Abstract
Stromal elements present within the tumor microenvironment may suppress host immunity and promote the growth of malignant lymphocytes in B cell–derived non-Hodgkin lymphoma (NHL). In contrast, little is known about the microenvironment's role in T cell–derived NHL. B7-H1 (PD-L1, CD274), a member of the B7 family of costimulatory/coinhibitory ligands expressed by both malignant cells and stromal cells within the tumor microenvironment, has emerged as an important immune modulator capable of suppressing host immunity. Therefore, B7-H1 expression and function were analyzed in cutaneous and peripheral T-cell NHL. B7-H1 was expressed by tumor cells, monocytes, and monocyte-derived cells within the tumor microenvironment in T-cell NHL and was found to inhibit T-cell proliferation and promote the induction of FoxP3+ regulatory T cells. Collectively, the data presented provide the first evidence implicating B7-H1 in the suppression of host immunity in T-cell lymphoproliferative disorders and suggest that the targeting of B7-H1 may represent a novel therapeutic approach.
Introduction
Tumorigenesis is associated with a wide array of both genetic and epigenetic changes that give rise to tumor-associated antigens capable of eliciting a host antitumor immune response. Although host immune surveillance may prevent tumor outgrowth during the earliest stages of tumor growth, locally invasive or metastatic tumors must evade host immunity.1 Immune escape is not merely a passive process of immune evasion but an active one by which both tumor cells and stromal cells present within the tumor microenvironment actively suppress the antitumor immune response. This distinction between immune evasion and suppression is an important one and may explain the paradoxical observation that many tumor immunotherapy clinical trials, despite eliciting an antitumor immune response, are not associated with a meaningful clinical response.2 Improved mechanistic understanding of tumor-associated immune suppression is needed if the next generation of immunotherapeutic strategies is to be rationally designed.
Malignant cells may suppress host immunity directly, by producing immunoregulatory cytokines or expressing inhibitory ligands on their cell surface. In addition, malignant cells may influence the tumor microenvironment leading to the induction or recruitment of immunoregulatory cells capable of suppressing host immunity.3 Both myeloid-derived cells (including tumor-associated macrophages, dendritic cells [DCs], and myeloid-derived suppressor cells) and lymphocyte subsets, most notably regulatory T (Treg) cells, present within the tumor microenvironment, collaborate with their malignant counterparts to suppress host immunity.3,4 The microenvironment's role in promoting tumor growth in non-Hodgkin lymphoma (NHL) was recently highlighted by both gene expression profiling and immunohistochemistry-based approaches.5-7 Therapeutic approaches capable of targeting the tumor microenvironment are currently being translated into clinical practice in hematologic malignancies and may be associated with improved outcomes.8,9 Fundamentally, 2 distinct approaches capable of targeting the tumor microenvironment may be imagined. The first seeks to eliminate immunosuppressive cells present within the tumor microenvironment and is highlighted by recent attempts to eliminate Treg. As different stromal cells may use common immunosuppressive mediators, the alternative approach seeks to identify and neutralize these shared molecular mediators of host immune suppression.
Members of the B7 family have emerged as important mediators of host immune suppression. In contrast to B7-1 (CD80) and B7-2 (CD86), which play an important role in T-cell activation and costimulation, the B7 homologs (B7-H, including B7-H1, B7-H2, B7-H3, and B7-H4), which have been described more recently may function as important “coinhibitors” of host T-cell immunity and have been associated with poor clinical outcomes in a variety of human tumors.10,11 B7-H1, for example, may be inducibly expressed on tumor cells and confer resistance to killing mediated by cytotoxic T lymphocytes (CTLs), induce apoptosis of tumor-specific T cells, and contribute to the induction of T-cell unresponsiveness, including T-cell anergy and exhaustion.11,12 In addition, B7-H1 expressed by myeloid-derived cells and Treg within the tumor microenvironment may further contribute to the suppression of host immunity. For example, B7-H1+ Treg infiltrating B cell–derived NHLs inhibit the proliferation of conventional T cells in a B7-H1–dependent manner.13 In contrast to B cell–derived NHLs, which represent the majority of NHLs in Western nations, T-cell NHLs are derived from mature (ie, postthymic) T cells and are generally, with rare exceptions, associated with a poor prognosis. Therefore, we sought to examine the role of B7-H1 in the suppression of host immunity in T-cell lymphoproliferative disorders.
Methods
Cell lines, proliferation, and cytotoxicity assays
The T-cell NHL cell line Karpas 299 (ATCC) was grown in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum and maintained at 37°C in 5% CO2. To generate allogeneic CD4+ or CD8+ T-cell lines, 4 × 106 purified CD4+ or CD8+ T cells were cocultured with 5 × 105 irradiated (16 000 cGy) Karpas 299 cells in 24-well plates. CD4+ or CD8+ T cells were enriched by negative selection from apheresis cones using enrichment kits (StemCell Technologies). Some cultures were supplemented with interleukin-2 (IL-2; 50 IU/mL). T cells were harvested; viable cells counted by Trypan Blue exclusion and restimulated with irradiated Karpas 299 every 6 to 8 days. T cells were restimulated at least 3 times before use. In some cultures, either an isotype control or blocking anti–B7-H1 monoclonal antibody (clone MIH1; obtained from eBioscience) was included at a concentration of 4 μg/mL. The concentration of the B7-H1 blocking antibody used was shown to block B7-H1 binding to both recombinant human PD-1 and B7-1 in an enzyme-linked immunosorbent assay system (data not shown). For proliferation assays, the T-cell lines generated were harvested and viable cells counted by Trypan Blue exclusion. T cells (4 × 105/well) were cocultured with irradiated Karpas 299 (5 × 104/well) in 96-well plates. T cells and Karpas 299 cells were cultured alone in each experiment. In some experiments, an isotype control or anti–B7-H1 antibody was included (4 μg/mL). For determination of cell proliferation, cells were pulsed with 1 μCi/well 3H-tritium deoxyribonucleotide for the last 12 to 15 hours of a 72-hour culture before determination of thymidine incorporation.
For CD107a (LAMP-1) staining, stimulated (ie, effector) CD8+ T cells (106/well) were cultured alone or cocultured with irradiated Karpas 299 cells (2.5 × 105/well) for 6 hours in a 48-well plate. A phycoerythrin-conjugated CD107a antibody and monensin were added to each well, as previously described.14 After culture, cells were washed and stained with anti-CD8 before fluorescence-activated cell sorter (FACS) analysis. In some experiments, freshly purified or effector CD8+ T cells (4 × 105/well, 96-well plate) were cocultured with carboxyfluorescein succinimidyl ester (CFSE) labeled, nonirradiated Karpas 299 at various T cell to tumor cell (E/T) ratios. Inhibition of tumor cell outgrowth was directly proportional to the E/T ratio in all experiments. The optimal E/T ratio (range, 80:1-200:1) for a given experiment is shown. CFSE+ tumor cells were subsequently identified by flow cytometry after 4 days of culture.
DCs
Purified monocytes (2 × 106/mL) from normal donors were cultured in 6-well plates in 3 mL total volume of complete RPMI supplemented with granulocyte-macrophage colony-stimulating factor (100 μg/mL) and IL-4 (10 μg/mL). Fresh media was added every 48 to 72 hours, and nonadherent DCs were collected for use on day 6 to day 7 of culture. To obtain mature DCs, 1 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich) was added for the final 24 hours of culture. Of note, immature DCs were observed to express B7-H1, which was further up-regulated on DC maturation. Allogeneic mixed leukocyte response was performed by culturing 4 × 105 DCs with 4 × 106 purified, CFSE-labeled CD3+ in 24-well plates. For the induction of FoxP3 expression, 4 × 106 Treg-depleted CD4 cells were cocultured with 106 DCs for 6 to 7 days. In some experiments, culture media was supplemented with 25 ng/mL transforming growth factor-β1 (TGF-β1; PeproTech). For Treg depletion experiments, CD25hi cells were removed from purified CD4+ cells isolated from peripheral blood mononuclear cells (PBMCs) using a CD4+CD25+ Treg isolation kit (Miltenyi Biotec). Monocytes were enriched by negative selection from apheresis cones using a monocyte enrichment kit (StemCell Technologies) and subsequently positively selected using RoboSep (StemCell Technologies), as per the manufacturer's instructions. Purity, as assessed by CD14 staining by flow cytometry, was at least 95%. CD3+ and CD4+ T cells were isolated in a similar fashion, by either negative or positive selection using RoboSep (StemCell Technologies) and were at least 90% pure.
Antibodies and flow cytometry
Unless otherwise indicated, fluorochrome-conjugated antibodies used for flow cytometry were obtained from BD Biosciences. Cells were analyzed on a FACSCalibur instrument (BD Biosciences) and analyzed using CellQuest or FACSDiva Software (BD Biosciences).
Patient samples, immunohistochemistry, and immunofluorescence assay
Malignant T cells were obtained from peripheral blood and were identified by the expression of the relevant T-cell receptor (TCR) Vβ chain. The TCR Vβ specific antibodies used were obtained from Beckman Coulter. In cases where TCR Vβ usage was unknown, malignant T cells were identified as CD4+CD7− cells, as these cells may lose the expression of T-cell markers, including CD7.15 The aberrant loss of CD7 expression was confirmed at the time of diagnosis for all patients whose specimens were used in the current study. Frozen plasma samples were obtained from the Lymphoma SPORE Biospecimens Core Facility. Paraffin tissue microarrays were constructed from peripheral T-cell lymphoma (PTCL) biopsy specimens, as previously described.16 In cases with insufficient tissue, and for all the cutaneous T-cell lymphoma (CTCL) cases examined, whole-tissue sections were stained. Immunohistochemical staining was performed, as previously described. The primary antibodies used for staining include: CD3 (Novocastra), FoxP3 (Novus Biologicals), CD68 (clone PGM-1; Dako North America), S-100 (Dako North America), and CD11c (Novocastra). Malignant T cells were visualized by morphology and CD3 staining, whereas histiocytic cells (ie, macrophages and DCs) were identified as CD3 negative, by their characteristic histiocytic morphology and by staining for histiocytic markers (ie, CD68, CD11c, and S-100), where indicated. Immunohistochemistry for B7-H1 (clone 5H1) was performed after slides had been deparaffinized in xylene, as previously described.17 Samples were scored as B7-H1+ if more than 30% of cells were positive. All slides were reviewed by an expert hematopathologist (A.L.F.). Normal skin, lymph node, and tonsil tissue were used as both positive controls and to ensure the specificity of staining for the markers used. Slides were viewed with an Olympus BX51 microscope and pictures taken with an Olympus DP71 camera. Olympus BSW with DP Controller software was used for image acquisition and storage. Immunofluorescent costaining for CD11c and B7-H1 expression was performed on paraffin-embedded tissue as previously described.18 Briefly, biopsy specimens obtained from PTCL patients were deparaffinized and stained with anti–B7-H1 (clone 5H1) and anti-CD11c (rabbit IgG; Abcam) using the CSA system (Dako North America) in accordance with the manufacturer's instructions. Sections were visualized by fluorescent microscopy (Olympus Provis AX70) and images captured with an Olympus DP71 camera and software. This study was approved by the Mayo Clinic Institutional Review Board, and informed consent was obtained in accordance with the Declaration of Helsinki.
Statistical methods
Comparisons among groups were evaluated using a Student t test and Wilcoxon rank-sum tests. Statistical analyses were performed using JMP6 and SigmaPlot Software, and P values less than .05 were considered statistically significant.
Results
Tumor cell–associated B7-H1 promotes T-cell unresponsiveness in T-cell lymphoproliferative disorders
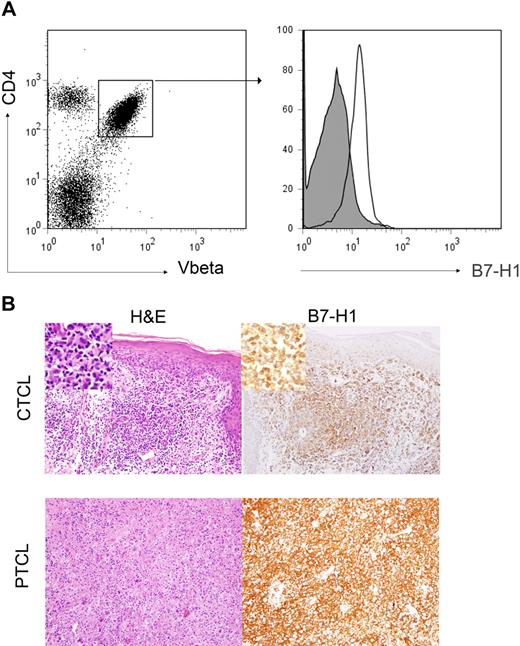
To determine tumor cell expression of B7-H1 in T-cell NHL, PBMCs from patients with circulating malignant T cells (ie, Sezary syndrome) were analyzed by flow cytometry for B7-H1 expression. Malignant T cells were identified by staining for the appropriate TCR Vβ chain specific for the patient's malignant clone (Figure 1A). B7-H1 was expressed by malignant T cells in the patients (n = 11) analyzed (Figure 1A). We next performed immunohistochemical staining for B7-H1 in biopsy specimens obtained from CTCL (n = 11) and PTCL (n = 144) patients, representative examples of which are shown in Figure 1B. In contrast to the results obtained by flow cytometry in Sezary syndrome, tumor cell–associated B7-H1 was appreciated in only 27% of the CTCL cases examined (Table 1). Although modest differences in the frequency of B7-H1+ tumors were noted among the various PTCL subsets, overall, tumor cell expression of B7-H1 was noted in 15% of cases (Table 1).
B7-H1 is expressed by malignant T cells. (A) PBMCs from patients with Sezary syndrome were analyzed by flow cytometry. Malignant T cells were identified by the expression of both CD4 and the clonotypic TCR Vβ chain, as shown in the representative dot plot. Samples were stained with an isotype control (closed histogram) or anti–B7-H1 (open histogram) and B7-H1 expression on malignant CD4+Vβ+ cells determined. For cases in which TCR Vβ use was unknown, malignant CD4+ T cells were identified by the aberrant down-regulation of CD7. A representative example (n = 11) is shown. (B) Immunohistochemical staining for B7-H1 was performed in paraffin-embedded biopsy specimens obtained from CTCL (n = 11) and PTCL (n = 144) patients and tumor-associated B7-H1 expression analyzed. Tumor cells were scored as B7-H1+ when B7-H1 and T cell–specific antibodies (eg, CD3, CD4, and CD8) both stained an identical population of cells, as determined by an expert hematopathologist. Two representative examples are shown (original magnification ×200).
B7-H1 is expressed by malignant T cells. (A) PBMCs from patients with Sezary syndrome were analyzed by flow cytometry. Malignant T cells were identified by the expression of both CD4 and the clonotypic TCR Vβ chain, as shown in the representative dot plot. Samples were stained with an isotype control (closed histogram) or anti–B7-H1 (open histogram) and B7-H1 expression on malignant CD4+Vβ+ cells determined. For cases in which TCR Vβ use was unknown, malignant CD4+ T cells were identified by the aberrant down-regulation of CD7. A representative example (n = 11) is shown. (B) Immunohistochemical staining for B7-H1 was performed in paraffin-embedded biopsy specimens obtained from CTCL (n = 11) and PTCL (n = 144) patients and tumor-associated B7-H1 expression analyzed. Tumor cells were scored as B7-H1+ when B7-H1 and T cell–specific antibodies (eg, CD3, CD4, and CD8) both stained an identical population of cells, as determined by an expert hematopathologist. Two representative examples are shown (original magnification ×200).
B7-H1 expression in T-cell lymphoproliferative disorders
| . | Tumor B7-H1 . | Stromal histiocyte B7-H1 . | ||||
|---|---|---|---|---|---|---|
| − . | + . | % positive . | − . | + . | % positive . | |
| CTCL (MF/SS) | 8 | 3 | 27 | 3 | 8 | 73 |
| PTCL-U | 54 | 11 | 17 | 37 | 28 | 43 |
| AITL | 20 | 1 | 5 | 9 | 12 | 57 |
| ALCL, ALK− | 17 | 3 | 15 | 13 | 7 | 35 |
| ALCL, ALK+ | 6 | 3 | 33 | 7 | 2 | 22 |
| Other | 26 | 3 | 10 | 22 | 7 | 24 |
| Total | 131 | 24 | 15 | 91 | 64 | 41 |
| . | Tumor B7-H1 . | Stromal histiocyte B7-H1 . | ||||
|---|---|---|---|---|---|---|
| − . | + . | % positive . | − . | + . | % positive . | |
| CTCL (MF/SS) | 8 | 3 | 27 | 3 | 8 | 73 |
| PTCL-U | 54 | 11 | 17 | 37 | 28 | 43 |
| AITL | 20 | 1 | 5 | 9 | 12 | 57 |
| ALCL, ALK− | 17 | 3 | 15 | 13 | 7 | 35 |
| ALCL, ALK+ | 6 | 3 | 33 | 7 | 2 | 22 |
| Other | 26 | 3 | 10 | 22 | 7 | 24 |
| Total | 131 | 24 | 15 | 91 | 64 | 41 |
Although work performed in a variety of solid tumor models has demonstrated that B7-H1 may inhibit tumor-specific T cells, little is known about its potential contribution to the suppression of host antitumor immunity in NHL. To address this question, sublethally irradiated Karpas 299 cells, a B7-H1+ and MHC class II+ T-cell lymphoma line (data not shown), were used to stimulate either CD4+ or CD8+ T cells isolated from normal donors. Allogeneic T cells isolated from normal donors were cocultured with irradiated Karpas 299 cells. After 6 to 8 days of culture, T cells were harvested and restimulated with newly irradiated Karpas 299 cells. After repeated cycles of in vitro restimulation, CD4+ and CD8+ T-cell lines were generated, as demonstrated by their ability to proliferate on restimulation with irradiated tumor cells (Figure 2A). Because B7-H1 may inhibit the proliferation of activated T cells, a blocking anti–B7-H1 monoclonal antibody was included in the cultures on restimulation with Karpas 299. As shown in Figure 2A, B7-H1 blockade significantly increased the proliferation of both CD4+ and CD8+ T cells after antigenic stimulation with Karpas 299 cells. In addition to its ability to directly inhibit T-cell proliferation, B7-H1 may also impair the T-cell proliferative response on antigenic restimulation by multiple mechanisms, including: (1) the induction of T-cell apoptosis,19 (2) the induction of T-cell anergy,12 or (3) promoting T-cell exhaustion in terminally differentiated T cells.20 To determine whether B7-H1 expression in T-cell NHL may impair the T-cell proliferative response on antigenic restimulation, CD4+ and CD8+ T cells were stimulated with irradiated Karpas 299 cells, as described in Figure 2A. However, during each stimulation cycle, either an isotype control or blocking B7-H1 antibody was included in the cultures. In contrast to T-cell lines that had been generated in the presence of a control antibody, a robust proliferative response was observed after restimulation of T cells that had been generated in the presence of a blocking B7-H1 antibody (Figure 2B). Therefore, tumor-associated B7-H1 inhibits T-cell proliferation directly and promotes the induction of T-cell hyporesponsiveness in T-cell NHL.
Tumor-associated B7-H1 inhibits T-cell immunity. (A) Purified allogeneic CD4+ or CD8+ T cells (4 × 106) were stimulated with irradiated B7-H1+ Karpas 299 cells (5 × 105) in 24-well plates. T-cell lines were harvested, washed, counted, and restimulated with newly irradiated tumor cells every 6 to 8 days, at least 3 times. The T-cell lines thus generated were harvested; viable cells counted by exclusion of Trypan Blue and either cultured alone (4 × 105) or restimulated with irradiated Karpas 299 (5 × 104) in triplicate in a 96-well plate and thymidine incorporation determined after 72 hours. On restimulation, either an isotype control or anti–B7-H1 (4 μg/mL) was included, as indicated. Any background thymidine incorporation by irradiated Karpas 299 cells was subtracted from the values shown. (B) CD4+ and CD8+ T-cell lines were generated, as described in panel A. An isotype control or anti–B7-H1 (4 μg/mL) was included during each stimulation cycle, as indicated. Cells were subsequently harvested and restimulated with irradiated Karpas 299 cells. (C) A CD8+ T-cell line (106/well), generated as described in panel A, was left unstimulated or restimulated with irradiated Karpas 299 cells (2.5 × 105/well) for 6 hours in a 48-well plate in the presence of anti-CD107a, as described in “Cell lines, proliferation, and cytotoxicity assays.” An isotype control or anti–B7-H1 (4 μg/mL) was included, as shown. CD107a+CD8+ cells, identified by flow cytometry, are included in the gates shown (n = 3, P = .048). (D) CFSE-labeled Karpas 299 cells were cocultured with freshly purified CD8+ T cells or activated effector CD8+ T cells (4 × 105/well) at various E/T ratios (E/T ratio of 200:1 is shown). CFSE+ tumor cells were identified by flow cytometry after 4 days of culture. All data shown are representative of at least 3 independently performed experiments.
Tumor-associated B7-H1 inhibits T-cell immunity. (A) Purified allogeneic CD4+ or CD8+ T cells (4 × 106) were stimulated with irradiated B7-H1+ Karpas 299 cells (5 × 105) in 24-well plates. T-cell lines were harvested, washed, counted, and restimulated with newly irradiated tumor cells every 6 to 8 days, at least 3 times. The T-cell lines thus generated were harvested; viable cells counted by exclusion of Trypan Blue and either cultured alone (4 × 105) or restimulated with irradiated Karpas 299 (5 × 104) in triplicate in a 96-well plate and thymidine incorporation determined after 72 hours. On restimulation, either an isotype control or anti–B7-H1 (4 μg/mL) was included, as indicated. Any background thymidine incorporation by irradiated Karpas 299 cells was subtracted from the values shown. (B) CD4+ and CD8+ T-cell lines were generated, as described in panel A. An isotype control or anti–B7-H1 (4 μg/mL) was included during each stimulation cycle, as indicated. Cells were subsequently harvested and restimulated with irradiated Karpas 299 cells. (C) A CD8+ T-cell line (106/well), generated as described in panel A, was left unstimulated or restimulated with irradiated Karpas 299 cells (2.5 × 105/well) for 6 hours in a 48-well plate in the presence of anti-CD107a, as described in “Cell lines, proliferation, and cytotoxicity assays.” An isotype control or anti–B7-H1 (4 μg/mL) was included, as shown. CD107a+CD8+ cells, identified by flow cytometry, are included in the gates shown (n = 3, P = .048). (D) CFSE-labeled Karpas 299 cells were cocultured with freshly purified CD8+ T cells or activated effector CD8+ T cells (4 × 105/well) at various E/T ratios (E/T ratio of 200:1 is shown). CFSE+ tumor cells were identified by flow cytometry after 4 days of culture. All data shown are representative of at least 3 independently performed experiments.
In addition to its ability to inhibit T-cell proliferation, B7-H1 may also protect tumor cells from cell death mediated by cytotoxic T cells. To address this possibility, allogeneic CD8+ T cells were stimulated with Karpas 299 cells and T-cell degranulation determined by CD107a staining. Karpas 299 cells were able to stimulate T-cell degranulation (3.1% vs 8.5% in Figure 2C). However, blocking B7-H1 resulted in a modest increase in T-cell degranulation (Figure 2C). Whether or not the suppression of T-cell cytotoxicity may promote the outgrowth of tumor cells was tested in an in vitro system in which fluorescently labeled Karpas 299 cells were cocultured at various E/T ratios with either naive CD8+ T cells or with effector CD8+ T cells, like those used in Figure 2C, which had been generated after multiple cycles of stimulation with irradiated Karpas 299 cells. Inhibition of tumor cell outgrowth was directly proportional to the E/T ratio used (data not shown) with diminished tumor cell outgrowth being observed on B7-H1 blockade (Figure 2D). Collectively, the data are consistent with observations made in multiple solid tumor models and demonstrate that T-cell lymphoma associated B7-H1 contributes to the suppression of host antitumor immunity.
B7-H1 is expressed by monocytes and their progeny present within the tumor microenvironment in T-cell lymphoproliferative disorders
Recent work has demonstrated that T-cell NHLs are characterized by a rich infiltrate of monocyte-derived cells, including DCs and macrophages.21 Monocytes and their progeny have been shown to inducibly express B7-H1 in a variety of infectious diseases.22 A population of HLA-DRlo monocytes have been shown to inhibit T-cell proliferation in solid tumors, and the presence of these cells is an adverse prognostic factor in patients with sepsis.23-26 IL-10, a known B7-H1 inducer,27 has been implicated in the development of these cells and may contribute to their ability to suppress T-cell immunity.28 Therefore, we sought to determine whether monocytes, either DR+ or DRlo subsets, and their progeny may express B7-H1 in T-cell NHL. To address this question, peripheral blood monocytes were identified in both normal donors and CTCL patients by the coexpression of both CD14 and HLA-DR. PBMCs from normal donors contain a population of CD14+ monocytes that are exclusively HLA-DR+ (Figure 3A). In contrast, 2 populations of CD14+ monocytes, including an HLA-DRlo subset, were detected in CTCL patients, both of which were found to express B7-H1 (Figure 3A). HLA-DRlo monocytes were detected in the peripheral blood in a significant number of CTCL patients (Figure 3B) and were found to highly express B7-H1 (Figure 3C-D). B7-H1 blockade in monocyte and T-cell cocultures resulted in an approximately 10% increase in both T-cell proliferation and IL-2 production. This modest increase, however, was not statistically significant (data not shown) and may suggest that additional factors may contribute to the suppression mediated by these cells.
B7-H1 is expressed by CD14+HLA-DRlo monocytes in CTCL. (A-B) PBMCs from both normal donors (n = 23) and CTCL patients (n = 11) were stained with anti-CD14, anti–HLA-DR, and either an isotype control (closed histogram) or anti–B7-H1 (open histogram). The representative dot plot shown demonstrates a unique population of CD14+HLA-DRlo cells present in CTCL patients. The frequency (percentage of total PBMCs) of HLA-DRlo monocytes for normal donors and CTCL patients is shown in panel B. B7-H1 expression by the gated populations shown in panel A was determined. (C-D) B7-H1 expression (compared with an isotype control for each sample) by CD14+HLA-DR+ and CD14+HLA-DRlo cells was analyzed (± 95% confidence interval is shown).
B7-H1 is expressed by CD14+HLA-DRlo monocytes in CTCL. (A-B) PBMCs from both normal donors (n = 23) and CTCL patients (n = 11) were stained with anti-CD14, anti–HLA-DR, and either an isotype control (closed histogram) or anti–B7-H1 (open histogram). The representative dot plot shown demonstrates a unique population of CD14+HLA-DRlo cells present in CTCL patients. The frequency (percentage of total PBMCs) of HLA-DRlo monocytes for normal donors and CTCL patients is shown in panel B. B7-H1 expression by the gated populations shown in panel A was determined. (C-D) B7-H1 expression (compared with an isotype control for each sample) by CD14+HLA-DR+ and CD14+HLA-DRlo cells was analyzed (± 95% confidence interval is shown).
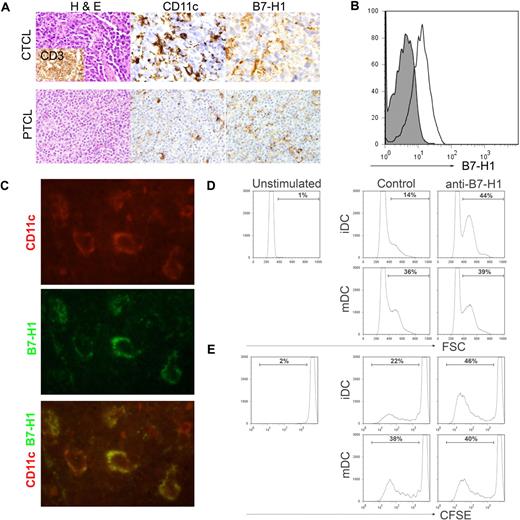
A more systematic analysis of B7-H1 expression on monocyte-derived cells, including DCs, was subsequently performed by immunohistochemistry. Multiple biopsy specimens obtained from CTCL (n = 11) and PTCL (n = 144) patients were stained for B7-H1, as described in Figure 1B, and B7-H1 expression on histiocytic cells infiltrating the tumor determined. Consecutive tissue sections on selected cases were stained for macrophage (CD68) and DC (CD11c) markers to aid in the identification of B7-H1+ histiocytes. All samples were stained with T-cell markers (CD3 and CD4) to aid in the identification of malignant T cells. B7-H1 was expressed on tumor-infiltrating histiocytic cells in 22% to 73% of cases, depending on disease histology (Figure 4A; Table 1). To confirm B7-H1 expression on CD11c+ DCs, single-cell suspensions from patient biopsy specimens (lymph node or spleen) were stained for CD11c, CD14, and B7-H1 expression. As shown in Figure 4B, CD11c+CD14− DCs within the tumor microenvironment were found to express B7-H1. In addition, biopsy specimens were costained with antibodies against human CD11c and B7-H1 and visualized by fluorescent microscopy. Tumor cells did not express B7-H1 in the cases selected. B7-H1 and CD11c were coexpressed in all of the cases examined (Figure 4C), further demonstrating B7-H1 expression on tumor-infiltrating DCs.
B7-H1 is expressed by tumor-associated DCs and inhibits T-cell proliferation. (A) As described in Figure 1, immunohistochemical staining for B7-H1 was performed in patient biopsy specimens. B7-H1 expression by stromal monocyte-derived cells, identified by CD11c (shown), S-100, or CD68 staining, was determined. (B) Single-cell suspensions were generated from spleen (shown) or lymph node biopsy specimens obtained from PTCL patients (n = 5). Cells were stained with an isotype control (closed histogram) or anti–B7-H1 (open histogram) and B7-H1 expression on CD11c+CD14− DCs determined. A representative example is shown. (C) Tissue specimens obtained from PTCL patients (n = 7) were double stained with antibodies to human CD11c (red) and B7-H1 (green) and viewed by fluorescent microscopy, as described in “Patient samples, immunohistochemistry, and immunofluorescence assay.” A representative example is shown (original magnification ×100). (D-E) Immature or LPS-matured DCs (4 × 105/well) generated from normal-donor monocytes were cocultured with allogeneic, CFSE-labeled T cells (4 × 106/well) for 6 days in the presence of an isotype control or anti–B7-H1 (4 μg/mL), as shown. T-cell activation, demonstrated by increasing forward scatter (D), and proliferation, indicated by CFSE dilution (E), were analyzed. The data shown are representative of at least 3 similarly performed experiments.
B7-H1 is expressed by tumor-associated DCs and inhibits T-cell proliferation. (A) As described in Figure 1, immunohistochemical staining for B7-H1 was performed in patient biopsy specimens. B7-H1 expression by stromal monocyte-derived cells, identified by CD11c (shown), S-100, or CD68 staining, was determined. (B) Single-cell suspensions were generated from spleen (shown) or lymph node biopsy specimens obtained from PTCL patients (n = 5). Cells were stained with an isotype control (closed histogram) or anti–B7-H1 (open histogram) and B7-H1 expression on CD11c+CD14− DCs determined. A representative example is shown. (C) Tissue specimens obtained from PTCL patients (n = 7) were double stained with antibodies to human CD11c (red) and B7-H1 (green) and viewed by fluorescent microscopy, as described in “Patient samples, immunohistochemistry, and immunofluorescence assay.” A representative example is shown (original magnification ×100). (D-E) Immature or LPS-matured DCs (4 × 105/well) generated from normal-donor monocytes were cocultured with allogeneic, CFSE-labeled T cells (4 × 106/well) for 6 days in the presence of an isotype control or anti–B7-H1 (4 μg/mL), as shown. T-cell activation, demonstrated by increasing forward scatter (D), and proliferation, indicated by CFSE dilution (E), were analyzed. The data shown are representative of at least 3 similarly performed experiments.
B7-H1, expressed by DCs, inhibits T-cell proliferation
Overall, almost 50% of T-cell NHL cases examined were associated with an abundant infiltrate of B7-H1+ monocyte-derived cells, including DCs. Therefore, we sought to determine whether DC-associated B7-H1 may contribute to the suppression of T-cell activation and proliferation. Therefore, monocyte-derived DCs were generated and used to stimulate allogeneic T cells in the presence of a control or blocking B7-H1 monoclonal antibody. Both immature DCs (iDCs) and LPS-matured DCs (mDCs) were cocultured with allogeneic T cells for 6 days and T-cell activation and proliferation determined. As shown in Figure 4D, an almost 3-fold increase in T-cell activation was observed on B7-H1 blockade in cocultures containing iDCs and was associated with a significant increase in T-cell proliferation, as demonstrated by CFSE dilution (Figure 4E). Whereas 22% of T cells proliferated in response to iDCs, a 2-fold increase in T-cell proliferation was noted on B7-H1 blockade, demonstrating that DC-associated B7-H1 inhibits T-cell activation and proliferation. As expected, mDCs were more potent stimulators of both T-cell activation and proliferation compared with iDCs, and any increase in T-cell proliferation observed after B7-H1 blockade in the mDC cocultures was modest, suggesting that B7-H1–mediated inhibition of T-cell activation may be overcome, at least partially, by costimulation provided by mDCs. B7-H1, whether present on tumor cells or DCs within the tumor microenvironment, contributes to the suppression of host antitumor immunity by inhibiting T-cell proliferation and promoting their unresponsiveness.
B7-H1 promotes the induction of FoxP3+ Treg
One mechanism by which DCs regulate the balance between self-tolerance and host immunity is via the induction of FoxP3+ Treg.29 As many T-cell lymphoproliferative disorders are characterized by a rich infiltrate of FoxP3+ Tregs,30 we sought to determine whether DC-associated B7-H1 may promote the induction of FoxP3+ Treg in human T cells. DCs were cultured with freshly purified CD4+ T cells, which had been depleted of CD25hi natural Treg. TGF-β was included as it plays a central role in the peripheral conversion of conventional T cells into Treg31 and is probably present within the tumor microenvironment in T-cell lymphoproliferative disorders.32-34 DCs were capable of inducing FoxP3 expression in these cells (Figure 5A). However, B7-H1 blockade greatly inhibited the induction of CD25hiFoxP3+ Treg (Figure 5A-B), suggesting that B7-H1 is critically important in the induction of human Treg within the tumor microenvironment. As natural Treg had been depleted, this probably does not represent the expansion of preexisting natural Treg. To determine whether B7-H1 may contribute to the generation of Treg in T-cell lymphoproliferative disorders, 1-mm cores were obtained, in triplicate, from each of 48 paraffin-embedded biopsy specimens obtained from PTCL patients. B7-H1 and FoxP3 expression was determined by immunohistochemical staining of adjacent tissue sections. Interestingly, FoxP3+ cells were found to be located in areas of the tumor infiltrated with abundant B7-H1+ DCs (Figure 5C). When biopsy specimens were stratified according to the presence or absence of B7-H1 expression, significantly more FoxP3+ cells were present in the B7-H1+ sections (Figure 5D). Therefore, DC-associated B7-H1 inhibits T-cell proliferation both directly and indirectly via the induction of FoxP3+ Treg, further contributing to the suppression of host immunity. Collectively, the data presented demonstrate that B7-H1 may contribute to the suppression of host immunity in T-cell lymphoproliferative disorders and represents a novel therapeutic target in T-cell NHL.
DC-associated B7-H1 promotes the induction of FoxP3+regulatory T cells. (A-B) Purified CD4+ T cells were depleted of CD25hi natural Tregs and cultured alone or with normal donor monocyte-derived iDCs in triplicate for 6 days. TGF-β (25 ng/mL) was included in the cultures shown in panel A or as indicated. Either an isotype control or blocking anti–B7-H1 (4 μg/mL) antibody was included. The frequency of CD25hiFoxP3+ cells was determined by flow cytometry. Representative dot plots are shown in panel A. Data shown are representative of at least 3 similarly performed experiments. (C-D) Immunohistochemical staining for both B7-H1 and FoxP3 was performed on paraffin-embedded PTCL biopsy specimens (n = 48; in triplicate), as described in “Patient samples, immunohistochemistry, and immunofluorescence assay.” B7-H1 and FoxP3 staining from multiple areas of the same biopsy specimen are shown in panel C. (Inset) Original magnification ×200. (D) FoxP3+ cells in each high-power field (± 95% confidence interval is shown) were counted and DC staining for B7-H1 determined. For the purpose of our analysis, core biopsy specimens were considered B7-H1+ if any portion of the specimen contained B7-H1+ DCs.
DC-associated B7-H1 promotes the induction of FoxP3+regulatory T cells. (A-B) Purified CD4+ T cells were depleted of CD25hi natural Tregs and cultured alone or with normal donor monocyte-derived iDCs in triplicate for 6 days. TGF-β (25 ng/mL) was included in the cultures shown in panel A or as indicated. Either an isotype control or blocking anti–B7-H1 (4 μg/mL) antibody was included. The frequency of CD25hiFoxP3+ cells was determined by flow cytometry. Representative dot plots are shown in panel A. Data shown are representative of at least 3 similarly performed experiments. (C-D) Immunohistochemical staining for both B7-H1 and FoxP3 was performed on paraffin-embedded PTCL biopsy specimens (n = 48; in triplicate), as described in “Patient samples, immunohistochemistry, and immunofluorescence assay.” B7-H1 and FoxP3 staining from multiple areas of the same biopsy specimen are shown in panel C. (Inset) Original magnification ×200. (D) FoxP3+ cells in each high-power field (± 95% confidence interval is shown) were counted and DC staining for B7-H1 determined. For the purpose of our analysis, core biopsy specimens were considered B7-H1+ if any portion of the specimen contained B7-H1+ DCs.
Discussion
Multiple lines of evidence highlight the importance of host immune surveillance in preventing the widespread growth and survival of malignant lymphocytes during lymphomagenesis. For example, as many as 20% of patients with indolent NHL may experience a spontaneous remission of their disease in the absence of treatment.35 Although the reasons for this are unclear, the development of a host antitumor immune response seems quite plausible and consistent with more recent gene expression profiling studies demonstrating that the nonmalignant constituents of the tumor stroma predict outcomes independently of conventional clinical prognostic factors.5 Similar studies, using gene expression profiling or immunohistochemistry-based approaches, demonstrate that characteristics of the host antitumor immune response are of prognostic importance in a variety of solid tumors and hematologic malignancies.6,36,37 T cells, comprising approximately 50% of lymphocytes, function as key regulatory and effector cells during the host antitumor immune response. Not surprisingly then, lymphopenia portends a poor prognosis in NHL.38-40 Factors associated with the tumor-bearing host, like HIV infection or the administration of immunosuppressants in recipients of solid organ transplantations, may contribute to the suppression of host immunity and the subsequent development of NHL.41 Similarly, tumor-associated factors may likewise contribute to the suppression of host immunity but are largely unexplored in many NHL, particularly those that are T-cell derived.
B7-H1, on engagement of either of its 2 receptors, PD-1 (CD279) or B7-1 (CD80), plays a central role in the suppression of T cell–mediated immunity and has emerged as an important mediator of tumor-associated immune suppression.11 PD-1 ligation on T cells results in the recruitment of protein tyrosine phosphatases (ie, SHP-1 and SHP-2) and inhibition of signaling events downstream of the TCR, which culminate in the inhibition of T-cell proliferation, cytokine production, differentiation, and survival.11 Murine studies, performed in either PD-1–deficient mice or with blocking B7-H1 or PD-1 monoclonal antibodies, demonstrate that tumors may exploit B7-H1 to suppress host immunity by the induction of T-cell unresponsiveness. First, B7-H1 may inhibit T-cell proliferation and cytokine production directly and is consistent with the observation that blockade of T-cell NHL-associated B7-H1 increased proliferation of both CD4+ and CD8+ T cells in vitro (Figure 2A). B7-H1 may likewise induce apoptosis in effector T cells19 or promote the induction of T-cell anergy,12 thus promoting T-cell unresponsiveness. We found that B7-H1 blockade, on repeated antigenic restimulation, as may occur in the tumor-bearing host, could inhibit the induction of T-cell hyporesponsiveness (Figure 2B). In addition to its ability to suppress T-cell immunity, tumor-associated B7-H1 may confer resistance to CTL-mediated lysis (Figure 2C-D).42 PD-1 ligation inhibits CTL degranulation (Figure 2C) and promotes the outgrowth of malignant T cells cultured with tumor-reactive CTL (Figure 2D). Interestingly, recent work has demonstrated that reverse signaling via B7-H1 may prevent tumor cell apoptosis in response to a variety of stimuli, including tumor-specific CTL and pharmacologic agents, and may represent an alternative explanation for our findings.43 As both B7-H1 and PD-1 (data not shown) are expressed within the tumor microenvironment, B7-H1 ligation may occur in an autocrine or paracrine manner and confer resistance to CTL-mediated lysis and apoptosis induced by chemotherapeutic agents. This hypothesis warrants further study and, if correct, may have therapeutic implications.
B7-H1 is expressed in several solid tumors and, probably because of its immunosuppressive effects, is an adverse prognostic factor when present.10 Therefore, monoclonal antibodies capable of blocking B7-H1 and PD-1 binding are currently being investigated in the clinical trial setting. Improved understanding of the mechanisms involved in the expression of B7-H1 on malignant cells and the development of pharmacologic inhibitors capable of preventing its aberrant up-regulation represents an equally rational therapeutic approach. Although B7-H1 expression is not commonly appreciated in B cell–derived NHL (data not shown), its expression was previously reported in a small number of PTCL cases (n = 7), including anaplastic large-cell lymphomas (ALCLs).44 Anaplastic lymphoma kinase (ALK) positive ALCLs (ALK+ ALCLs) express an oncogenic chimeric nucleophosmin-ALK protein, which has been shown to up-regulate B7-H1.45 This may explain our finding of a 2-fold increase in B7-H1 expression among ALK+ ALCLs compared with their ALK− counterparts (Table 1). Work performed in several solid tumors has shown that activation of the PI3 kinase/AKT/mammalian target of rapamycin (mTOR) pathway leads to the posttranscriptional regulation of B7-H1 and its aberrant up-regulation on tumor cells.46,47 As the mTOR pathway is activated by nucleophosmin-ALK, this raises the possibility that mTOR inhibitors may inhibit B7-H1 expression in T-cell lymphoproliferative disorders in a fashion similar to that observed in solid tumors.48,49 B7-H1 expression was observed in a variety of ALK− T-cell lymphoproliferative disorders, demonstrating that B7-H1 expression is not strictly ALK dependent. The extent to which its expression may be dependent on mTOR or STAT3 activation45 in these tumors is unknown.
Although B7-H1 expression on tumor cells was observed in only a minority of cases, B7-H1 may be expressed by a variety of nonmalignant cells present within the tumor microenvironment, including myeloid-derived cells, like DCs. Furthermore, we have recently shown that T-cell lymphoproliferative disorders are characterized by an abundant infiltrate of iDCs and other monocyte-derived cells.21 Therefore, we sought to determine the extent to which tumor-infiltrating monocyte-derived cells may express B7-H1 in T-cell NHL. In contrast to its expression on tumor cells, B7-H1 expression was far more common on tumor-infiltrating monocyte-derived cells, being observed in anywhere from 22% to 73% of cases, depending on the T-cell NHL subset analyzed. B7-H1, expressed by iDCs, was found to inhibit T-cell activation (Figure 4B), proliferation (Figure 4C), and cytokine production (data not shown). Interestingly, the effect of B7-H1 blockade was most pronounced when iDCs, as opposed to mDCs, were used as stimulators. This may be explained by the ability of T-cell costimulation, provided on DC maturation, to overcome PD-1–mediated inhibition and is consistent with previous observations.27,50 Whether B7-H1 may influence T-cell differentiation after antigen presentation by DCs is less well understood, but perhaps best supported by the finding that B7-H1 promotes the induction of FoxP3+ Treg and B7-H1+ DCs are associated with an abundant infiltrate of these cells in vivo. These findings are further supported by the recent observation that B7-H1–deficient DCs were unable to promote the induction of FoxP3+ Treg in a murine model.29 In addition, these findings using B7-H1–deficient DCs seemingly render the alternative interpretation that the effects we observed after B7-H1 blockade are the result of blocking B7-1 or PD-1 engagement on DCs by T cell–associated B7-H1 less plausible. In addition, no alteration in DC maturation or cytokine production was noted after B7-H1 blockade. Whereas immature DCs were able to promote the induction of FoxP3+ Treg, B7-H1+ Karpas 299 cells, in similarly performed experiments, were unable to support the induction of FoxP3+ Treg (data not shown), suggesting that the role of B7-H1 in the regulation of Treg is context dependent. Malignant T cells may express PD-1 and FoxP3 expression has been observed in several T-cell lymphoproliferative disorders, leading some to suggest that particular subsets of T-cell lymphoproliferative disorders represent a malignancy of regulatory T cells.34 This is an intriguing possibility, as suppression of nonmalignant conventional T cells and loss of T-cell repertoire diversity is observed in some patients.51 Therefore, the possibility that B7-H1–expressing DCs may contribute to the induction of a suppressive phenotype in these cells may warrant further investigation, as B7-H1 blockade may reverse suppression of conventional T cells mediated by their malignant counterparts.
Just as DC-associated B7-H1 may suppress host immunity, DC precursors in the peripheral blood, including monocytes, may express B7-H1 and suppress host immunity during chronic inflammatory states. DC-associated B7-H1 may be up-regulated within the tumor microenvironment; alternatively, B7-H1+ DC precursors may be preferentially recruited to the tumor microenvironment. Although the work presented here cannot distinguish between these 2 possibilities, neither of which is mutually exclusive, the finding that B7-H1 was most highly expressed on a subset of HLA-DRlo peripheral blood monocytes makes the latter hypothesis quite plausible. In contrast, we also observed that B7-H1 was up-regulated and HLA-DR down-regulated on monocytes cocultured with T-cell NHL cell lines, suggesting that the expansion of HLA-DRlo monocytes in T-cell NHL is driven by tumor-derived factors (data not shown). The subset of HLA-DRlo monocytes, described in sepsis and melanoma, is thought to inhibit T-cell proliferation in a cytokine-dependent manner.23 Whether the frequency of these cells is of prognostic significance in T-cell lymphoproliferative disorders, as demonstrated in sepsis, deserves consideration. Collectively, the data presented demonstrate that B7-H1, expressed by either tumor cells or tumor-infiltrating DCs, contributes to the suppression of host immunity; consequently, targeting B7-H1, and the cells on which it is expressed, represents a novel therapeutic strategy in T-cell lymphoproliferative disorders.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported in part by the National Institutes of Health (grants CA92104 and CA97274).
National Institutes of Health
Authorship
Contribution: R.A.W. designed and performed research, analyzed data, and wrote the manuscript; A.L.F. performed research and analyzed data; D.A.W., Z.-Z.Y., N.I.C., H.D., A.J.N., M.R.P., and T.E.W. analyzed data and critically reviewed the manuscript; E.D.K. contributed vital reagents; S.N.M. contributed vital reagents and critically reviewed the manuscript; and S.M.A. assisted with research design, data analysis, and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen M. Ansell, Division of Hematology and Internal Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: ansell.stephen@mayo.edu.