Abstract
Dasatinib and nilotinib are tyrosine kinase inhibitors (TKIs) developed to overcome imatinib resistance in Philadelphia-positive leukemias. To assess how Bcr-Abl kinase domain mutation status evolves during sequential therapy with these TKIs and which mutations may further develop and impair their efficacy, we monitored the mutation status of 95 imatinib-resistant patients before and during treatment with dasatinib and/or nilotinib as second or third TKI. We found that 83% of cases of relapse after an initial response are associated with emergence of newly acquired mutations. However, the spectra of mutants conferring resistance to dasatinib or nilotinib are small and nonoverlapping, except for T315I. Patients already harboring mutations had higher likelihood of relapse associated with development of further mutations compared with patients who did not harbor mutations (23 of 51 vs 8 of 44, respectively, for patients who relapsed on second TKI; 13 of 20 vs 1 of 6, respectively, for patients who relapsed on third TKI).
Introduction
Resistance to the tyrosine kinase inhibitor (TKI) imatinib mesylate (IM) in chronic myeloid leukemia (CML) and Philadelphia-positive (Ph+) acute lymphoblastic leukemia (ALL) is often caused by selection of mutations in the Bcr-Abl kinase domain (KD),1-9 altering residues that are directly or indirectly critical for IM binding. To overcome this problem, novel TKIs have been developed. Dasatinib and nilotinib10-13 have been the first ones to enter clinical evaluation and to receive marketing approval in IM-resistant patients. Preclinical experience with these agents showed that they are active against several IM-resistant Bcr-Abl mutants, with the exception of T315I.10,11,14 However, both TKIs have been hypothesized to retain their own “Achilles heels.” Recent studies have tried to profile mutations that will probably emerge under dasatinib and nilotinib, inducing random mutagenesis and then selecting for cell clones retaining viability when cultured in the presence of the inhibitors.15-18 Results suggested that, besides T315I, other mutants might be critical. To assess how Bcr-Abl KD mutation status evolves under the selective pressure of sequential therapy with novel TKIs and which mutations among those predicted by in vitro studies may indeed develop in patients who relapse on dasatinib or nilotinib, we have monitored the mutation status of 95 IM-resistant patients before and during sequential treatment with one or both of these agents.
Methods
Patients and definitions
This report focuses on 95 patients (Table 1) who were referred to our laboratory for mutation analysis at the time of IM failure and who received up to 2 subsequent TKIs (dasatinib and/or nilotinib). Thirty-eight patients had chronic-phase (CP) CML, 46 patients had advanced-phase CML (accelerated-phase [AP], n = 11; myeloid blast crisis [BC], n = 18; lymphoid BC, n = 17), and 11 patients had Ph+ ALL. For CML patients, IM failure was defined according to European LeukemiaNet recommendations.19 Similar criteria were applied to define IM failure in Ph+ ALL. All 95 patients received a second TKI (dasatinib, n = 55; nilotinib, n = 40). Twenty-six of 95 patients received a third TKI after relapse on second TKI (dasatinib, n = 16; nilotinib, n = 10). For the purpose of this analysis, response to dasatinib or nilotinib was defined as at least complete hematologic response. Informed consent was obtained from all patients, in accordance with the Declaration of Helsinki. This study received approval from the Review Board of S Orsola-Malpighi Hospital and all other participating institutions.
Patient characteristics and treatment
| Characteristic . | Value . |
|---|---|
| No. of patients | 95 |
| CP CML, n (%) | 38 (40) |
| AP CML, n (%) | 11 (11.5) |
| BC CML, n (%) | 35 (37) |
| Myeloid | 18 |
| Lymphoid | 17 |
| Ph+ ALL, n (%) | 11 (11.5) |
| Median age, y (range) | 56 (18-77) |
| Median time from diagnosis, mo (range) | 49 (4-181) |
| Median time on IM, mo (range) | 32 (4-66) |
| Patients positive for Bcr-Abl KD mutations at the time of IM failure, n (%) | 51 (54) |
| Patients who received dasatinib as second TKI, n | 55 |
| Patients who received nilotinib as second TKI, n | 40 |
| Patients who received dasatinib as third TKI, n | 19 |
| Patients who received nilotinib as third TKI, n | 7 |
| Characteristic . | Value . |
|---|---|
| No. of patients | 95 |
| CP CML, n (%) | 38 (40) |
| AP CML, n (%) | 11 (11.5) |
| BC CML, n (%) | 35 (37) |
| Myeloid | 18 |
| Lymphoid | 17 |
| Ph+ ALL, n (%) | 11 (11.5) |
| Median age, y (range) | 56 (18-77) |
| Median time from diagnosis, mo (range) | 49 (4-181) |
| Median time on IM, mo (range) | 32 (4-66) |
| Patients positive for Bcr-Abl KD mutations at the time of IM failure, n (%) | 51 (54) |
| Patients who received dasatinib as second TKI, n | 55 |
| Patients who received nilotinib as second TKI, n | 40 |
| Patients who received dasatinib as third TKI, n | 19 |
| Patients who received nilotinib as third TKI, n | 7 |
CML indicates chronic myeloid leukemia; CP, chronic phase; AP, accelerated phase; BC, blast crisis; Ph+ ALL, Philadelphia-positive acute lymphoblastic leukemia; IM, imatinib mesylate; KD, kinase domain; and TKI, tyrosine kinase inhibitor.
Abl KD mutation monitoring
All patients were analyzed for the presence of Bcr-Abl KD mutations at the time of IM failure and then monitored monthly during subsequent TKI treatment. Mutation analysis was performed by denaturing high-performance liquid chromatography followed by sequencing of denaturing high-performance liquid chromatography–positive cases, as previously reported.5,20
Statistical analysis
Fisher exact test was used to test for differences in mutation frequency among categories of patients.
Results and discussion
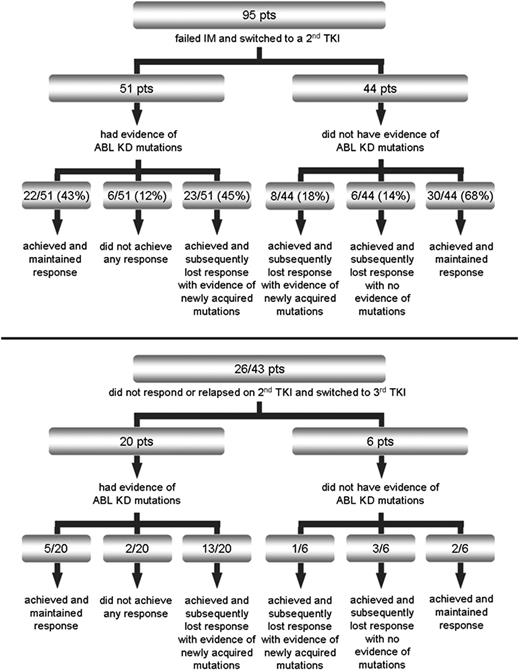
At the time of IM failure, 51 of 95 (54%) patients had evidence of KD mutations. Response after switching to a second TKI is summarized in Figure 1. With a median duration of second TKI therapy of 10 months (range, 1-32 months), 23 of 51 (45%) patients who had evidence of Bcr-Abl KD mutations at baseline versus 8 of 44 (18%) patients with wild-type Bcr-Abl relapsed after an initial response with newly acquired mutations (P = .01). Median time to relapse was 9 months (range, 1-20 months). Median time to first detection of the new mutations was 6 months (range, 1-20 months). Twenty-six of the 43 patients who did not respond or relapsed on second TKI switched to a third TKI. Twenty of 26 patients had Bcr-Abl KD mutations. With a median duration of third TKI therapy of 4 months (range, 2-9 months), 13 of 20 mutated patients versus 1 of 6 nonmutated patients achieved a response and then relapsed again with newly acquired mutations. Median time to relapse was 3 months (range, 2-6 months). Median time to first detection of the new mutations was 2 months (range, 1-6 months). Responses and frequency of newly acquired mutations according to the mutation status at baseline in the separate subsets of CP CML, AP/BC CML, and Ph+ ALL are detailed in supplemental Figures 1 through 6 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Patient treatment after imatinib failure and response patterns according to baseline Bcr-Abl kinase domain mutation status after switching to second (top panel) or third (bottom panel) tyrosine kinase inhibitor.
Patient treatment after imatinib failure and response patterns according to baseline Bcr-Abl kinase domain mutation status after switching to second (top panel) or third (bottom panel) tyrosine kinase inhibitor.
Interestingly, all patients with mutations at baseline who lost response to second or third TKI developed new mutations (36 of 36). In contrast, none of the patients who achieved and maintained a response was found to develop new mutations (0 of 59). In addition, none of the patients who never achieved a response to second or third TKI was found to develop new mutations (0 of 8).
Overall, 31 patients relapsed on dasatinib as second or third TKI with evidence of newly acquired mutations: T315I (n = 13), F317L (n = 9), V299L (n = 4), T315A (n = 3), F317I (n = 2), F317C (n = 1), and F317V (n = 1). One patient had evidence of multiple mutations (F317I + F317V + F317C). In addition, 4 patients already harboring mutations (T315I, n = 2; F317L, n = 2) did not achieve any response to dasatinib as second TKI. Fourteen patients relapsed on nilotinib as second or third TKI with evidence of newly acquired mutations; they were Y253H (n = 4), T315I (n = 3), E255V (n = 3), E255K (n = 2), F359V (n = 1), and L273M (n = 1). In addition, 4 patients already harboring mutations (Y253H, n = 2; E255K + F317L, n = 1; T315I, n = 1) did not achieve any response to nilotinib as second (Y253H) or third TKI (T315I, E255K + F317L).
In this analysis, we focused on the mutations that were found to have emerged at the time of relapse on dasatinib or nilotinib because they can reasonably be considered as being the main cause for the development of resistance. In our series of IM-resistant patients treated with a second or third TKI, relapse after an initial response was associated with the emergence of Bcr-Abl KD mutations in 45 of 54 (83%) cases. The T315I was the single most frequent mutation that outgrew and led to relapse during dasatinib or nilotinib treatment (16 of 45, 36% of patients). Other mutations, however, were found to have emerged at the time of relapse in the remaining two-thirds of patients, confirming that, as predicted by in vitro mutagenesis screenings15-18 and subsequently observed in some dasatinib- and nilotinib-resistant patients,21-25 the T315I is not the only mutant that confers insensitivity to these inhibitors. Some of these are known, IM-resistant mutants; this is the case of the F359V and the P-loop mutants Y253H and E255K/V that in our study were associated with relapse to nilotinib, and of the F317L that we and others have reported in several dasatinib-resistant patients.21,22,24,25 The others are either novel mutations (V299L) or novel substitutions at residues already implicated in IM resistance (T315A, F317I/C/V). Interestingly, we detected an L273M at the time of relapse to nilotinib in a patient who had no previous evidence of mutations. L273M has never been reported in association with resistance either to nilotinib or to imatinib, but a different amino acid substitution at the same residue, L273F, was recovered in an in vitro screening for mutations conferring resistance to nilotinib,18 suggesting that also the L273M might confer a certain degree of insensitivity.
Overall, the spectra of “critical” mutants for each single inhibitor are much smaller with respect to that observed for IM and seem to not overlap with each other, with the exception of T315I. This is important and, if confirmed by observation of larger series of dasatinib- and nilotinib-resistant patients, will allow in the future the selection of the most effective second TKI also on the basis of individual mutation status.
Our data also suggest that IM-resistant patients already harboring mutations have a higher likelihood of developing further mutations under the selective pressure of novel TKIs. It can be hypothesized that, in this specific subset of patients, a higher genetic instability may foster rapid emergence of multiple mutations over time within the same or different Bcr-Abl–positive subclones, which are selected or deselected depending on the specific TKI used. In this clinical setting, combination therapies (possibly including a T315I inhibitor) would probably be more effective than single-agent treatment for long-term disease control.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by European LeukemiaNet, Associazione Italiana contro le Leucemie, Associazione Italiana per la Ricerca sul Cancro, Programmi di Ricerca di Rilevante Interesse Nazionale projects, and Fondazione del Monte di Bologna e Ravenna.
Authorship
Contribution: S.S. designed and performed the research, analyzed and interpreted the data, and wrote the manuscript; A. Gnani, S.C., I.I., A.P., and M.A. performed the research; F.C., E.A., S.P., S.M., E.O., S.d.M., A. Gozzini, F.P., G.G., C.P., D.C., and G.R. collected and provided the clinical data; and M.B. and G.M. designed the research, analyzed the data, and critically revised the manuscript before submission.
Conflict-of-interest disclosure: G.M. received research funding from Novartis. M.B. received a consultancy and honoraria from Novartis and Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Simona Soverini, S Orsola-Malpighi Hospital, Via Massarenti 9, 40138 Bologna, Italy; e-mail: simona.soverini@tin.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal