In this issue of Blood, Dominici and colleagues use a murine model to demonstrate that after lethal TBI, there is a transient significant increase in the number of host osteoblasts accompanied by a maintenance of megakaryocytes, which produce multiple key cytokines known to regulate osteogenesis.1
The fate of endosteal stem cell niches after preparative ablation for bone marrow transplantation (BMT) regarding their ability to accommodate and regulate engrafting donor hematopoietic stem cells (HSCs) remains unclear. Yet, it is critical to understand this variable for the optimal success of clinical stem cell transplantation. These data are used to suggest an increase in the size of the niche available for engrafting transplanted HSCs.
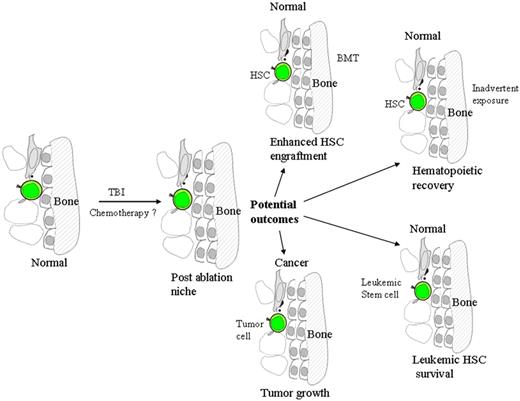
Model of potential clinical outcomes postablation of an endosteal niche. If the niche is either a cancer niche, or harbors a leukemic hematopoietic stem cell (HSC), the outcome could be detrimental. However, for normal niches and BMT or after inadvertent exposure to ablation, the outcome could be enhanced hematopoietic reconstitution.
Model of potential clinical outcomes postablation of an endosteal niche. If the niche is either a cancer niche, or harbors a leukemic hematopoietic stem cell (HSC), the outcome could be detrimental. However, for normal niches and BMT or after inadvertent exposure to ablation, the outcome could be enhanced hematopoietic reconstitution.
Whereas bone marrow transplantation has been successfully used to treat hematologic diseases for many decades, the effects of the preparative ablation on the microenvironment into which HSCs are landing have not been thoroughly investigated. Important factors could include both the severity and permanency of the damage as well as the impact of the timing of cell infusion on transplantation outcome. As a consequence, we are potentially missing opportunities to enhance transplantation outcomes by manipulating either the preparative ablation or the timing of HSC transplantation. Although the mechanism for transient 2-fold increase in endosteal osteoblasts observed by Dominici et al after total body irradiation (TBI) remains unclear, the authors suggest it occurs via the maintenance of functional host megakaryocytes that transiently continue to produce critical growth factors known to stimulate osteogenesis. Although the study highlights the creation of a larger and seemingly receptive microenvironment for engrafting transplanted HSCs, with an increase in the expression of 2 putative critical niche proteins, n-cadherin and osteopontin,2-4 it stops well short of demonstrating that an increase in osteoblasts results directly in an increase of functional support and, hence, expansion of engrafting HSCs. In addition, the role of the niche in regulating HSCs is multifactorial, relying on many cell types and their biosynthesized products. As a consequence, further studies are required to analyze the effects of preparative ablation on other key aspects of the HSC niche.
The study by Dominici et al also highlights the natural response of the endosteal niche to significant injury. In this context, the increase in osteoblasts in the absence of transplantation may be performing dual physiologic roles. First, these cells likely maintain osteogenesis and the structural integrity of the skeletal system. Second and equally important is that the increase in osteoblasts could be a mechanism to stimulate recovery of the endogenous HSC pool and thus rescue hematopoiesis. A correlation between the number of osteoblasts and the size of the HSC pool has previously been demonstrated in other models, for instance, following the administration of parathyroid hormone.5 Understanding the extent of microenvironmental recovery and impact on the survival and proliferation of the endogenous HSC pool has significant implications in clinical transplantation, especially for applications in the treatment of hematopoietic malignancies including leukemia. In this context, whereas the recovery of the damaged hematopoietic microenvironment is essential for a successful BMT, it may also result in the survival and proliferation of leukemic stem cells.
The study by Dominici et al also has significant implications for the treatment of other malignancies involving the bone marrow. For example, if a putative cancer stem cell niche6 includes many of the cell types of a putative normal HSC niche, such as the osteoblast, then the use of preparative TBI may not only protect the abnormal cells but stimulate their proliferation. This is more likely in diseases that potentially affect the bone marrow microenvironment, such as metastatic breast cancer.
Whereas it is very difficult to demonstrate increased functional support of an ablated niche due to the increased numbers of osteoblasts, the work by Dominici et al highlights the critical need to fully understand the effects of ablation on the hematopoietic microenvironment, if we are to be able to manipulate or take advantage of these effects to improve specific BMT outcomes in the clinic. Ideally these will include an ability to assess the “receptiveness” of the niche to HSCs and its ability to regulate the proliferation and differentiation of engrafted resident HSCs. In addition, it remains to be determined if preparative high-intensity chemo-ablation will have the same effects. It is only through such analysis that we will be able to address the question of whether “a bigger niche is necessarily a better niche.”
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal