Abstract
In a clinical trial for adeno-associated virus serotype 1 (AAV-1)–mediated gene transfer to muscle for lipoprotein lipase (LPL) deficiency, 1 subject from the high-dose cohort experienced a transient increase in the muscle enzyme creatine phosphokinase (CPK) 4 weeks after gene transfer. Simultaneously, after an initial downward trend consistent with expression of LPL, plasma triglyceride levels returned to baseline. We characterized B- and T-cell responses to the vector and the transgene product in the subjects enrolled in this study. IFN-γ enzyme-linked immunosorbent spot (ELISpot) and intracellular cytokine staining assays performed on peripheral blood mononuclear cells (PBMCs) from the subject who experienced the CPK elevation showed the activation of capsid-specific CD4+ and CD8+ T cells. Four of 8 subjects had detectable T-cell responses to capsid with dose-dependent kinetics of appearance. Subjects with detectable T-cell responses to capsid also had higher anti–AAV-1 IgG3 antibody titer. No subject developed B- or T-cell responses to the LPL transgene product. These findings suggest that T-cell responses directed to the AAV-1 capsid are dose-dependent. Whether they also limit the duration of expression of the transgene at higher doses is unclear, and will require additional analyses at later time points.
Introduction
Adeno-associated virus (AAV) vector–mediated gene transfer has been successfully demonstrated in small and large animal models,1-3 and translation of animal results into clinical studies is currently the major goal of the field. In a phase 1 study of AAV-2–mediated gene transfer to liver in hemophilia B subjects, therapeutic levels of factor IX (FIX, > 10% normal) were achieved, but eventually fell to baseline (< 1%), accompanied by a transient and asymptomatic rise in liver enzymes that occurred simultaneously with expansion of a population of circulating AAV capsid–specific CD8+ T cells.4,5 We hypothesized that this set of findings, observed in human subjects but not in animal models,2,6 arose from reactivation by vector infusion of a population of capsid-specific memory CD8+ T cells generated originally in response to an infection by wild type AAV-2. The implications of these studies for AAV-mediated gene transfer have been unclear, because comprehensive prospective studies of the immune response to capsid in AAV vector–injected human subjects are lacking.
In the current study, we characterized the immune response to both vector capsid and transgene product in a group of adult subjects undergoing AAV-1–mediated gene transfer to skeletal muscle for lipoprotein lipase (LPL) deficiency. Building on proof-of-concept studies in animal models,7,8 Stroes et al conducted an open-label dose escalation study in which an AAV-1 vector expressing a naturally occurring variant of the LPL transgene (LPLS447X, a truncated version of the LPL protein associated with improved lipid profile, carried by 20% of the general population7 ) was introduced by direct intramuscular injection into the lower extremities in subjects with LPL deficiency.9
Subjects were enrolled into 2 dose cohorts (n = 4 each), receiving either 1011 genome copies (gc)/kg or 3 × 1011 gc/kg; vector was administered by direct intramuscular injection as previously described.10 Vector administration was shown to be safe and well tolerated at all doses. Median plasma triglyceride (TG) initially decreased in all subjects, with 40% reduction in median TG levels in 3 subjects, and detection of LPL transgene in biopsies from injected muscle of 2 subjects from the high-dose cohort. However, long-term follow up of triglycerides showed loss of efficacy in both dose cohorts after 18 to 31 months.9
In this study, we show that (1) none of the subjects demonstrated T-cell or B-cell responses to the LPL transgene product; (2) 4 of 8 injected subjects showed a T-cell response to AAV-1 capsid after vector injection, with kinetics that are dose-dependent; (3) 1 of 8 subjects showed a rise in the muscle enzyme CPK (beginning 4 weeks after vector injection) coinciding with an apparent loss of transgene expression, suggestive of T cell–mediated destruction of transduced muscle cells; (4) 4 of 8 subjects, those with documented T-cell responses to capsid, showed a rapid rise in anti–capsid IgG3 after vector injection, whereas the other 4 showed a slower, more modest rise. These results are consistent with previous findings of T-cell responses to capsid in human subjects undergoing hepatic gene transfer with an AAV vector,4,5 and extend the observations to a serotype other than AAV-2, with low affinity for heparin11 and another route of administration. Whether these T-cell responses to capsid limit long-term transgene expression in some or all cases requires further investigation. If so, a general solution to overcoming host immune responses to gene therapy vectors may be needed to reach the goal of long-term transgene expression in muscle or liver in human subjects.
Methods
Subjects
LPL-deficient subjects with missense mutations in both LPL alleles were enrolled in the clinical trial; 8 subjects were enrolled in 2 dose cohorts (4 subjects per cohort) receiving 1011 gc/kg and 3 × 1011 gc/kg. Vector was administered intramuscularly into multiple sites at a dose of 1.6 to 4.2 × 1011 gc/site of injection. The study was performed at the Academic Medical Center, Amsterdam.9 High-resolution HLA typing of all subjects was performed at the Department of Pathology and Laboratory Medicine, University of Pennsylvania Medical Center using the polymerase chain reaction sequence–specific primer kit Olerup by GenoVision and LifeMATCH kit (Tepnel Lifecodes) on a Luminex 100 detection system (Luminex Corp). Peripheral blood mononuclear cell (PBMC) isolation was performed by standard gradient techniques12 at the Academic Medical Center, Amsterdam; frozen PBMCs were sent to the Children's Hospital of Philadelphia on dry ice. Experiments involving specimen collection or testing were approved, respectively, by the Institutional Review Board and the local Ethical Committees at the Children's Hospital of Philadelphia and the Academic Medical Center, and informed consent was obtained in accordance with the Declaration of Helsinki.
Muscle biopsies analysis
Muscle biopsies were collected from subjects A, B, C, E, F, G, and H between 10 and 36 weeks after gene transfer. Genomic DNA was isolated from injected muscle tissue using the Gentra Puregene genomic DNA purification tissue kit (QIAGEN). One microgram of DNA was analyzed for AAV1-LPLS447X vector DNA sequence by quantitative polymerase chain reaction using primers and probe located in the boundary between the LPL cDNA and the WPRE element.
IFN-γ ELISpot
A library of 146 15-mers overlapping in sequence by 10 amino acids was synthesized (Mimotope) based on the AAV-1 VP1 amino acid sequence. Peptides were arranged in a matrix of 24 pools, 12 to 14 peptides per pool. Recombinant human LPL protein resuspended in glycerol was used at 1.7 μg/mL, final concentration. Cytomegalovirus, Epstein-Barr virus, and influenza A–derived (CEF13 ) peptide pool (Mabtech) and a mix of phorbol 12-myristate 13-acetate (PMA) and ionomycin (Sigma-Aldrich) served as positive controls. In an IFN-γ enzyme-linked immunosorbent spot (ELISpot) assay, PMA/ionomycin control usually gives more than 1000 spot-forming units (SFU)/million cells, unless cell viability is below acceptable levels.
A positive response to an antigen or control was defined by several spots per million PBMCs of at least 50 and at least 3 times the number of spots measured for the medium only control.
T-cell responses were measured using one-color ELISpot assay for IFN-γ (Mabtech) as previously described4,5 ; each antigen and control were tested in triplicate.
Depletion of CD4+ or CD8+ T cells was performed by magnetic bead-conjugated antibodies according to the manufacturer's protocol (Dynabeads; Invitrogen-Dynal). The fraction of PBMCs containing the untouched CD4− or CD8− cells was used in an IFN-γ ELISpot assay.
Perforin ELISpot
Perforin ELISpot was performed as previously described.14 Briefly, anti–human perforin-precoated plates (Mabtech) were blocked for 1 hour at room temperature with AIM-V (Invitrogen-Gibco) containing 10% heat-inactivated FBS. Cells were thawed, washed twice, and counted. PBMCs were plated at 2.5 × 105 cells/well in triplicate; test conditions included medium only, a pool of irrelevant peptides (human retinal pigment epithelial 65 protein; Mimotopes) at a final concentration of 5 μg/mL per peptide, AAV-1 capsid particles at a final concentration of 40 μg/mL, and CEF (Mabtech) at a final dilution of 1:100, according to the manufacturer's instructions.
PBMCs were incubated for 24 hours at 37°C, 5% CO2; afterward incubation plates were washed 5 times with PBS and a biotin-conjugated anti–human perforin antibody (Mabtech) was added to wells. Spot detection was performed using streptavidin-conjugated alkaline phosphatase (Mabtech) and BCIP/NBT substrate (KPL).
Intracellular staining for IFN-γ and polyfunctional analysis of T-cell responses
Intracellular cytokine staining for IFN-γ was performed as previously described15 ; PBMCs were incubated at 37°C with 10 μg/mL peptide, or 50 ng/mL PMA, 1 μg/mL ionomycin (Sigma-Aldrich), or medium alone for 5 hours before surface and intracellular staining.
Polyfunctional analysis of T cells was performed as previously described.16,17 PBMCs were rested overnight at 2 × 106 cells/mL; the following morning, cells were resuspended at 106 cells/mL with 1 μg/mL αCD28 and αCD29d antibodies and 10 μg/mL CD107a FITC. Fifteen-mer overlapping peptides covering the AAV capsid sequence were added to 1 mL cells at a concentration of 2 μg/mL each. Cells were then incubated for 1 hour at 37°C and 5% CO2 before adding 1 μL per tube each of brefeldin A and Monensin (BD Biosciences). Cells were incubated for an additional 5 hours before washing once in PBS. An Invitrogen amine reactive live/dead aqua dye was added to cells followed by surface stain with CD3 Qdot 585, CD8 Texas Red PE, CD4 PeCy5.5, CD27 PeCy5, CD57 Qdot 565, CD45RO Qdot 705, CD14 PacBlue, CD16 PacBlue, and CD19 PacBlue. Cells were then stained intracellularly with IL-2 APC, IFN-γ Alexa 700, perforin PE, and TNF-α PeCy7 for 1 hour at room temperature. All antibodies were purchased from BD Biosciences; Quantum Dots were obtained from Invitrogen. Cells were run on a modified LSR II A (BD Biosciences) and analyzed using FlowJo 8.8 (TreeStar).
Anti-AAV capsid antibody assays
Anti–AAV-1 capsid Ig subclasses were measured with a capture assay; enzyme-linked immunosorbent assay plates were coated overnight at 4°C with 5 × 1010 capsid particles/mL AAV-1. Plates were blocked with 2% BSA, 0.05% Tween 20 in PBS for 2 hours at room temperature; serial dilutions of samples in blocking buffer were loaded and incubated overnight at 4°C. Biotin-conjugated anti–human IgG1 (BD Biosciences), IgG2 (Sigma-Aldrich), IgG3 (Sigma-Aldrich), IgG4 (Sigma-Aldrich), or IgM (Sigma-Aldrich) was used as detecting antibodies at a dilution of 1:4000. Immunoglobulin concentration was determined against standard curves made with serial dilution of human purified IgG1 (Sigma-Aldrich), IgG2 (Sigma-Aldrich), IgG3 (Sigma-Aldrich), IgG4 (Millipore-Chemicon), or IgM (Sigma-Aldrich). Anti–AAV-1 neutralizing antibody titer was determined as previously described.18
Bioinformatics and statistical analysis
Results
Transient elevation of CPK following vector administration
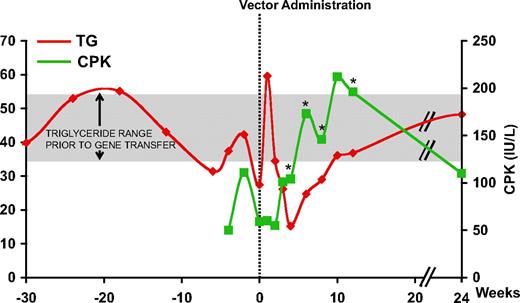
Intramuscular administration of the AAV-1 vector encoding LPL (AAV-1-LPLS447X) proceeded uneventfully in all subjects.9 However, in the first subject, subject E, receiving a dose of 3 × 1011 gc/kg, an increase in CPK was observed beginning around week 4 and lasting for several weeks, returning to baseline by week 24 (Figure 1). Peak levels of CPK were twice that of baseline and above the upper limit of normal (190 IU/L). In this subject, plasma TG decreased in the first 4 weeks following AAV-1- LPLS447X gene transfer, the expected result with successful LPL expression. However the trend reversed beginning at week 6, with kinetics similar to that of CPK levels (Figure 1), suggesting a relationship between the 2 phenomena. No CPK elevation was observed in any of the other subjects enrolled in the study with the exception of a transient elevation, still within the normal range, around week 4 observed in subject H (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Plasma TG and serum CPK levels in subject E. Vertical dotted line represents time of vector administration; TG levels are expressed in millimoles per liter (mM) and CPK in international units per liter (IU/L). *Positive capsid IFN-γ ELISpot. CPK: ULN 190 IU/L; TG: normal levels less than 2 mM, LPL study inclusion criteria more than 10 mM. Mean (± SD) TG prior to gene transfer (week −42 to 0) for subject E was 42.7 mM (± 9.3 mM) (shaded area).
Plasma TG and serum CPK levels in subject E. Vertical dotted line represents time of vector administration; TG levels are expressed in millimoles per liter (mM) and CPK in international units per liter (IU/L). *Positive capsid IFN-γ ELISpot. CPK: ULN 190 IU/L; TG: normal levels less than 2 mM, LPL study inclusion criteria more than 10 mM. Mean (± SD) TG prior to gene transfer (week −42 to 0) for subject E was 42.7 mM (± 9.3 mM) (shaded area).
Activation of capsid T-cell responses demonstrates kinetics that overlap with the rise and fall of serum CPK levels
PBMCs from subject E were isolated at baseline and after gene transfer. An IFN-γ ELISpot specific for the AAV-1 capsid and the LPL transgene product did not show any reactivity at baseline, however beginning at week 4 and up to 12 weeks after gene transfer T cells reacting to AAV-1 peptide pools became detectable, peaking around week 6 at approximately 300 SFU/106 PBMCs (Figure 2). No B- or T-cell responses to the LPL transgene product were detectable (data not shown, and9 ). T-cell responses returned to negative by week 24 (Figure 2). Of note is the similarity in kinetics between the detectable T-cell response to AAV-1 capsid and the elevation in serum CPK (Figure 1).
Capsid-specific IFN-γ ELISpot on PBMCs from subject E. (A) Capsid-specific IFN-γ ELISpot; results are expressed in SFU/106 PBMCs (average ± SD). Error bars represent SD. P 1 to P 24, AAV peptide matrix pools. Positive pools are indicated (positive defined as at least 3-fold above the medium control and at least 50 SFU/106 PBMCs). Horizontal lines represent the cutoff for positivity. PMA indicates positive control (> 1000 SFU/106 PBMCs for all time points); M, medium only. (B) Matrix analysis of results. Peptides identified by positive pools at any time point are indicated in black boxes. Note that peptides 145 and 146 were included in the matrix pools 12 and 13.
Capsid-specific IFN-γ ELISpot on PBMCs from subject E. (A) Capsid-specific IFN-γ ELISpot; results are expressed in SFU/106 PBMCs (average ± SD). Error bars represent SD. P 1 to P 24, AAV peptide matrix pools. Positive pools are indicated (positive defined as at least 3-fold above the medium control and at least 50 SFU/106 PBMCs). Horizontal lines represent the cutoff for positivity. PMA indicates positive control (> 1000 SFU/106 PBMCs for all time points); M, medium only. (B) Matrix analysis of results. Peptides identified by positive pools at any time point are indicated in black boxes. Note that peptides 145 and 146 were included in the matrix pools 12 and 13.
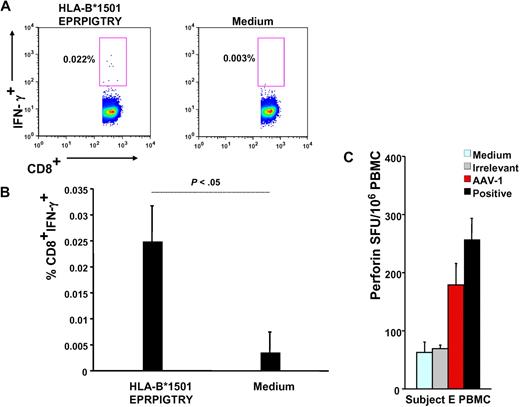
Subject E's HLA haplotype was obtained (supplemental Table 1) and used in a bioinformatics program to identify HLA class I and II epitopes within the AAV-1 capsid VP-1 protein sequence predicted to bind to the subject's alleles (Table 1); this analysis led to identification of a 9-mer contained in peptide 145 (EPRPIGTRY), identified by IFN-γ ELISpot (Figure 2B), binding to the HLA-B*1501 allele carried by this subject; the 9-mer peptide was used in an IFN-γ intracellular cytokine staining assay with PBMCs isolated 6 weeks after gene transfer, confirming the presence of CD8+ T cells reactive to this AAV-derived epitope (Figure 3A-B).
AAV-1 peptide epitopes identified
| Peptides identified by IFN-γ ELISpot . | Amino acid position . | Peptide library pools . | Peptide library no. . | HLA binding prediction . | MHC restriction . |
|---|---|---|---|---|---|
| Subject A | |||||
| SSFYCLEYFPSQMLRTGNNF | 390 | 7-19/20 | 79/80 | N/D | II† |
| Subject D | |||||
| TTSTRTWALPTYNNH | 247 | 5-13 | 49 | HLA-DR*0801 | II† |
| TTSTRTWALPTYNNH | 243 | 5-13 | 49 | HLA-DR*1302 | II† |
| Subject E | |||||
| TTSTRTWALPTYNNH | 240 | 5-13 | 49 | N/D | II† |
| YTEPRPIGTRYLTRP | 724 | 12-13 | 145 | HLA-B*1501 | I‡ |
| EFSATKFASFITQYS | 665 | 12-13 | 133 | HLA-DR*1501 | II |
| Subject H | |||||
| VQVFSDSEYQLPYV | 341 | 6-21 | 69 | HLA-A*3301 | I |
| Peptides identified by IFN-γ ELISpot . | Amino acid position . | Peptide library pools . | Peptide library no. . | HLA binding prediction . | MHC restriction . |
|---|---|---|---|---|---|
| Subject A | |||||
| SSFYCLEYFPSQMLRTGNNF | 390 | 7-19/20 | 79/80 | N/D | II† |
| Subject D | |||||
| TTSTRTWALPTYNNH | 247 | 5-13 | 49 | HLA-DR*0801 | II† |
| TTSTRTWALPTYNNH | 243 | 5-13 | 49 | HLA-DR*1302 | II† |
| Subject E | |||||
| TTSTRTWALPTYNNH | 240 | 5-13 | 49 | N/D | II† |
| YTEPRPIGTRYLTRP | 724 | 12-13 | 145 | HLA-B*1501 | I‡ |
| EFSATKFASFITQYS | 665 | 12-13 | 133 | HLA-DR*1501 | II |
| Subject H | |||||
| VQVFSDSEYQLPYV | 341 | 6-21 | 69 | HLA-A*3301 | I |
Bold, underlined amino acid sequences represent the epitopes predicted using online MHC-binding prediction programs.19,20 Amino acid position indicates the position of the first amino acid of the epitope in the AAV-1 VP1 capsid protein sequence. MHC restriction indicates whether CD8+ (class I) or CD4+ (class II) T cells recognize the epitopes identified.
N/D indicates not determined.
Confirmed by IFN-γ ELISpot assay.
Confirmed by IFN-γ intracellular staining.
Functional characterization of capsid-specific T cells. (A) Intracellular IFN-γ staining on subject E's week-6 PBMCs; numbers indicate the percentage of CD4−CD8+ T cells that are IFN-γ+. Cells are gated on forward and side scatter, on singlets, and on CD4−CD8+ T cells. (B) Representation of 3 independent intracellular IFN-γ staining experiments (average ± SD of % CD4−CD8+IFN-γ+ T cells). (C) Perforin ELISpot on subject E's PBMCs. Medium indicates medium only control; irrelevant, human retinal pigment epithelium 65 protein; AAV-1, AAV-1 capsids; and positive, CEF peptide pool. Data are expressed in SFU per 106 PBMCs. SFU per 106 PBMCs were compared between AAV-1 and medium control (P = .008), AAV-1, and irrelevant control (P = .007), and AAV-1 and positive controls (P > .05, not significant).
Functional characterization of capsid-specific T cells. (A) Intracellular IFN-γ staining on subject E's week-6 PBMCs; numbers indicate the percentage of CD4−CD8+ T cells that are IFN-γ+. Cells are gated on forward and side scatter, on singlets, and on CD4−CD8+ T cells. (B) Representation of 3 independent intracellular IFN-γ staining experiments (average ± SD of % CD4−CD8+IFN-γ+ T cells). (C) Perforin ELISpot on subject E's PBMCs. Medium indicates medium only control; irrelevant, human retinal pigment epithelium 65 protein; AAV-1, AAV-1 capsids; and positive, CEF peptide pool. Data are expressed in SFU per 106 PBMCs. SFU per 106 PBMCs were compared between AAV-1 and medium control (P = .008), AAV-1, and irrelevant control (P = .007), and AAV-1 and positive controls (P > .05, not significant).
In addition, a perforin ELISpot14 on subject E's PBMCs was used to confirm the cytolytic potential of CD8+ T cells upon encounter of AAV capsid antigen but not when exposed to an irrelevant antigen or medium-only controls (Figure 3C). The magnitude of response to AAV measured by perforin ELISpot was comparable with the response directed against the CEF positive control, a pool of MHC class I epitopes derived from cytomegalovirus, Epstein-Barr virus, and flu antigens.
Administration of AAV-1-LPLS447X results in capsid-specific T-cell activation in 4 of 8 subjects with dose-dependent kinetics
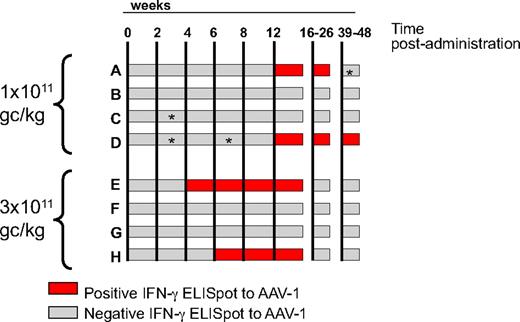
Two of 4 subjects from the low-dose cohort (1011 gc/kg) and 2 of 4 from the high-dose cohort (3 × 1011 gc/kg) had detectable T-cell responses to capsid on an IFN-γ ELISpot screening (Figure 4 and supplemental Figure 2). Although the magnitude of response at week 12 was similar among subjects (with the exception of subject H, who had a weaker response; supplemental Figure 3), the kinetics of appearance of these responses differed between the 2 dose cohorts (Figure 4); subjects A and D, who received a low vector dose, had detectable T-cell responses to AAV capsid beginning at week 12, whereas in subjects E and H the delay between vector administration and detection of a response by ELISpot was only 4 and 6 weeks, respectively (Figure 4). Duration of detection of T-cell responses also differed between the 2 cohorts of subjects, low-dose cohort subjects having detectable responses up to week 36, and high-dose cohort subjects only up to week 12 (Figure 4). Long-term follow up of capsid T-cell responses by IFN-γ ELISpot (up to 24 months for some of the subjects) confirmed persistence of these responses only in subjects A and D from the low-dose cohort (data not shown).
Time course of T-cell responses to AAV-1 capsid measured by IFN-γ ELISpot. Each horizontal bar represents an individual subject; time after intramuscular administration (in weeks) is indicated by the vertical lines. Gray bars indicate a negative ELISpot result; red bars indicate a positive ELISpot result. A time point was considered positive when the average SFU/106 PBMCs was higher than 3 × the medium control and at least 50 SFU/106 PBMCs for both the AAV-1 peptide pools and the AAV-1 empty particles antigens. The vector dose, in genome copies per kilogram (gc/kg), received by the subjects in the 2 cohorts is indicated on the left of the graph. *Negative for AAV-1 empty capsids.
Time course of T-cell responses to AAV-1 capsid measured by IFN-γ ELISpot. Each horizontal bar represents an individual subject; time after intramuscular administration (in weeks) is indicated by the vertical lines. Gray bars indicate a negative ELISpot result; red bars indicate a positive ELISpot result. A time point was considered positive when the average SFU/106 PBMCs was higher than 3 × the medium control and at least 50 SFU/106 PBMCs for both the AAV-1 peptide pools and the AAV-1 empty particles antigens. The vector dose, in genome copies per kilogram (gc/kg), received by the subjects in the 2 cohorts is indicated on the left of the graph. *Negative for AAV-1 empty capsids.
In contrast to results of anti–AAV-1 neutralizing antibody assay (supplemental Table 3), no subject had detectable T-cell responses to the AAV-1 capsid before vector administration, confirming that primed capsid-specific T cells, if present prior to vector injection, are not easily detectable in PBMCs.4,5
None of the subjects enrolled in the clinical study had detectable B- or T-cell responses to the LPL transgene9 (and data not shown) before or after vector administration. Vector genome copy number was evaluated on muscle biopsies collected between weeks 10 and 36. Notably, vector genome copy number was lower in subject E compared with subjects F and G from the same dose cohort (Table 2); analysis of the biopsy in subject H showed no detectable levels of vector genomes, indicating either complete clearance of transduced muscle fibers or, alternatively, that the injected area was missed during muscle biopsy.
Vector genome copy number per diploid genome in muscle homogenates from AAV1-LPL–injected subjects
| Subject . | Weeks after vector administration . | Vector genome copy no., gc/diploid genomes . |
|---|---|---|
| A | 36 | 0.37 |
| B | 36 | 0.03 |
| C | 28 | 1.08 |
| D | na | na |
| E | 10 | 1.14 |
| F | 32 | 5.58 |
| G | 26 | 14.4 |
| H | 28 | und |
| Subject . | Weeks after vector administration . | Vector genome copy no., gc/diploid genomes . |
|---|---|---|
| A | 36 | 0.37 |
| B | 36 | 0.03 |
| C | 28 | 1.08 |
| D | na | na |
| E | 10 | 1.14 |
| F | 32 | 5.58 |
| G | 26 | 14.4 |
| H | 28 | und |
na indicates not available, subject withdrew consent; and und, undetectable.
Identification of MHC class I and II epitopes within the AAV-1 capsid sequence
A matrix of 24 pools of 15-mers overlapping by 10 amino acids spanning the entire sequence of AAV-1 VP-1 capsid protein was used to screen PBMCs collected from all subjects enrolled in the clinical study (Figure 2B). For each subject, ELISpot results (supplemental Figure 2) were matched with the high-resolution HLA haplotype (supplemental Table 2) to identify class I and II peptide epitope candidates (Table 1). CD4+ and CD8+ depletion, followed by IFN-γ ELISpot assay, was used to confirm the subset of T cells responding to the identified epitopes (Table 1); interestingly, subjects A and D, who were enrolled in the low vector dose cohort and had a delayed detection of capsid T-cell responses in PBMCs, showed predominantly a CD4+ T-cell response (Figure 5) in contrast to subjects E and H, in whom both CD4+ and CD8+ T-cell responses were observed (Figure 3 and Table 1).
CD4+ and CD8+ T-cell depletion experiments. IFN-γ ELISpot on PBMCs, or CD4- or CD8-depleted fraction of PBMCs (CD4−CD8+ or CD4+CD8−, respectively). Samples were collected at week 19 (subject A) and week 12 (subject D). Results are expressed in SFU/106 cells as average (± SD) of 3 replicates. Peptide 79/80 indicates 15-mers previously identified in subject A by screening ELISpot; peptide 49, 15-mer previously identified in subject D by screening ELISpot; medium, negative control; and PMA, positive control (PMA and ionomycin).
CD4+ and CD8+ T-cell depletion experiments. IFN-γ ELISpot on PBMCs, or CD4- or CD8-depleted fraction of PBMCs (CD4−CD8+ or CD4+CD8−, respectively). Samples were collected at week 19 (subject A) and week 12 (subject D). Results are expressed in SFU/106 cells as average (± SD) of 3 replicates. Peptide 79/80 indicates 15-mers previously identified in subject A by screening ELISpot; peptide 49, 15-mer previously identified in subject D by screening ELISpot; medium, negative control; and PMA, positive control (PMA and ionomycin).
AAV capsid administration results in the activation of both CD4+ and CD8+ T cells with production of TNF-α
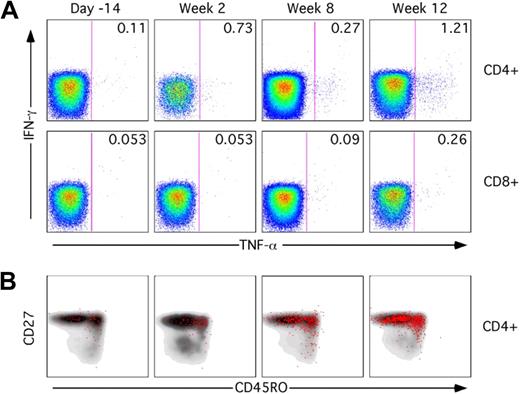
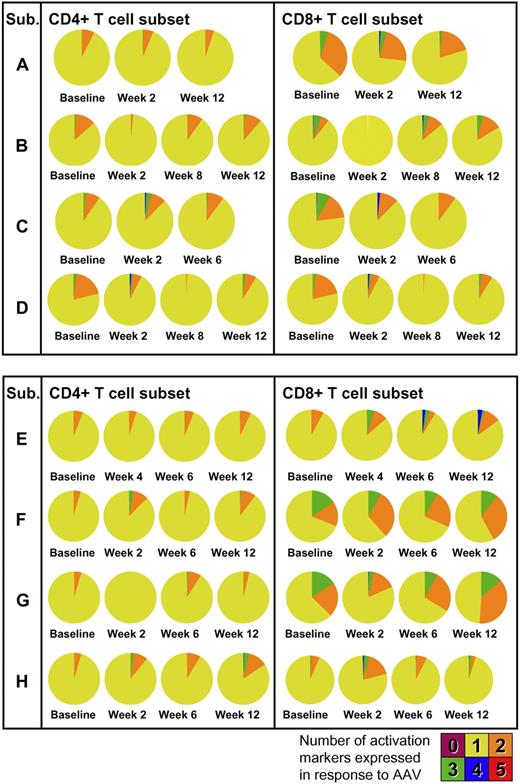
Phenotypic and functional analysis of T-cell responses was performed by multicolor flow cytometry. PBMCs collected at baseline and after gene transfer were restimulated in vitro with peptides derived from the AAV capsid and stained for T-cell activation markers. In all subjects, upon vector administration, activation of CD4+ and, to a lesser extent, CD8+ T cells was observed with production of TNF-α (Figure 6A) in addition to IFN-γ. Phenotypic analysis showed a mix of naive (CD27+CD45RO−) and memory (CD27+CD45RO+) CD4+ T cells reacting to the AAV capsid (Figure 6B). The polyfunctional analysis of T-cell responses to the AAV capsid is summarized in Figure 7; pie charts show the degree of T-cell activation as measured by the number of T-cell activation and functional markers (IL-2, IFN-γ, perforin, TNF-α, and CD107) expressed by CD4+ or CD8+ T cells. Notably, some level of reactivity of CD4+ and CD8+ T cells was measured at baseline before vector administration. This could be accounted for by pre-exposure to wild-type AAV, as documented by baseline neutralizing antibody titer (supplemental Table 2). The highest level of activation (expression of 4/5 activation markers upon restimulation with AAV-1 peptides) was measured for subject E's CD8+ T cells in PBMCs collected at 6 and 12 weeks after gene transfer; details of the polyfunctional analysis of T-cell responses to capsid in subject E are shown in supplemental Figure 3.
T-cell response to AAV capsid proteins. PBMCs were stimulated for 6 hours with a set of 15-mer peptides covering the AAV capsid protein sequence and cytokine responses assessed by polychromatic flow cytometry. (A) TNF-α response to capsid proteins in CD4+ and CD8+ T cells. Numbers in the top right corner indicate the percentage of responding CD4+ or CD8+ T cells. A minimum of 240 000 events were collected for each time point. (B) Immunophenotyping of responding TNF-α+ cells (red dots) compared with total CD4+ T cells (gray density plots). Cells were gated on forward and side scatter, CD3+ cells, CD14−CD16−CD20− cells. Cells were also stained with an Invitrogen amine reactive live/dead aqua dye.
T-cell response to AAV capsid proteins. PBMCs were stimulated for 6 hours with a set of 15-mer peptides covering the AAV capsid protein sequence and cytokine responses assessed by polychromatic flow cytometry. (A) TNF-α response to capsid proteins in CD4+ and CD8+ T cells. Numbers in the top right corner indicate the percentage of responding CD4+ or CD8+ T cells. A minimum of 240 000 events were collected for each time point. (B) Immunophenotyping of responding TNF-α+ cells (red dots) compared with total CD4+ T cells (gray density plots). Cells were gated on forward and side scatter, CD3+ cells, CD14−CD16−CD20− cells. Cells were also stained with an Invitrogen amine reactive live/dead aqua dye.
Polyfunctional analysis of T-cell responses to the AAV-1 capsid. Concurrent expression of IL-2, IFN-γ, perforin, TNF-α, and CD107 is measured at baseline and after gene transfer. Pie charts represent the proportion of capsid-specific CD4+ (left) or CD8+ (right) T cells expressing 1, 2, 3, 4, or 5 markers. Cells were gated on forward and side scatter, CD3+ cells, CD14−CD16−CD20− cells. Cells were also stained with an Invitrogen amine reactive live/dead aqua dye. CD4+ or CD8+ T cells were analyzed for IL-2, IFN-γ, perforin, TNF-α, and CD107 expression in response to AAV-1 peptides against medium control.
Polyfunctional analysis of T-cell responses to the AAV-1 capsid. Concurrent expression of IL-2, IFN-γ, perforin, TNF-α, and CD107 is measured at baseline and after gene transfer. Pie charts represent the proportion of capsid-specific CD4+ (left) or CD8+ (right) T cells expressing 1, 2, 3, 4, or 5 markers. Cells were gated on forward and side scatter, CD3+ cells, CD14−CD16−CD20− cells. Cells were also stained with an Invitrogen amine reactive live/dead aqua dye. CD4+ or CD8+ T cells were analyzed for IL-2, IFN-γ, perforin, TNF-α, and CD107 expression in response to AAV-1 peptides against medium control.
Subjects with a detectable IFN-γ response to AAV capsid develop higher titer of anti–AAV-1 IgG3 antibodies
No clear correlation between the titer of pre-existing neutralizing antibodies to the AAV-1 capsid and the detection of T-cell responses to the capsid after gene transfer was detected (supplemental Figure 2) in agreement with previous findings in the context of a study for AAV-2–mediated hepatic gene transfer in severe hemophilia subjects.4
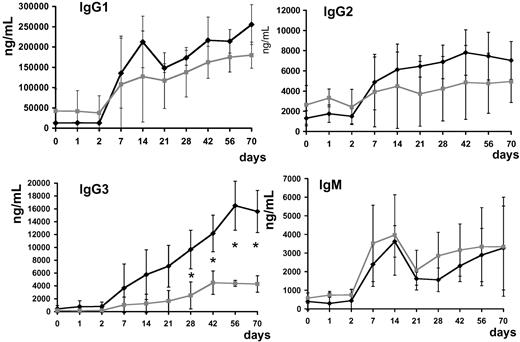
Anti–AAV-1 IgG1, IgG2, IgG3, and IgG4 subclasses and IgM were determined. Antibody titer for IgG subclasses and IgM rose in all subjects upon vector infusion; subjects with positive capsid IFN-γ ELISpot had statistically significantly higher (P < .05 at day 28 after injection) anti–AAV-1 IgG3 titers (Figure 8).
Anti–AAV-1 antibody subclass analysis. Serum levels of IgG1, IgG2, IgG3, IgG4, and IgM (average ± SD, ng/mL). ♦ indicates subjects with positive T-cell response to the AAV capsid measured by ELISpot (subjects A, D-E, and H);  , subjects with no detectable T-cell response to the AAV capsid measured by ELISpot (subjects B-C, F-G); *P < .05.
, subjects with no detectable T-cell response to the AAV capsid measured by ELISpot (subjects B-C, F-G); *P < .05.
Anti–AAV-1 antibody subclass analysis. Serum levels of IgG1, IgG2, IgG3, IgG4, and IgM (average ± SD, ng/mL). ♦ indicates subjects with positive T-cell response to the AAV capsid measured by ELISpot (subjects A, D-E, and H);  , subjects with no detectable T-cell response to the AAV capsid measured by ELISpot (subjects B-C, F-G); *P < .05.
, subjects with no detectable T-cell response to the AAV capsid measured by ELISpot (subjects B-C, F-G); *P < .05.
Discussion
The potential of AAV vectors as a therapeutic tool has been established in several preclinical models of disease.1,21-23 In retinal gene transfer, compelling data in dog models of inherited blindness scaled up uneventfully to human subjects.24,25 However, recent findings in a clinical study for hepatic gene transfer with an AAV-2 vector expressing coagulation FIX in severe hemophilia B subjects highlighted an issue previously unidentified in experimental animals. In this study, 2 subjects developed a transient, self-limited elevation of liver enzymes following gene transfer; concomitantly, a population of capsid-specific CD8+ T cells expanded and contracted with similar kinetics, suggesting immune destruction of transduced hepatocytes.4,5 Our hypothesis is that pre-existing memory T cells to the AAV capsid are responsible for the loss of transgene expression observed in humans but not in experimental animals.5
The present study represents the first complete prospectively analyzed characterization of B- and T-cell responses to the AAV capsid and to the transgene product in a clinical trial of AAV-mediated gene transfer.
Several conclusions can be drawn from this study. First, subject E is of special interest. The constellation of findings in his case, an initial approximately 40% drop of serum triglyceride levels, followed by a return to baseline coincident with a rise in the muscle enzyme CPK between weeks 4 and 12, is suggestive of a process in which transduced muscle fibers (expressing LPL) are destroyed. The fact that these events overlap chronologically with the activation of T-cell responses to AAV capsid (as measured by IFN-γ ELISpot), and that gene copy number in subject E is considerably lower than F and G, the 2 subjects who received the higher dose of vector and did not have detectable capsid T-cell responses, is also consistent with destruction of transduced cells, and moreover suggests that these phenomena may be linked. The fact that residual levels of muscle transduction were still detectable in subject E may be accounted for by the timing of muscle biopsy, which occurred at week 10 after vector injection for subject E. A biopsy at a later time point would be required to determine whether the donated DNA had persisted.
The lack of evidence of T-cell responses to the transgene product is another finding shared by both the AAV-2 intrahepatic gene transfer for hemophilia and the AAV-1 LPL intramuscular gene transfer clinical studies. Based on animal studies,2,26 the absence of a response to the transgene product would be predicted for liver-directed gene transfer, which tends to promote tolerance to the transgene product,27-29 but not necessarily for muscle-directed gene transfer, where immune responses to the transgene product have more commonly occurred.30,31 The absence of immune responses is likely at least partly accounted for by the exclusion from this study of all subjects except those with a missense mutation as the cause of LPL deficiency, that is, the study was limited to subjects with some degree of tolerance to the transgene product.
It is important to note that, at the levels of muscle enzyme elevation reported here, and the levels of liver enzyme elevation reported in the AAV-2-FIX trial, these are safety signals, rather than adverse events, because the changes were short-term and resolved without medical intervention. Tissues such as liver and skeletal muscle are capable of regeneration after damage, an advantage not shared by certain other target tissues of interest including cardiac muscle, where there is limited capacity to regenerate after damage. This highlights the critical nature of a careful analysis of this problem before dose escalation in more vulnerable tissues.
The analysis of the subjects in this trial highlights other findings of interest. First is that administration of higher doses of vector to skeletal muscle results not in a higher readout on the IFN-γ ELISpot, but in a faster generation of a detectable immune response to capsid. This may have important clinical consequences, based on the current understanding of the fate of the capsid after vector transduction of the target cells. If, as has been proposed, transduced cells are characterized by presentation of capsid antigens via MHC class I, then the cells would be vulnerable to destruction by cognate CD8+ T cells for as long as capsid is presented on the cell surface; a recent study32 showing up-regulation of MHC class I expression on muscle fibers after AAV-1 gene transfer supports this hypothesis. However, immune responses that develop slowly and do not become detectable until later time points33 may in fact encounter few or no targets for immune-mediated destruction.
These data also suggest that there may be a dose dependence to development of a CD8+ T-cell response, based on the finding that further analysis of the positive IFN-γ ELISpots in the low-dose subjects indicated that these are driven by CD4+ rather than CD8+ T-cell epitopes. This is in contrast to the data from the higher dose group, where intracellular cytokine staining, perforin ELISpot, and bioinformatics analysis of the epitopes identified are indicative of a mixed CD4+ and CD8+ T-cell–mediated immune response to the AAV capsid.
Not surprisingly, the administration of an AAV-1 vector results in activation of both CD4+ and CD8+ T cells; CD4+ T-cell activation is required for helper functions both in humoral and cytotoxic responses. Furthermore, it is clear that CD4+ T cells play an important role in disease control in viral infections,34 where this population of virus-specific T cells shows predominantly production of IFN-γ and TNF-α35 as we documented here. Successful immune response to a virus is characterized by a complex pattern of activation, and looking only at IFN-γ may underestimate the total antigen-specific CD8+ T-cell frequency.36
Consistent with previous population studies,5,37 PBMCs of all 8 subjects were negative for responses to AAV capsid by IFN-γ ELISpot before vector infusion, independent of pre-exposure to wild-type AAV as indicated by anticapsid neutralizing antibodies measured at baseline. Moreover, even in those who developed a response after vector infusion, the duration of detectable response was quite short in some (eg, subject E), suggesting that (1) screening of PBMCs will not be useful for predicting which subjects are likely to develop a T-cell response to capsid; and (2) capsid-specific CD8+ T cells, if already present, likely reside in compartments other than the peripheral blood. For example, in an earlier study we were able to expand capsid-specific T cells from up to 60% of subjects when lymphocytes isolated from the spleen were used as starting material.5
Interestingly, the average IgG3 titer of subjects who had detectable IFN-γ responses to the AAV capsid was higher than the rest of the subjects. IgG3 together with IgG1 are involved in humoral responses to acute infections with parvovirus B19,38 and are the prevalent IgG subclasses in diseases characterized by IFN-γ response.39 Whether IFN-γ helps to drive an immunoglobulin class switch under these circumstances is unknown. It should be noted also that IgG1 and IgG3 are involved in antibody-dependent cell-mediated cytotoxicity, although it is not clear whether antibody-dependent cell-mediated cytotoxicity played a role in the findings noted here.
It is perhaps critical to underscore the dose dependence of the T-cell response with AAV vectors, with 2 points deserving emphasis. Injection of low doses of vector, such as occurs in early phase gene transfer for genetic disease, or in vaccine studies, is not likely to result in capsid-specific T-cell activation. Thus presence or absence of T-cell activation should be carefully correlated with the doses being injected. Second, the findings have implications for vector design; vectors that gain access to target cells more efficiently may result in increased presentation of antigen to the immune system, and more efficient activation of T cells.
Improvements in the expression cassette itself, which allow greater protein expression per introduced virion, are likely to decrease the vector dose required and thus lessen the risk for activation of circulating capsid-specific T cells. Thus more efficient serotypes may enhance risk, whereas more efficient expression cassettes will reduce it.
At a dose of 1.6 to 4.2 × 1011 gc per site of injection, the AAV-1 vector used in this study resulted in muscle transduction at a level of 0.5 to 7.0 vector gene copies per diploid genome and evidence for a capsid-specific T-cell response in 4 of 8 subjects; differently, in an AAV-2 muscle gene transfer study for hemophilia B, a dose of 1.5 × 1012 gc per site of injection gave 0.5 to 4.0 vector gene copies per diploid genome, no apparent T-cell response to capsid and long-term expression of transgene.10,40 The 3- to 10-fold higher performance of AAV-1 over AAV-2 in transducing muscle, leading to a higher capsid antigen load and thus better antigen presentation, may explain the different immunogenic profiles of the 2 serotypes.
Continuing dose escalation may address the question as to whether responses are detected in greater numbers of subjects at higher doses. Alternatively, previous exposure to wild-type AAV may account for the different outcomes of gene transfer in subjects in this study; the percentage of positive T-cell responses observed here is consistent with the observation that approximately 50% of the population is positive for anti-AAV antibodies.5 Polyfunctional analysis of T-cell responses to capsid in this study revealed some baseline levels of CD4+ and CD8+ activation, which, together with the memory phenotype exhibited by AAV-specific T cells, is consistent with this last hypothesis. It is also important to note that examining circulating PBMCs is only a surrogate for examining T cells in the target tissues, so that the readout here might underestimate numbers of subjects with T-cell infiltrates in injected muscle.
An unanswered question regarding these findings is whether T-cell responses predict clinical outcomes. The relationship of T-cell responses, changes in muscle enzymes, and long-term expression of the donated gene requires further study. Recent studies in mice and humans32,41 showed apoptosis of lymphocytes infiltrating skeletal muscle after AAV-mediated gene transfer, a phenomenon that may play a role in shaping clinical responses to T-cell activation, and may also account for variability of responses among subjects receiving equivalent vector doses.
In summary, we demonstrated that the intramuscular administration of an AAV-1 vector in humans results in T-cell activation in 4 of 8 subjects. The results here extend previous observations made in the context of an AAV-2 administered intravascularly in hemophilia B subjects to another serotype, AAV-1, with low affinity to heparin,11 another target tissue, and another route of administration. Whether these T-cell responses limit long-term expression in every case is not yet clear. Potential solutions such as the use of transient immunomodulation around the time of gene transfer may be required to achieve sustained, persistent expression of the transgene product.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant P01 HL078810 to K.A.H. and the Howard Hughes Medical Institute. N.C.H. was supported by training grant NIH T32 HL007150, and D.J.H. by training grant NIH T32 HL07439. The AAV-lipoprotein lipase clinical study was supported by Amsterdam Molecular Therapeutics.
National Institutes of Health
Authorship
Contribution: F.M. designed and performed the experiments, and drafted the paper; J.J.M. coordinated sample collection, participated in experimental design, and drafted the paper; D.J.H., E.B.-T., N.C.H., and S.A.E. participated in experimental activities; N.A.H. performed multifunctional analysis of T cells; M.R.B. provided critical insights to paper preparation; J.J.K. and E.S.S. performed clinical work; and K.A.H. supervised experimental design and execution, performed data analysis, and drafted the paper.
Conflict-of-interest disclosure: F.M., D.J.H., and K.A.H. hold patents related to AAV gene therapy. The remaining authors declare no competing financial interests.
The current address for Dr Meulenberg is ORCA Therapeutics, Amsterdam, The Netherlands.
Correspondence: Katherine A. High, The Children's Hospital of Philadelphia, ARC Suite 302, 3615 Civic Center Blvd, Philadelphia, PA 19104; e-mail: high@email.chop.edu.