyzed by immunoblot for phosphorylation of Erk1/2 and p38. The lowest panel shows the same blot stripped and reprobed with total p38 antibody to confirm that similar amounts of protein extracts were analyzed in each lane. (B) Cells were stimulated with SCF (50 ng/mL) for 10 minutes with and without preincubation with PD98059 (20 μM), SB202109 (5 μM), 2-ME (500 μM), or NAC (100 μM) for 30 minutes, and whole cell lysates were analyzed by immunoblot for phosphorylation of Erk1/2 and p38. The lowest panel shows the same blot stripped and reprobed with total Erk2 antibody to confirm that similar amounts of protein extracts were analyzed in each lane. Representative immunoblots from 3 independent experiments are shown.
SCF-induced activation of Erk1/2 and p38 MAPK. CD34+ cells were cultured in EPO-containing medium for 6 days before the addition of SCF. (A) Cells were incubated with SCF (50 ng/mL) for the indicated times, and whole cell lysates (30 μg) were analyzed by immunoblot for phosphorylation of Erk1/2 and p38. The lowest panel shows the same blot stripped and reprobed with total p38 antibody to confirm that similar amounts of protein extracts were analyzed in each lane. (B) Cells were stimulated with SCF (50 ng/mL) for 10 minutes with and without preincubation with PD98059 (20 μM), SB202109 (5 μM), 2-ME (500 μM), or NAC (100 μM) for 30 minutes, and whole cell lysates were analyzed by immunoblot for phosphorylation of Erk1/2 and p38. The lowest panel shows the same blot stripped and reprobed with total Erk2 antibody to confirm that similar amounts of protein extracts were analyzed in each lane. Representative immunoblots from 3 independent experiments are shown.
SCF-induced γ-globin reactivation is mediated by activation of Erk1/2 through down-regulation of COUP-TFII. CD34+ cells were cultured with or without SCF (50 ng/mL) in the presence or absence of the indicated inhibitors from day 6 to day 14. On day 14, whole cell lysates (30 μg) were analyzed by immunoblot for phosphorylation of PP2A, COUP-TFII, γ-hemoglobin, and β-actin (A), total RNA was analyzed by quantitative real-time PCR to determine the expression of γ-globin mRNA level (B), the expression of β-globin mRNA level (C), the ratios of γ/(γ + β) globin percentages (D). Results are shown as mean ± SD from 3 independent donors that were analyzed in separate experiments. *P < .05 versus SCF-stimulated cells.
SCF-induced γ-globin reactivation is mediated by activation of Erk1/2 through down-regulation of COUP-TFII. CD34+ cells were cultured with or without SCF (50 ng/mL) in the presence or absence of the indicated inhibitors from day 6 to day 14. On day 14, whole cell lysates (30 μg) were analyzed by immunoblot for phosphorylation of PP2A, COUP-TFII, γ-hemoglobin, and β-actin (A), total RNA was analyzed by quantitative real-time PCR to determine the expression of γ-globin mRNA level (B), the expression of β-globin mRNA level (C), the ratios of γ/(γ + β) globin percentages (D). Results are shown as mean ± SD from 3 independent donors that were analyzed in separate experiments. *P < .05 versus SCF-stimulated cells.
Discussion
This study provides for the first time detailed molecular evidence for the downstream transcriptional factor COUP-TFII that is involved in SCF-induced increase in γ-globin gene expression during erythroid differentiation. The data show what we think is a novel role for SCF to promote replacement of the COUP-TFII from the γ-globin promoter by suppression of the COUP-TFII expression, followed by a simultaneous and proportional recruitment of the basal transcription machinery RNA polymerase II to the γ-globin promoter, resulting in γ-globin gene reactivation. The results of this study provide several new insights into the mechanisms that contribute to SCF-induced γ-globin reactivation that might act in part through effects on downstream transcriptional elements. In the present work, we show that the expression of the COUP-TFII is up-regulated in terminal erythroid differentiation. When SCF was added to the culture medium, the expression of the COUP-TFII was reduced compared with its expression in unstimulated cells, and it was inversely correlated with γ-globin induction during terminal erythroid differentiation (Figure 1). Furthermore, we found that decreased expression of COUP-TFII was associated with phosphorylation of PP2A (Figure 2B), and this decrease was mediated by Erk1/2 MAPK signaling (Figure 7A). Indeed, previous studies have shown that the inhibition of protein phosphatase PP2A prevented sonic hedgehog signaling–induced COUP-TFII expression.25 Moreover, the phosphatase activity of PP2A is functionally inactivated by FoxO transcription factors.32 Recent studies have shown that inhibition of FoxO1 reduces the expression of COUP-TFII.33 Whether SCF suppression of COUP-TFII is through inhibition of FoxO transcript factors will be required for further study. Although it has been reported that PP2A activity also can be inhibited by inhibition of its B56 subunit by the immediate early response gene X-1 protein (IEX-1),34,35 we could not detect induction of IEX-1 in response to SCF in this system (data not shown). Notably, our results have shown that SCF-induced phosphorylation of PP2A correlated well with SCF-induced suppression of COUP-TFII, resulting in an increase of fetal hemoglobin in erythroblasts (Figure 2B), further supporting our hypothesis. To examine the local effects of SCF on γ-globin gene promoters, we investigated the occupancy of transcription factor COUP-TFII at the human γ-globin promoter in SCF-stimulated CD34+ cells. In agreement with our hypothesis, ChIP assays showed that COUP-TFII was displaced from the γ-globin promoter on SCF stimulation, and RNA polymerase II was recruited to the promoter by SCF stimulation (Figure 3A). Importantly, knockdown of COUP-TFII by siRNA is sufficient to significantly increase γ-globin mRNA and the ratio of γ/(γ + β) globin (Figure 3C). Although (partial) silencing of COUP-TFII results in comparatively less induction of γ-globin than occurred in SCF stimulation, our data clearly show a correlation between the decrease in COUP-TFII protein expression and reactivation of γ-globin expression during erythroid maturation (Figure 2B). Taken together, our finding suggests that COUP-TFII is a potent repressor of γ-globin and, at least in part, is involved in γ-globin reactivation.
It has been reported that NF-Y specifically binds to the γ-globin CCAAT box, and this binding facilitates the recruitment of the basal transcription machinery and communication between the γ-globin promoter and the locus control region complex.36 Our finding that NF-YA expression was increased in SCF-stimulated erythroblasts (Figure 4A) was consistent with a recent study that showed that NF-YA is highly expressed in immature c-kit+ bone marrow CD34+ cells, but its levels decline rapidly with hematopoietic differentiation.37 Furthermore, to determine whether the cellular redox state was modulated by SCF, we studied the effect of SCF on the regulation of Trx and Ref-1 in erythroblasts, given that NF-Y subunit DNA-binding ability is regulated by the cellular redox state.31 We found that SCF increased Trx and Ref-1 protein expression levels in erythroblasts, but only the Ref-1 expression pattern correlated with that of the SCF-induced NF-YA protein levels (Figure 4B). Moreover, SCF-induced Trx did not appear to translocate into the nucleus (Figure 4C), and Trx treatment did not induce γ-globin expression (data not shown). However, SCF-induced Ref-1 appeared to be localized in both the cytoplasm and the nucleus (Figure 4C), in agreement with recent studies that showed that hemin induced Ref-1 translocation to the nucleus during K562 cell differentiation.38 Thus, we propose that SCF-induced Ref-1 plays a role in SCF-induced γ-globin expression through enhancing the redox-sensitive transcription factors and other regulators binding to the γ-globin promoter. Moreover, our studies with reducing agents NAC (glutathione donor) and 2-ME showed that these 2 molecules enhanced SCF-induced γ-globin reactivation. These data indicate that cellular redox status is regulated through different antioxidant systems, and all these redox regulators might provide the environment that favors transcriptional regulators binding to the γ-globin promoter region, resulting in reactivation of its expression.
A major challenge in studying γ-globin reactivation is integrating transcriptional effectors into the existing signaling and transcriptional cascades. This work for the first time describes a direct connection between upstream signaling events and effects on the transcriptional cascade in the modulation of γ-globin expression. SCF stimulation results in reorganization of proteins at the γ-globin gene promoter, with displacement of the repressor COUP-TFII and recruitment of RNA polymerase II. It is not yet clear whether additional factors are recruited to the promoter to permit gene activation after these events. Our results clearly establish that SCF-induced phosphorylation of Erk1/2 plays a critical event for γ-globin reactivation by down-regulation of COUP-TFII. These findings will help to unravel the full complexity of γ-globin reactivation and will provide a new molecular target for the development of effective pharmacologic strategies for treatment of patients with sickle cell disease or other β-hemoglobinopathies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
We thank Dr Zhigang Gao from the Molecular and Clinical Hematology Branch/National Institute of Diabetes and Digestive and Kidney Diseases, NIH, for his skillful help with flow cytometry analyses.
Authorship
Contribution: G.P.R. and W.A. designed the research; W.A., J.Z., and C.K. participated in performing the research; K.C. contributed vital new reagents; W.A. and Z.J. analyzed data; and W.A. and G.P.R. wrote the paper. All authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Griffin P. Rodgers, Molecular and Clinical Hematology Branch, Bldg 10, Rm 9N-113A, NIH, Bethesda, MD 20892-2560; e-mail: gr5n@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal