Abstract
Leukocyte adhesion deficiency (LAD)–III is associated with homozygous stop codon mutations in Kindlin-3, the hematopoietic member of the Kindlin family of integrin coactivators. In addition, a subgroup of LAD-III patients has a homozygous splice junction mutation in and reduced expression of the Rap-1 guanine nucleotide exchange factor, CalDAG-GEFI (CDGI). In this study, we compared the adhesive properties of the leukocyte function-associated antigen-1 (LFA-1) and very late activation antigen-4 (VLA-4) integrins in both primary and activated leukocytes derived from these 2 LAD-III subgroups. Primary lymphocytes lacking both Kindlin-3 and CDGI lost all firm T-cell receptor–stimulated LFA-1 adhesiveness, in contrast to LAD-III lymphocytes deficient in Kindlin-3 alone. Effector T cells expanded from all tested LAD-III variants expressed normal CDGI, but lacked Kindlin-3. These Kindlin-3–null effector T cells exhibited total loss of inside-out LFA-1 activation by chemokine signals as well as abrogated intrinsic LFA-1 adhesiveness. Surprisingly, VLA-4 in Kindlin-3–null resting or effector lymphocytes retained intrinsic rolling adhesions to vascular cell adhesion molecule-1 and exhibited only partial defects in chemokine-stimulated adhesiveness to vascular cell adhesion molecule-1. Deletion of the putative β1 Kindlin-3 binding site also retained VLA-4 adhesiveness. Thus, our study provides the first evidence that Kindlin-3 is more critical to LFA-1 than to VLA-4–adhesive functions in human lymphocytes.
Introduction
Integrins constitute the major and largest family of cell adhesion receptors.1 In hematopoietic cells, these heterodimers rapidly undergo dramatic allosteric conformational changes in response to various activation signals.2 Talins are key integrin activators implicated in these processes in essentially all integrin-containing cell types.3 Activated integrins are essential for platelet aggregation, firm leukocyte adhesiveness to vascular endothelium, and lymphocyte arrest on antigen-presenting cells.4 Recent evidence suggests that Kindlins, a group of 3 structurally related adaptors, cooperate with talin in activating integrins in different cell types through binding to distinct motifs on the short tails of the integrin β subunits.5-8 In contrast to Kindlins-1 and -2, Kindlin-3 expression is restricted to the hematopoietic system.7 Whereas loss of talin1 is embryonically lethal, deletion of Kindlin-3 is not;7 yet, deletion of Kindlin-3 in mice results in severe defects in platelet and leukocyte integrin activation and in reduced lymphocyte counts,7,9 osteoporosis,7 and abnormal erythrocyte function, which is ultimately fatal.10 Although a recent study has directly implicated murine Kindlin-3 in neutrophil and monocyte integrin adhesiveness to inflamed endothelium in vitro and in vivo,9 the role of Kindlin-3 in inside-out (chemokine-mediated) and outside-in (ligand-induced) activation of lymphocyte integrins has remained unexplored.
Leukocyte adhesion deficiency (LAD)–III is a rare autosomal recessive syndrome, which is manifested as a combined defect in β3, β2, and β1 integrin activation in platelets, neutrophils, and lymphocytes.11 We previously reported 3 LAD-III cases from families of Turkish origin, associated with a homozygous splice junction mutation that results in defective expression of a key Rap-1–specific guanine nucleotide exchange factor (Rap-1/2 GEF), CalDAG-GEFI (CDGI), in platelets, neutrophils, and resting lymphocytes.12 We recently showed that these patients suffer from an additional mutation, a homozygous nonsense stop codon in the kindlin-3 gene.13 Most recently, we identified a new patient with a normal CDGI gene and a homozygous stop mutation in Kindlin-3, distinct from the Kindlin-3 mutation of the Turkish patients. LAD-III patients from non-Turkish origin with a normal CDGI gene were recently identified to bear various homozygous nonsense stop codon mutations in Kindlin-3,14-16 and transformed lymphocytes derived from these patients were shown to lack Kindlin-3 expression.15,16 These lines display various defects in integrin spreading and motility, but the outcome of Kindlin-3 deficiency on rapid spontaneous versus chemokine-triggered integrin adhesion under physiologic conditions of shear flow relevant for LAD-III pathophysiology has not been dissected. These recent studies also did not assess these questions in subgroups of LAD-III patients of distinct genetic background, and therefore, could not compare the contribution of Kindlin-3 and CDGI to rapid integrin activation processes.
In this study, we describe the effect of genetically distinct LAD-III mutations on integrin-mediated adhesive processes in multiple types of primary leukocytes, and activated lymphocytes. Interestingly, expanded patient peripheral blood lymphocytes (PBLs) that originally lacked CDGI expression gain normal CDGI levels in both activated T cells and Epstein-Barr virus (EBV)–transformed B cells. Whereas lack of Kindlin-3 alone in either resting or activated lymphocytes results in major defects in both intrinsic (spontaneous) and inside-out activation of leukocyte function-associated antigen-1 (LFA-1) under physiologic conditions of shear flow, the intrinsic adhesiveness of the second major lymphocyte integrin, very late activation antigen-4 (VLA-4), is largely conserved in LAD-III Kindlin-3–deficient lymphocytes. Collectively, our results suggest that lymphocyte Kindlin-3 is more critical to LFA-1 than to VLA-4 adhesiveness, raising functional hierarchies of Kindlin-3 usage in the coactivation of distinct integrins coexpressed in the same cellular background.
Methods
Reagents and antibodies
Recombinant 7–domain human vascular cell adhesion molecule (VCAM)–1, soluble VCAM-1, and BIO-1211, a VLA-4–specific blocker,17 were kindly provided by B. Pepinsky (Biogen Idec). Intercellular adhesion molecule (ICAM)–Fc, VCAM-1-Fc, and CXC chemokine ligand (CXCL)12 were purchased from R&D Systems. Bovine serum albumin (fraction V), protein A, Ca2+- and Mg2+-free Hanks balanced salt solution, and anti-talin monoclonal antibody (mAb; clone 8d4) were from Sigma-Aldrich. The anti-ß2 integrin subunit mAb TS1.18, the anti–LFA-1 TS2.4, and the anti–Mac-1 integrin mAb CBRM1/218 were gifts from T. Springer (Harvard University, Cambridge, MA). Anti-β2 integrin neoepitope 327C mAb19 was a gift from D. Staunton (ICOS Corporation). The KIM127 mAb20 was a gift of M. Robinson (UCB Celltech). The β1 activation reporter HUTS2121 was a kind gift from C. Cabanas (Ciudad University). Anti–Kindlin-3 antibody was raised against peptide 156-170 of human Kindlin-3.16 Monoclonal (clone 18B11) and rabbit polyclonal anti-CDGI antibodies were provided by Ann Graybiel (Massachusetts Institute of Technology, Cambridge, MA). The anti-CD3 mAb OKT3 was from Biolegend. Anti-extracellular signal-regulated kinase (ERK)–2 antiserum (clone C-14) and anti-actin antibody (sc-1616) were from Santa Cruz Biotechnology. Anti-phospho–AKT antibody was purchased from Cell Signaling Technology. R-phycoerythrin–conjugated mouse anti–human CD49d (α4) or anti–human CD11 (αL) were from Southern Biotechnology Associates. Peroxidase-labeled, RPE-conjugated, and phycoerythrin-labeled secondary antibodies were all from Jackson ImmunoResearch Laboratories.
Cell isolation and culture
Human PBLs were isolated and purified from citrate anticoagulated whole blood from healthy donors, as described.22 All lymphocytes used consisted of more than 90% CD3+ T lymphocytes. Stably transfected β1 integrin-deficient Jurkat A1 lines were maintained, as described.23 Effector T cells were derived by T-cell receptor (TCR)/CD28 activation for 3 days, followed by expansion in IL-2–supplemented medium for 7 days, as described.24 The Weizmann Review Board–approved research and informed consent were obtained according to the Declaration of Helsinki.
Immunofluorescence and flow cytometry
Cells were washed once with cation-free H/H medium (Hanks balanced salt solution containing 2 mg/mL bovine serum albumin and 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4), resuspended in binding medium (H/H medium supplemented with 1 mM CaCl2 and 1 mM MgCl2), and incubated with primary antibodies (10 μg/mL) for 30 minutes at 4°C. The samples were then washed, incubated with secondary antibodies, and analyzed immediately on a FACScan flow cytometer (BD Biosciences). To probe the basal and agonist-stimulated expression of the activation reporter epitopes mAbs 327C, KIM127, and HUTS21, cells were incubated with the mAbs (10 μg/mL) in binding medium for 5 minutes at 37°C in the presence or absence of agonists. Cells were then washed, incubated with secondary antibodies at 4°C, and analyzed, as above.
Western blot analyses
For Western blot studies, 107 cells (treated or untreated) were solubilized in 100 μL lysis buffer,25 and 20 μL lysates was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis in reducing buffer. Blots were developed using enhanced chemiluminescence (Sigma-Aldrich).
Soluble VCAM-1-Fc–binding assay
Cells (0.5 × 106) were incubated with VCAM-1-Fc for 30 minutes at room temperature in H/H-binding medium, washed twice, incubated with phycoerythrin-donkey anti–human immunoglobulin G (Jackson ImmunoResearch Laboratories) for an additional 20 minutes, washed, and analyzed by fluorescence-activated cell sorter (FACS).
Laminar flow adhesion assays
Adhesion assays were performed on directly coated soluble VCAM or on ICAM-Fc or VCAM-1-Fc overlaid on precoated protein A polystyrene plates.12,22 Site densities were determined by radiolabeled mAbs, as described.22 Substrate-coated polystyrene plates were each assembled on the lower wall of a standard flow chamber (260-μm gap), as described.22 Cells were washed with H/H medium, resuspended in binding medium, pretreated for 1 minute with agonists of interest, and perfused through the flow chamber at 37°C. Tethers were defined as transient if cells attached briefly (< 2 seconds) to the substrate, and as arrests if they remained stationary during at least 3 seconds of continuous flow.22
Statistical analysis
For statistical comparison between groups, the paired 2-tailed Student t test was used. Analyses were performed using the statistics tool of Microsoft Excel.
Results
Kindlin-3 expression is lost in genetically distinct LAD-III patients
A female baby with a LAD-III phenotype, a sibling of a previously reported LAD-III boy of Palestinian origin,11 was shown to carry an autosomal recessive stop codon in Kindlin-3 (nucleotide 687, TG[b]G > TG[b]A; Figure 1A); no Kindlin-3 could be detected in her blood (Figure 1B). Interestingly, the patient (in this study, patient B) expressed normal levels of CDGI. Kindlin-3 expression was also lost in a previously characterized Turkish girl, LAD-III patient A (Figure 1C),12 recently reported by us to display a distinct autosomal recessive mutation in Kindlin-3 (nucleotide 1632 [CGA > TGA]; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). This patient and a second Turkish patient (LAD-III-K) share identical homozygous splice junction mutations in CDGI12,13 and in Kindlin-3 with a large group of Turkish patients.14,16 Kindlin-3 is expressed in the same locus as CDGI (11q13), although the 2 genes are 503 029 bp apart,14 suggesting that both mutations were inherited as a common allele in all of these patients. Indeed, a third Turkish patient, LAD-III-C, with identical mutations to all other published Turkish patients, lacked expression of both CDGI and Kindlin-3 in his resting T cells (Figure 1C, supplemental Figure 2, and data not shown). Unfortunately, this patient died before further functional analysis of his leukocytes could be performed. Importantly, LAD-III-B, as well as in other LAD-III patients of non-Turkish origin, expresses intact CDGI14,16 (supplemental Figure 2). We therefore conclude that the Turkish patients who lack both CDGI and Kindlin-3 expression in their primary leukocytes (Figure 1C) constitute a LAD-III subgroup.
Kindlin-3 is lost in LAD-III patients, whereas CDGIexpression is restricted to primary leukocytes of a subset of LAD-III patients. (A) Multiple alignment of genomic DNA sequence (chr11:63735681-63735711, National Center for Biotechnology Information Build 36) surrounding the putative stop codon mutation within the Kindlin-3 gene. (B) Lysates of whole blood cells either derived from a control donor or from LAD-III-B patient were immunoblotted with anti–Kindlin-3 Ab, anti-CDGI mAb, or anti–total ERK2 antibody as a control. (C) Lysates of resting T cells from control, LAD-III-A, or LAD-III-C patients were immunoblotted with anti–Kindlin-3 antibody, anti-CDGI mAb (Mono), anti-CDGI antibody (Poly), and anti–total ERK2 Ab. (Di) Lysates of effector T cells derived from control and LAD-III-A patients after the indicated number of days of in vitro expansion were each immunoblotted with anti–Kindlin-3, anti-CDGI mAb, anti-CDGI polyclonal Ab, and anti-actin antibody as a control. (Dii) Lysates of control and LAD-III-A effector T cells derived after 8 days of expansion in vitro were immunoblotted with anti–Kindlin-3 Ab, anti-CDGI mAb, and anti–total ERK2 Ab, as in panel B.
Kindlin-3 is lost in LAD-III patients, whereas CDGIexpression is restricted to primary leukocytes of a subset of LAD-III patients. (A) Multiple alignment of genomic DNA sequence (chr11:63735681-63735711, National Center for Biotechnology Information Build 36) surrounding the putative stop codon mutation within the Kindlin-3 gene. (B) Lysates of whole blood cells either derived from a control donor or from LAD-III-B patient were immunoblotted with anti–Kindlin-3 Ab, anti-CDGI mAb, or anti–total ERK2 antibody as a control. (C) Lysates of resting T cells from control, LAD-III-A, or LAD-III-C patients were immunoblotted with anti–Kindlin-3 antibody, anti-CDGI mAb (Mono), anti-CDGI antibody (Poly), and anti–total ERK2 Ab. (Di) Lysates of effector T cells derived from control and LAD-III-A patients after the indicated number of days of in vitro expansion were each immunoblotted with anti–Kindlin-3, anti-CDGI mAb, anti-CDGI polyclonal Ab, and anti-actin antibody as a control. (Dii) Lysates of control and LAD-III-A effector T cells derived after 8 days of expansion in vitro were immunoblotted with anti–Kindlin-3 Ab, anti-CDGI mAb, and anti–total ERK2 Ab, as in panel B.
To further compare integrin properties on leukocytes derived from the 2 subgroups, represented by the LAD-III-B patient (Kindlin-3 negative, CDGI normal) and by patient LAD-III-A (defective in both Kindlin-3 and CDGI), we expanded in vitro resting T cells from both patients. Surprisingly, within a day of activation by anti-CD3/anti-CD28 mAbs, T blasts derived from LAD-III patient A (Kindlin-3 and CDGI negative) acquired partial expression of CDGI, which reached normal levels after 4 days of activation, as confirmed using 2 distinct CDGI antibody probes (Figure 1Di). These activated/effector T cells also maintained normal levels of CDGI mRNA (supplemental Figure 3), but, as expected, lacked any detectable Kindlin-3 (Figure 1Dii). As splice junction mutations are known to exert variable effects on mRNA stabilities depending on the cellular environment,26 these results suggest that upon activation by antigens, CDGI transcription and translation override the splice junction mutation of the Turkish LAD-III patients.12 Thus, in vivo, subsets of patient T cells may regain expression of CDGI and normal Rap-1 signaling.
T-cell Kindlin-3 is critical for LFA-1 activation, but not for VLA-4 activation by chemokines
A hallmark of the functional regulation of LFA-1 integrins by chemokine signals are 2 main conformational switches in the ectodomain of its β2 subunit.27,28 We next analyzed the ability of the LFA-1 integrin in normal and in Kindlin-3–null effector T cells to undergo these inside-out (chemokine-mediated) conformational switches induced by the prototypic chemokine, CXCL12. The expression of both LFA-1 integrin subunits, as well as of CXC chemokine receptor (CXCR)4, the Gi-coupled receptor for CXCL12, was normal on effector LAD-III T cells (Figure 2A, and data not shown). As LFA-1 is the predominant β2 integrin member expressed by these effectors, any conformational change in the β2 integrin subunit reflects an inside-out activation of LFA-1. Despite normal CXCR4 expression and intact CXCL12 signaling to CXCR4 in Kindlin-3–null LAD-III effectors (Figure 2B), the ability of LFA-1 to undergo extension and headpiece opening in response to CXCL12 signals, detected by the conformation-specific antibodies KIM127 and 327C, respectively, was dramatically reduced (Figure 2Ci). LFA-1 conformational activation by phorbol esters (phorbol myristate acetate [PMA]), potent agonists of chemokine-independent integrin activation,29 was also abrogated in all tested Kindlin-3–null LAD-III T cells (Figure 2Ci, and data not shown). Importantly, artificial activation of LFA-1 with Mg2+ ions was normal (Figure 2Cii). Notably, transient expression of murine green fluorescent protein (GFP)–Kindlin-3, but not of GFP alone, fully rescued the ability of LFA-1 in LAD-III patient T cells to undergo conformational activation by chemokine signals (Figure 2D). In agreement with the impaired ability of LFA-1 in Kindlin-3–null effector T cells to undergo inside-out conformational activation, LFA-1 also failed to undergo activation by rapid in situ signals from coimmobilized CXCL12 (Figure 2E). Because this inside-out activation takes place within less than 0.5-second contact periods,27 we next allowed healthy and LAD-III effector T cells to settle for 1 minute on the ICAM-1/CXCL12-coated substrate and tested whether such prolonged chemokine and ICAM-1 signaling to LFA-1 at the contact area can partially rescue the LFA-1 activation defect of LAD-III lymphocytes. Remarkably, even after prolonged contact with ICAM-1 and chemokine or PMA, Kindlin-3–null LAD-III cells failed to develop any detectable adhesion (Figure 2F). Thus, T cells deficient in Kindlin-3 expression exhibit major defects in both conformational activation and acquisition of both transient and firm adhesiveness of their LFA-1 by chemokine and phorbol ester signals transduced during a large range of contact periods.
Inside-out LFA-1 activation is lost in Kindlin-3–deficient LAD-III T cells, whereas partial VLA-4 activation is retained. (A) FACS staining of αL, β2, and CXCR4 of LAD-III and control effector T cells. (B) Chemokine signaling in LAD-III lymphocytes. Control and LAD-III effector T cells were left intact or stimulated for 1 to 5 minutes with CXCL12 (10 nM) at 37°C. Cell lysates were immunoblotted with anti–phospho-AKT and anti-talin mAb. (Ci) T cells were stimulated for 5 minutes with PMA (100 ng/mL) or with CXCL12 (10 nM), as in panel B. Expression levels of either the extension-specific epitope KIM127 or the high affinity β2 headpiece epitope 327C are shown for control and LAD effector T cells before or after stimulation with CXCL12 (c) or with PMA (p). (Cii) 327C epitope expression on control and LAD-III effector T cells suspended either in physiologic medium or in Mg2+-EGTA (ethyleneglycoltetraacetic acid) medium (Mg++). (D) Basal and CXCL12-stimulated induction of the 327C epitope in LAD-III effector T cells transfected with either GFP or Kindlin-3–GFP. For comparison, CXCL12-stimulated induction of 327C is shown on control effector T cells transfected with GFP. MFI indicates mean fluorescent intensity. (E) Attachment (tethering) and immediate arrest of control and LAD-III effector T cells on ICAM-1-Fc (95 cell adhesion molecule [CAM] sites/μm2) triggered by immobilized CXCL12 (2 μg/mL). The frequency of transient and firm tethers was determined in 2 fields of view, and the depicted values are the mean ± range. The experiment shown is representative of 4. (F) Effects of immobilized CXCL12 or of lymphocyte pretreatment with PMA on LFA-1–dependent adhesion strengthening of control and LAD-III effector T cells. Lymphocytes were settled on ICAM- 1 (95 CAM sites/μm2) for 1 minute and then subjected to incremented shear forces. The percentage of initially settled T cells that resisted detachment from the substrate at the indicated shear forces was determined in 2 fields of view, and values shown represent the mean ± range. The experiment shown is representative of 3. **P < .001 for the compared experimental groups. (G) Attachment and immediate arrest of control and LAD-III effector T cells on VCAM-1 (coated at 1 μg/mL) triggered by immobilized CXCL12 (2 μg/mL) measured at a shear stress of 0.75 dyn/cm2 in 2 fields of view. **P < .001 for firm tethers of LAD-III versus control cells. (Right panel) Expression of the activation β1 epitope HUTS21 on control effector T cells before and after stimulation with CXCL12, PMA, or Mg2+-EGTA medium, as described in panel C. (A-F) LAD-III effector lymphocytes were from patient B. (G) LAD-III effectors were from patient A.
Inside-out LFA-1 activation is lost in Kindlin-3–deficient LAD-III T cells, whereas partial VLA-4 activation is retained. (A) FACS staining of αL, β2, and CXCR4 of LAD-III and control effector T cells. (B) Chemokine signaling in LAD-III lymphocytes. Control and LAD-III effector T cells were left intact or stimulated for 1 to 5 minutes with CXCL12 (10 nM) at 37°C. Cell lysates were immunoblotted with anti–phospho-AKT and anti-talin mAb. (Ci) T cells were stimulated for 5 minutes with PMA (100 ng/mL) or with CXCL12 (10 nM), as in panel B. Expression levels of either the extension-specific epitope KIM127 or the high affinity β2 headpiece epitope 327C are shown for control and LAD effector T cells before or after stimulation with CXCL12 (c) or with PMA (p). (Cii) 327C epitope expression on control and LAD-III effector T cells suspended either in physiologic medium or in Mg2+-EGTA (ethyleneglycoltetraacetic acid) medium (Mg++). (D) Basal and CXCL12-stimulated induction of the 327C epitope in LAD-III effector T cells transfected with either GFP or Kindlin-3–GFP. For comparison, CXCL12-stimulated induction of 327C is shown on control effector T cells transfected with GFP. MFI indicates mean fluorescent intensity. (E) Attachment (tethering) and immediate arrest of control and LAD-III effector T cells on ICAM-1-Fc (95 cell adhesion molecule [CAM] sites/μm2) triggered by immobilized CXCL12 (2 μg/mL). The frequency of transient and firm tethers was determined in 2 fields of view, and the depicted values are the mean ± range. The experiment shown is representative of 4. (F) Effects of immobilized CXCL12 or of lymphocyte pretreatment with PMA on LFA-1–dependent adhesion strengthening of control and LAD-III effector T cells. Lymphocytes were settled on ICAM- 1 (95 CAM sites/μm2) for 1 minute and then subjected to incremented shear forces. The percentage of initially settled T cells that resisted detachment from the substrate at the indicated shear forces was determined in 2 fields of view, and values shown represent the mean ± range. The experiment shown is representative of 3. **P < .001 for the compared experimental groups. (G) Attachment and immediate arrest of control and LAD-III effector T cells on VCAM-1 (coated at 1 μg/mL) triggered by immobilized CXCL12 (2 μg/mL) measured at a shear stress of 0.75 dyn/cm2 in 2 fields of view. **P < .001 for firm tethers of LAD-III versus control cells. (Right panel) Expression of the activation β1 epitope HUTS21 on control effector T cells before and after stimulation with CXCL12, PMA, or Mg2+-EGTA medium, as described in panel C. (A-F) LAD-III effector lymphocytes were from patient B. (G) LAD-III effectors were from patient A.
LFA-1 and VLA-4 activation by chemokine signals involves distinct conformational changes.30 As LAD-III T cells retained normal expression of VLA-4 (supplemental Figure 4), we next tested the ability of this integrin to undergo rapid in situ stimulation by CXCL12 under shear flow. Surprisingly, in contrast to LFA-1, VLA-4 in LAD-III effector T cells underwent significant stimulation of transient adhesions (Figure 2G) by the same in situ CXCL12 signals, which failed to elicit any LFA-1 stimulation under shear flow (Figure 2E). Indeed, the total number of CXCL12-triggered VLA-4 interactions with VCAM-1 observed with Kindlin-3–null T cells was comparable with that observed in control T cells (Figure 2G). Nevertheless, only a diminished fraction of CXCL12-stimulated VLA-4–mediated attachments underwent stabilization in LAD-III effector T cells (Figure 2G). Kindlin-3–null T cells also failed to generate resistance to detachment upon prolonged contacts with VCAM-1 and CXCL12 (data not shown). Notably, we could not attribute chemokine-mediated stimulation of firm VLA-4 adhesions (arrests) in control T cells to a chemokine-induced conformational switch in the integrin ectodomain during physiologically relevant exposure periods of 1 to 5 minutes (Figure 2G inset). Consistent with this notion, chemokine stimuli also failed to enhance the binding of soluble VCAM-1 to effector T cells (data not shown). Collectively, our results suggest that normal chemokine-stimulated VLA-4 adhesions are initiated by Kindlin-3–null LAD-III effector T cells, but fail to undergo subsequent stabilization. Thus, Kindlin-3 deficiency leads to major defects in chemokine stimulation of all LFA-1–mediated adhesions, as well as in firm chemokine-stimulated VLA-4 arrests.
Kindlin-3 is critical for intrinsic LFA-1, but not for VLA-4 adhesiveness in effector T cells
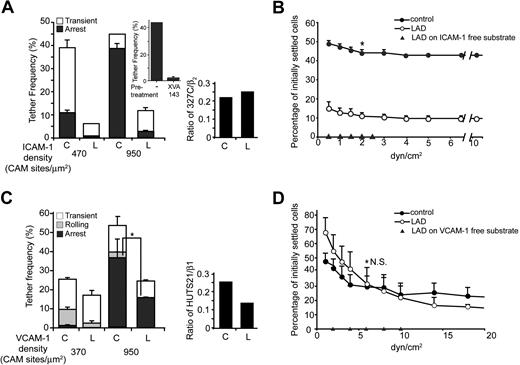
Chemokine stimulation of LFA-1 can facilitate outside-in (ligand-induced) conformational activation, which takes place more readily under shear forces.31,32 We therefore next assessed whether loss of Kindlin-3 in LAD-III effector T cells also interferes with this rapid outside-in LFA-1 activation, and therefore reduces spontaneous LFA-1–mediated tethering and arrest on ICAM-1 developed under continuous application of shear forces on the interacting T cells. As indicated in Figure 3A, the intrinsic adhesiveness of LFA-1 in Kindlin-3–null T cells to ICAM-1 under shear flow was almost eliminated (Figure 3A). In contrast, LFA-1 on healthy effector T cells readily tethered on ICAM-1, and these tethers were progressively stabilized into firm arrests on ICAM-1 via the rapid allosteric activation of the LFA-1 α-I-domain. Indeed, interference with the opening of this domain in the presence of the allosteric inhibitor XVA143 completely abolished these LFA-1–mediated arrests (Figure 3A top inset). Nevertheless, the fraction of LFA-1 that pre-exists in the high affinity conformation before contact with ICAM-1, although small, was similar in Kindlin-3–null LAD-III and in healthy T cells (Figure 3A right inset). Thus, LFA-1 on Kindlin-3–null T cells, although conformationally active before ICAM-1 binding, is nonadhesive to ICAM-1, in addition to its inability to undergo conformational activation by inside-out chemokine signals (Figure 2Ci). Nevertheless, this defect in adhesiveness and ligand-mediated activation of LFA-1 by ICAM-1 could be partially rescued by prolonged interaction with ICAM-1, because a fraction of LAD-III effector T cells settled for 1 minute on high density ICAM-1 developed significant adhesion strengthening, that is, resistance to detachment from ICAM-1 by incremented shear forces (Figure 3B).
Intrinsic LFA-1 adhesiveness is dramatically reduced in Kindlin-3–deficient T cells, whereas VLA-4 adhesiveness is retained. (A) Spontaneous LFA-1 adhesiveness of control and LAD-III–derived effector T cells to medium and high densities of ICAM-1. Frequencies of transient and firm attachments (tethers) measured at a shear stress of 0.5 dyn/cm2 in 2 fields are depicted for the indicated groups. C indicates control; L, LAD-III. (Top inset) Effect of blocking ICAM-1–induced I-domain activation of LFA-1 with the allosteric inhibitor, XVA143, on the arrest fraction of control effector lymphocytes attaching to the high density ICAM-1. (Right panel) The fraction of total β2 integrin (stained by the TS1.18 mAb) that expresses the 327C epitope was compared between control and LAD-III–derived effector T cells. (B) Spontaneous LFA-1–dependent adhesion strengthening developed by effector cells settled for 1 minute on ICAM-1 and subjected to detachment by increasing shear forces was determined, as in panel D. *P < .05 for the percentage of initially settled control versus LAD-III cells that remained adhered at 2 dyn/cm2. (C) Spontaneous tethering (transient, rolling, or firm arrest) of cells interacting with medium and high density VCAM-1 determined at a shear stress of 0.75 dyn/cm2. The frequency and strength of all tethers were determined in 2 fields. Results are given as the mean ± range. *P < .05 for firm tethers of LAD-III versus control cells. (Right panel) The fraction of total β1 integrin (stained by the β1 mAb TS2.16) that expresses the activation neoepitope HUTS21 was compared between control and LAD-III–derived effector T cells. (D) VLA-4–dependent adhesion strengthening of control and LAD-III effector T cells. Lymphocytes were settled on VCAM-1 for 1 minute and then subjected to incremented shear forces. The percentage of initially settled T cells that resisted detachment from the substrate at the indicated shear forces was determined, as in panel B. N.S. indicates a nonsignificant P value. Data shown in panels A through D are each representative of 3 experiments, and all LAD-III effector lymphocytes were from patient B.
Intrinsic LFA-1 adhesiveness is dramatically reduced in Kindlin-3–deficient T cells, whereas VLA-4 adhesiveness is retained. (A) Spontaneous LFA-1 adhesiveness of control and LAD-III–derived effector T cells to medium and high densities of ICAM-1. Frequencies of transient and firm attachments (tethers) measured at a shear stress of 0.5 dyn/cm2 in 2 fields are depicted for the indicated groups. C indicates control; L, LAD-III. (Top inset) Effect of blocking ICAM-1–induced I-domain activation of LFA-1 with the allosteric inhibitor, XVA143, on the arrest fraction of control effector lymphocytes attaching to the high density ICAM-1. (Right panel) The fraction of total β2 integrin (stained by the TS1.18 mAb) that expresses the 327C epitope was compared between control and LAD-III–derived effector T cells. (B) Spontaneous LFA-1–dependent adhesion strengthening developed by effector cells settled for 1 minute on ICAM-1 and subjected to detachment by increasing shear forces was determined, as in panel D. *P < .05 for the percentage of initially settled control versus LAD-III cells that remained adhered at 2 dyn/cm2. (C) Spontaneous tethering (transient, rolling, or firm arrest) of cells interacting with medium and high density VCAM-1 determined at a shear stress of 0.75 dyn/cm2. The frequency and strength of all tethers were determined in 2 fields. Results are given as the mean ± range. *P < .05 for firm tethers of LAD-III versus control cells. (Right panel) The fraction of total β1 integrin (stained by the β1 mAb TS2.16) that expresses the activation neoepitope HUTS21 was compared between control and LAD-III–derived effector T cells. (D) VLA-4–dependent adhesion strengthening of control and LAD-III effector T cells. Lymphocytes were settled on VCAM-1 for 1 minute and then subjected to incremented shear forces. The percentage of initially settled T cells that resisted detachment from the substrate at the indicated shear forces was determined, as in panel B. N.S. indicates a nonsignificant P value. Data shown in panels A through D are each representative of 3 experiments, and all LAD-III effector lymphocytes were from patient B.
We next addressed whether loss of Kindlin-3 in LAD-III T cells may also affect spontaneous VLA-4 adhesiveness to VCAM-1 developed under distinct dynamic conditions. In contrast to the diminished intrinsic LFA-1 adhesiveness in Kindlin-3–null T cells observed under continuous shear flow (Figure 3A), VLA-4 in these T cells could still support nearly normal tethering and considerable level of arrests on VCAM-1 (Figure 3C). Expression of the activation epitope, HUTS21, associated with high affinity conformation of β1 integrins was also reduced in LAD-III cells (Figure 3C inset). Nevertheless, and in sharp contrast to LFA-1 (Figure 3B), VLA-4 on Kindlin-3–null effector T cells developed normal adhesion strengthening and resistance to detachment by incremented shear forces after settling for 1 minute on VCAM-1 under shear-free conditions (Figure 3D). These results suggest that the ability of VLA-4 to undergo activation by VCAM-1 is largely conserved in Kindlin-3–null LAD-III effector T cells. Thus, VLA-4 adhesiveness to VCAM-1 developed under shear flow conditions is only marginally perturbed in LAD-III effector T cells, although inside-out stimulation of VLA-4 by chemokine signals is significantly reduced (Figure 2G).
Kindlin-3 is essential for intrinsic LFA-1, but not for VLA-4 adhesiveness in activated B cells
To gain further insight into these distinct impairments in VLA-4 versus LFA-1 adhesiveness displayed by Kindlin-3–deficient LAD-III T cells, we next analyzed these 2 integrins in another cell type, EBV-transformed B lymphoblasts derived from either LAD-III or control PBL. Similar to effector T cells, B blasts derived from LAD-III patient A carrying the splice junction CDGI mutation expressed normal levels of CDGI protein, but lacked Kindlin-3 expression (Figure 4A). LAD-III EBV cells also expressed normal CXCR4, α4 integrins, and β2 integrins (Figure 4B, and data not shown). The predominant β2 molecule on these cells was LFA-1, because Mac-1 expression was negligible. LAD-III EBV cells also expressed normal levels of the β2 integrin activation epitope, 327C, and of the β1 activation epitope, HUTS21 (Figure 4Bii, and data not shown). Interestingly, in spite of high CXCR4 levels, LFA-1 on healthy EBV-transformed B cells did not undergo detectable conformational activation when exposed to CXCL12 (Figure 4Bii), in sharp contrast to effector T cells (Figure 2C). Protein kinase C activation by PMA also marginally switched LFA-1 to high affinity conformation probed by 327C staining (data not shown), suggesting that LFA-1 chemokine-mediated activation is severely impaired in these cells. Further analysis was therefore focused on comparing intrinsic LFA-1 adhesiveness to ICAM-1 in EBV-transformed cells from healthy donors versus LAD-III patients. Similar to effector T cells (Figure 3A), LFA-1 on Kindlin-3–deficient LAD-III EBV blasts failed to attach to ICAM-1 even at the highest coating densities (Figure 4C). In contrast, in these LAD-III EBV blasts, VLA-4 interacted as efficiently as in healthy EBV cells, and supported efficient rolling adhesions, despite a defect in firm arrest (Figure 4D). Thus, intrinsic VLA-4–mediated tethering and rolling on VCAM-1 in B blasts are conserved also in Kindlin-3–deficient EBV cells, in contrast to intrinsic LFA-1 adhesiveness.
Intrinsic LFA-1 adhesiveness, but not VLA-4 adhesiveness is diminished in Kindlin-3–null LAD-III EBV cells. (A) Loss of Kindlin-3, but not of CDGI, in LAD-III EBV cells derived from patient A. Lysates of control and LAD-III EBV lymphoblasts were immunoblotted with anti–Kindlin-3 Ab, anti-CDGI mAb, and anti–total ERK2 Ab. (Bi) FACS staining of β2 and CXCR4 on EBV lymphoblasts. (Bii) Basal and CXCL12-stimulated (c) or Mg2+-EGTA–stimulated (Mg) induction of the 327C β2 epitope in control and LAD-III EBV lymphoblasts. (C) Spontaneous LFA-1 adhesiveness to high density ICAM-1-Fc by control and LAD-III EBV lymphoblasts under low shear stress (0.5 dyn/cm2). Frequency of transient and firm attachments determined in 2 fields of view for each group of cells is depicted as mean values ± range. (D) Spontaneous attachments (transient, rolling, or arrest) of EBV cells interacting with the indicated densities of VCAM-1-Fc at a shear stress of 0.75 dyn/cm2. The frequency and strength of all tethers were determined, as in panel C. Data shown in panels C and D are each representative of 3 experiments.
Intrinsic LFA-1 adhesiveness, but not VLA-4 adhesiveness is diminished in Kindlin-3–null LAD-III EBV cells. (A) Loss of Kindlin-3, but not of CDGI, in LAD-III EBV cells derived from patient A. Lysates of control and LAD-III EBV lymphoblasts were immunoblotted with anti–Kindlin-3 Ab, anti-CDGI mAb, and anti–total ERK2 Ab. (Bi) FACS staining of β2 and CXCR4 on EBV lymphoblasts. (Bii) Basal and CXCL12-stimulated (c) or Mg2+-EGTA–stimulated (Mg) induction of the 327C β2 epitope in control and LAD-III EBV lymphoblasts. (C) Spontaneous LFA-1 adhesiveness to high density ICAM-1-Fc by control and LAD-III EBV lymphoblasts under low shear stress (0.5 dyn/cm2). Frequency of transient and firm attachments determined in 2 fields of view for each group of cells is depicted as mean values ± range. (D) Spontaneous attachments (transient, rolling, or arrest) of EBV cells interacting with the indicated densities of VCAM-1-Fc at a shear stress of 0.75 dyn/cm2. The frequency and strength of all tethers were determined, as in panel C. Data shown in panels C and D are each representative of 3 experiments.
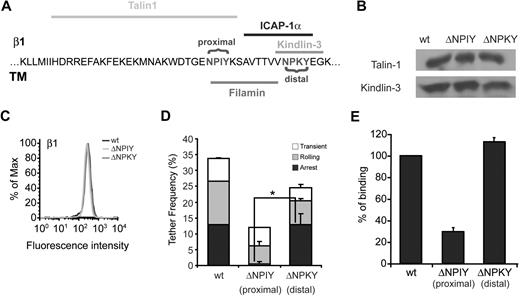
The Kindlin-3–binding NPKY motif on the β1 tail is not required for VLA-4 affinity and intrinsic adhesiveness
Intrinsic VLA-4 adhesiveness and outside-in activation by high density VCAM-1 in T cells require intact talin1.33 Talin1 binds VLA-4 at the β subunit tail near the membrane-proximal NPXY motif, NPIY, whereas the putative Kindlin-3 binding site on this subunit is located close to the membrane distal NPKY site8 (Figure 5A). Based on the retained VLA-4–mediated tethering and rolling on VCAM-1 displayed by both Kindlin-3–null T and B lymphoblasts, we hypothesized that deletion of this membrane distal NPKY site would not affect VLA-4 binding to VCAM-1, whereas deletion of the talin1 binding site was likely to abrogate binding. We therefore next analyzed VLA-4 affinity and adhesiveness to VCAM-1 in β1-deficient Jurkat T cells (Jurkat A134 ) transfected with either wild-type (wt) β1 integrin or the β1 tail mutants ΔNPIY and ΔNPKY,23 containing deletions comprising the proximal NPIY or the distal NPKY motifs, respectively. All β1-transfected Jurkat cells were validated to express normal talin1 and Kindlin-3 as well as normal α4 (Figure 5B, and data not shown). Notably, whereas the ΔNPIY β1 VLA-4 mutant poorly tethered and failed to firmly arrest on VCAM-1 under shear flow conditions (Figure 5D), consistent with previous results,23 all adhesive categories of the VLA-4 ΔNPKY mutant were only mildly reduced (Figure 5D). VLA-4 containing a deletion of the talin1-binding NPIY β1 motif also exhibited poor binding to soluble VCAM-1, whereas the VLA-4 containing the ΔNPKY β1 deletion bound normally to soluble VCAM-1 (Figure 5E and supplemental Figure 5). Thus, deletion of the putative Kindlin-binding membrane distal NPKY site of VLA-4 does not perturb VLA-4 affinity or adhesiveness to VCAM-1 under shear flow.
The putative Kindlin-3 binding site on the β tail of VLA-4 is not required for VLA-4 affinity or adhesiveness to VCAM-1. (A) Sequence of β1 integrin subunit, showing its putative association sites with Kindlin-3, talin1, filamin, and ICAP-1 in leukocytes. The 2 NPXY binding sites are highlighted. (B) Kindlin-3 and talin1 expression in Jurkat A1 cells (β1 integrin deficient) reconstituted with either wt β1 (wt), β1ΔNPIY (ΔNPIY), or β1ΔNPKY (ΔNPIY). Lysates of each Jurkat transfectant were immunoblotted with anti–Kindlin-3 antibody and anti-talin mAb. (C) FACS staining of β1 on the various Jurkat A1 transfectants. α4 expression was identical on all transfectants (data not shown). (D) Frequency and strength of tethers mediated by Jurkat β1 wt transfectants, Jurkat β1ΔNPIY, or Jurkat β1ΔNPKY transfectants perfused over medium density soluble VCAM-1 (370 sites/μm2). All interactions were determined at a shear stress of 0.75 dyn/cm2 in 2 fields of view, and results shown are the mean ± range. *P < .01 for firm tethers of the compared experimental groups. (E) Binding of soluble VCAM-1-Fc (at a saturating concentration of 60 μM) to Jurkat β1 wt cells, Jurkat β1ΔNPIY, and β1ΔNPKY transfectants, detected by fluorescence staining. No VCAM-1 binding could be detected in the presence of the VLA-4 blocker, Bio1211 (data not shown). Data are representative of 2 independent experiments.
The putative Kindlin-3 binding site on the β tail of VLA-4 is not required for VLA-4 affinity or adhesiveness to VCAM-1. (A) Sequence of β1 integrin subunit, showing its putative association sites with Kindlin-3, talin1, filamin, and ICAP-1 in leukocytes. The 2 NPXY binding sites are highlighted. (B) Kindlin-3 and talin1 expression in Jurkat A1 cells (β1 integrin deficient) reconstituted with either wt β1 (wt), β1ΔNPIY (ΔNPIY), or β1ΔNPKY (ΔNPIY). Lysates of each Jurkat transfectant were immunoblotted with anti–Kindlin-3 antibody and anti-talin mAb. (C) FACS staining of β1 on the various Jurkat A1 transfectants. α4 expression was identical on all transfectants (data not shown). (D) Frequency and strength of tethers mediated by Jurkat β1 wt transfectants, Jurkat β1ΔNPIY, or Jurkat β1ΔNPKY transfectants perfused over medium density soluble VCAM-1 (370 sites/μm2). All interactions were determined at a shear stress of 0.75 dyn/cm2 in 2 fields of view, and results shown are the mean ± range. *P < .01 for firm tethers of the compared experimental groups. (E) Binding of soluble VCAM-1-Fc (at a saturating concentration of 60 μM) to Jurkat β1 wt cells, Jurkat β1ΔNPIY, and β1ΔNPKY transfectants, detected by fluorescence staining. No VCAM-1 binding could be detected in the presence of the VLA-4 blocker, Bio1211 (data not shown). Data are representative of 2 independent experiments.
CDGI and Kindlin-3 play additive roles in rapid TCR-stimulated LFA-1 adhesion
TCR ligation is another robust modality of inside-out LFA-1 activation. LFA-1 on primary resting T cells deficient in both Kindlin-3 and CDGI derived from LAD-III-A was indeed completely deficient in its ability to undergo activation of shear-resistant adhesiveness to ICAM-1 by TCR ligation using the stimulatory mAb, OKT3 (Figure 6). Surprisingly, Kindlin-3–deficient, CDGI-expressing T cells derived from the LAD-III-B patient exhibited reduced, but substantial TCR-stimulated LFA-1–dependent adhesion to ICAM-1 (Figure 6). Thus, Kindlin-3 and CDGI additively contribute to optimal LFA-1 activation by rapid inside-out TCR signals. Hence, in vivo, partial TCR signaling to LFA-1 may be retained by Kindlin-3–null T cells. TCR signaling to LFA-1 is also predicted to be partially retained in patient T cells that carry mutations in both Kindlin-3 and CDGI once these cells have undergone in vivo activation and acquired CDGI expression (Figure 1Di).
TCR-stimulated LFA-1–dependent adhesion to ICAM-1 is abrogated only in LAD-III T cells deficient in both Kindlin-3 and CDGI. T cells from the indicated LAD-III patients and control donors were settled for 1 minute on identical ICAM-1-Fc–coated surfaces in the absence or the presence of anti-CD3 mAb (OKT3, 10 μg/mL), and the fractions of initially settled T cells that resisted detachment to a shear stress of 1 dyn/cm2 were each scored in 2 fields of view. Results shown are representative of 2 independent experiments.
TCR-stimulated LFA-1–dependent adhesion to ICAM-1 is abrogated only in LAD-III T cells deficient in both Kindlin-3 and CDGI. T cells from the indicated LAD-III patients and control donors were settled for 1 minute on identical ICAM-1-Fc–coated surfaces in the absence or the presence of anti-CD3 mAb (OKT3, 10 μg/mL), and the fractions of initially settled T cells that resisted detachment to a shear stress of 1 dyn/cm2 were each scored in 2 fields of view. Results shown are representative of 2 independent experiments.
Kindlin-3 deficiency alone is sufficient to abrogate β2 integrin, but not VLA-4 adhesiveness in primary leukocytes
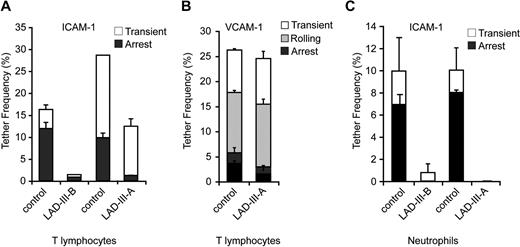
In light of these differences between Kindlin-3–null and Kindlin-3/CDGI–null T cells, we next compared the intrinsic LFA-1 adhesiveness of primary resting T cells derived from either the LAD-III-A or LAD-III-B patients. Interestingly, the defective capacity of LFA-1 to support transient tethers and firm arrests in primary LAD-III-B T cells deficient only in Kindlin-3 was similar to that in LAD-III-A T cells lacking both Kindlin-3 and CDGI (Figures 7A and 1C). Thus, the lack of CDGI did not further impair the primary defect in LFA-1 adhesiveness associated with loss of Kindlin-3. In contrast, VLA-4–mediated tethering, rolling, and arrest on VCAM-1 of resting T cells lacking both Kindlin-3 and CDGI were essentially normal (Figure 7B).
Intrinsic β2 integrin adhesiveness is diminished in both primary T cells and neutrophils derived from distinct LAD-III patients. (A) Spontaneous LFA-1–mediated adhesiveness of control and different LAD-III–derived resting T cells to medium density ICAM-1 (470 CAM sites/μm2). Frequencies of transient and firm attachments measured at a shear stress of 0.5 dyn/cm2 in 2 fields are depicted for the indicated experimental groups. Each LAD-III group was compared with T cells from an age-matched control on an identical ICAM-1-Fc–containing substrate. (B) Spontaneous tethering (transient, rolling, or firm arrest) of control and LAD-III resting T cells (deficient in both Kindlin-3 and CDGI) interacting with high density VCAM-1-Fc (740 CAM sites/μm2) at a shear stress of 0.75 dyn/cm2. The frequency and strength of all tethers were determined in 2 fields of view. All adhesive interactions were eliminated by VLA-4 blocking on T cells (data not shown). (C) Spontaneous β2 integrin-dependent adhesiveness of control and LAD-III–derived neutrophils to medium density ICAM-1 (470 CAM sites/μm2). Each LAD-III group was compared with neutrophils from an age-matched control on an identical ICAM-1–containing substrate. Frequency and strength of all tethers were measured at a shear stress of 0.5 dyn/cm2. All adhesive interactions were eliminated in the presence of the β2 blocking mAb, TS1.18. Results in panels A through C are given as the mean ± range of individual measurements, each a representative of 3 independent experiments.
Intrinsic β2 integrin adhesiveness is diminished in both primary T cells and neutrophils derived from distinct LAD-III patients. (A) Spontaneous LFA-1–mediated adhesiveness of control and different LAD-III–derived resting T cells to medium density ICAM-1 (470 CAM sites/μm2). Frequencies of transient and firm attachments measured at a shear stress of 0.5 dyn/cm2 in 2 fields are depicted for the indicated experimental groups. Each LAD-III group was compared with T cells from an age-matched control on an identical ICAM-1-Fc–containing substrate. (B) Spontaneous tethering (transient, rolling, or firm arrest) of control and LAD-III resting T cells (deficient in both Kindlin-3 and CDGI) interacting with high density VCAM-1-Fc (740 CAM sites/μm2) at a shear stress of 0.75 dyn/cm2. The frequency and strength of all tethers were determined in 2 fields of view. All adhesive interactions were eliminated by VLA-4 blocking on T cells (data not shown). (C) Spontaneous β2 integrin-dependent adhesiveness of control and LAD-III–derived neutrophils to medium density ICAM-1 (470 CAM sites/μm2). Each LAD-III group was compared with neutrophils from an age-matched control on an identical ICAM-1–containing substrate. Frequency and strength of all tethers were measured at a shear stress of 0.5 dyn/cm2. All adhesive interactions were eliminated in the presence of the β2 blocking mAb, TS1.18. Results in panels A through C are given as the mean ± range of individual measurements, each a representative of 3 independent experiments.
Finally, in light of the major deficiency in intrinsic adhesiveness of LFA-1 in resting T cells, we were prompted to compare intrinsic β2 integrin adhesiveness in resting neutrophils deficient in either Kindlin-3 alone (ie, from LAD-III-B) or in both Kindlin-3 and CDGI (ie, from LAD-III-A12 ). Assessing spontaneous β2 integrin-dependent attachments to ICAM-1 of neutrophils from both LAD-III patients, we observed a similar near-complete failure of these integrins to tether and arrest on isolated ICAM-1 (Figure 7C). Thus, lack of CDGI does not further impair β2 integrin adhesiveness in Kindlin-3–null neutrophils. These data further support a general rather than cell-type–dependent role of Kindlin-3 in intrinsic β2 integrin adhesiveness under shear flow.
Discussion
Integrin activation is a complex process regulated by conformational switches that involve unclasping of the integrin subunit tails.2,35 Recent data suggest that application of force on an anchored, ligand-occupied integrin is necessary to achieve maximal separation between the integrin subunit tails.36 Thus, the full acquisition of high affinity binding of the integrin's headpiece to its ligand may require accurate accommodation of unclasping molecules and actin-binding adaptors within the short cytoplasmic interface of integrins. Recent data establish both talin and Kindlins as 2 essential coactivators of integrins in both nonhematopoietic and hematopoietic cells.5-7,9,15 Indeed, both chemokine-triggered and ligand-mediated activation of LFA-1, shown in this study to be defective in LAD-III Kindlin-3–deficient T cells, are impaired in T cells lacking talin1.27 Similarly, VLA-4 activation by chemokines, which is also highly sensitive to talin1 depletion,33 is also impaired in Kindlin-3–deficient LAD T cells. Nevertheless, VLA-4 is far less susceptible than LFA-1 to loss of Kindlin-3, as considerable VLA-4 activation, but not of LFA-1 activation, is retained in Kindlin-3–null effector T cells (supplemental Figure 6). Kindlin-3 may associate less avidly with the VLA-4 β1 subunit than with the LFA-1 β2 subunit in both resting and activated T and B cells. Indeed, deletion of the Kindlin-3 binding site on the β1 tail of VLA-4 results in only mild impairment of VLA-4 adhesiveness to VCAM-1. In cell-free systems, Kindlin-3 binding to isolated β1 tails is, however, higher compared with isolated β2 and β3 tails,9 and it is therefore possible that in vivo, Kindlin-3 activation of VLA-4 is hampered. This could reflect either an endogenous Kindlin-3 competitor such as integrin cytoplasmic domain-associated protein 1 (ICAP-1), known to bind β1 tails with higher affinity than it binds other β subunits,37 and/or preformed binding of the paxillin adaptor to the α4 subunit tail of VLA-4.38 Interestingly, ICAP-1 deficiency in fibroblasts results in enhanced β1 integrin affinity and clustering,39 but whether ICAP-1 also negatively regulates VLA-4 in immune cells requires further investigation. Another possibility to account for this differential Kindlin-3 dependence of β2 and β1 integrins is hypertyrosine phosphorylation of the NPKY site of the β1 tail, which might reduce the affinity of the Kindlin-3 phosphotyrosine binding (PTB) domain to VLA-4.40 Kindlin-3 is the only Kindlin family member expressed in murine platelets, neutrophils, and lymphocytes.7,9 Absence of Kindlin-3 in human EBV cells derived from LAD-III patients16 and knockdown of Kindlin-3 by small interfering RNA in K562 erythroleukemia15 were recently shown to impair spreading and motility mediated by both β1 and β2 integrin ligands, while retaining integrin binding to nonphysiologic ligands.15 These recent studies did not assess, however, whether and how rapid integrin-mediated arrest is also affected in Kindlin-3–null LAD-III leukocytes encountering chemokines under shear flow conditions. The only results published to date on the consequence of Kindlin-3 deficiency on rapid integrin activation under shear flow has been on murine myeloid leukocytes.9 Our new data highlight for the first time an essential role of human Kindlin-3 in both inside-out LFA-1 activation by chemokines as well as outside-in LFA-1 activation during the earliest phases of firm arrests on ligand under shear flow conditions. Our data also suggest that inside-out activation of VLA-4 by chemokine signals is partially retained due to a largely intact ability of VLA-4 in Kindlin-3–null T cells to interact with VCAM-1 under shear flow, a property not shared by LFA-1 in these lymphocytes or in B lymphocytes. This unexpected retention of VLA-4 activities in Kindlin-3–null cells was not predicted in recent studies that analyzed β1 functions in the context of lymphocyte motility and spreading.15
Interestingly, in mice, loss of Kindlin-3 also severely impairs T-cell development, as suggested from reduced counts of T cells in blood and spleen, and from impaired lymph node development.9 In humans, in contrast, loss of Kindlin-3 expression and function results in elevated numbers of circulating lymphocytes, a possible result of their reduced extravasation capacities. Whether murine thymocyte activation and selection are more dependent on Kindlin-3 than human thymocyte activation is an open question. Notably, Kindlin-3 loss did not impair TCR-stimulated LFA-1–dependent spreading of primary T cells on ICAM-1, a process implicated in normal lymphocyte activation and effector functions.41 It therefore appears that Kindlin-3 can be compensated by talin1 during some TCR-mediated LFA-1 activation processes. Future studies will be required to further elucidate whether Kindlin-3 cooperates differently with talin1 in the coactivation of this and other hematopoietic integrins. In this context, the present study also reveals significant retention of the capacity of VLA-4 to support tethering, rolling, firm arrests, and adhesion strengthening in multiple types of lymphocytes deficient in Kindlin-3 expression. Because precursor T-cell entry to the thymus is VLA-4 dependent, it is possible that human T precursors deficient in Kindlin-3 in LAD-III patients, unlike their murine counterparts, retain sufficient VLA-4 adhesiveness to allow normal T-cell development and thymic egress.
The role of Kindlin-3 deficiency in the pathophysiology of LAD-III is generally accepted,42 yet a major group of LAD-III patients of Turkish origin carries a splice junction mutation in their CDGI gene, in addition to their Kindlin-3 stop codon mutation.12,14,16 CDGI is therefore not expressed in platelets, neutrophils, and primary lymphocytes of these patients. A recent study suggested that the integrin activation deficiency of LAD-III EBV cells that lack Kindlin-3 could be rescued by Kindlin-3 re-expression, although not by CDGI.16 However, these LAD-III EBV cells,16 similar to our LAD-III–derived EBV cells, express normal levels of CDGI. Therefore, ectopic expression of CDGI in these cells could not have corrected any integrin activation deficiency caused by Kindlin-3 deletion. Nevertheless, it is noteworthy that in all primary leukocytes tested to date, the loss of CDGI, a key Rap-1 GEF, alone is sufficient to impair integrin activation by chemokine signals.43,44 Therefore, single deletion of either Kindlin-3 or CDGI could result, in principle, in a similar defect of integrin activation and a LAD-III phenotype.42 Comparing patient lymphocytes and neutrophils that suffer from the loss of both Kindlin-3 and CDGI to cells from patients who are defective specifically in Kindlin-3, we show in this study that loss of Kindlin-3 alone is sufficient to severely impair both intrinsic and chemokine-triggered LFA-1 adhesiveness to ICAM-1, and to reduce chemokine triggering of VLA-4–mediated firm adhesions to VCAM-1 under shear flow. Because loss of Kindlin-3 on its own results in such severe integrin activation defects, the loss of CDGI in Kindlin-3–deficient patients may be masked, as both proteins operate in the same signaling axis. To date, CDGI deficiency alone has not been identified in humans. Therefore, one cannot rule out that a loss of this GEF or of another hematopoietic Rap-1 GEF may underlie other, possibly less severe, LAD-III variants.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr R. Fassler for helpful discussion and Dr S. Schwarzbaum for editorial assistance.
This work was supported by Israel Science Foundation, United States-Israel Binational Science Foundation, and Minerva Foundation, Germany (R.A.). R.A. is an incumbent of the Linda Jacobs Chair in Immune and Stem Cell Research.
Authorship
Contribution: E.M.-M. performed research, analyzed data, and assisted in manuscript preparation; S.W.F. designed parts of the study, performed research, and assisted in manuscript preparation; R.P. performed research and analyzed data; M.A. diagnosed LAD-III-B patient, supplied blood samples, and provided fruitful insights; V.G. and Z.S. performed research; S.S.K. diagnosed LAD-III-A and LAD-III-C patients and provided blood samples; M.A.R.-A. provided Jurkat cell lines; S.B.-D. provided assistance with the genetic mapping of all the Kindlin-3 mutations as well as helpful genetic discussion; A.M. sequenced the Kindlin-3 gene exons from LAD-III-B patient; A.B. provided Jurkat cell lines; M.M. prepared the antiserum and offered helpful suggestions; A.E. diagnosed patients, designed parts of the study, and assisted in manuscript preparation; and R.A. designed and supervised all aspects of the work and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronen Alon, Department of Immunology, Weizmann Institute of Science, Rehovot, Israel 76100; e-mail: ronen.alon@weizmann.ac.il; or Amos Etzioni, Department of Pediatrics, Meyer Children's Hospital, Rambam Medical Center and B. Rappaport School of Medicine, Technion, Haifa, Israel 31096; e-mail: Israeletzioni@rambam.health.gov.il.
References
Author notes
E.M.-M. and S.W.F. contributed equally to this study.


![Figure 2. Inside-out LFA-1 activation is lost in Kindlin-3–deficient LAD-III T cells, whereas partial VLA-4 activation is retained. (A) FACS staining of αL, β2, and CXCR4 of LAD-III and control effector T cells. (B) Chemokine signaling in LAD-III lymphocytes. Control and LAD-III effector T cells were left intact or stimulated for 1 to 5 minutes with CXCL12 (10 nM) at 37°C. Cell lysates were immunoblotted with anti–phospho-AKT and anti-talin mAb. (Ci) T cells were stimulated for 5 minutes with PMA (100 ng/mL) or with CXCL12 (10 nM), as in panel B. Expression levels of either the extension-specific epitope KIM127 or the high affinity β2 headpiece epitope 327C are shown for control and LAD effector T cells before or after stimulation with CXCL12 (c) or with PMA (p). (Cii) 327C epitope expression on control and LAD-III effector T cells suspended either in physiologic medium or in Mg2+-EGTA (ethyleneglycoltetraacetic acid) medium (Mg++). (D) Basal and CXCL12-stimulated induction of the 327C epitope in LAD-III effector T cells transfected with either GFP or Kindlin-3–GFP. For comparison, CXCL12-stimulated induction of 327C is shown on control effector T cells transfected with GFP. MFI indicates mean fluorescent intensity. (E) Attachment (tethering) and immediate arrest of control and LAD-III effector T cells on ICAM-1-Fc (95 cell adhesion molecule [CAM] sites/μm2) triggered by immobilized CXCL12 (2 μg/mL). The frequency of transient and firm tethers was determined in 2 fields of view, and the depicted values are the mean ± range. The experiment shown is representative of 4. (F) Effects of immobilized CXCL12 or of lymphocyte pretreatment with PMA on LFA-1–dependent adhesion strengthening of control and LAD-III effector T cells. Lymphocytes were settled on ICAM- 1 (95 CAM sites/μm2) for 1 minute and then subjected to incremented shear forces. The percentage of initially settled T cells that resisted detachment from the substrate at the indicated shear forces was determined in 2 fields of view, and values shown represent the mean ± range. The experiment shown is representative of 3. **P < .001 for the compared experimental groups. (G) Attachment and immediate arrest of control and LAD-III effector T cells on VCAM-1 (coated at 1 μg/mL) triggered by immobilized CXCL12 (2 μg/mL) measured at a shear stress of 0.75 dyn/cm2 in 2 fields of view. **P < .001 for firm tethers of LAD-III versus control cells. (Right panel) Expression of the activation β1 epitope HUTS21 on control effector T cells before and after stimulation with CXCL12, PMA, or Mg2+-EGTA medium, as described in panel C. (A-F) LAD-III effector lymphocytes were from patient B. (G) LAD-III effectors were from patient A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/11/10.1182_blood-2009-04-218636/4/m_zh89990941670002.jpeg?Expires=1765927451&Signature=bolbDPiBi-HVXj358Yv~AxKYhF6R1PeOadekT~4O9McQeF1uHpPQComXjTOXl94OEGqaBh0u4gYotwxAIGdjYdJBzH3Lkt9BvNvg1GFRopDZdOo7Q0320csJK~blcPrfk8tSgl9yBaTBtlISwdBWM0w1hnflr90jh8xp8I81x5HCEWy-PO-RSwM0VoLG2OzEDfhirDrYKkHdqViQmh2siQEu7AWcNISgB6FGixhYP4Rjuk7hXknJAH-G6P9cT1QYwSbP05DH1y3gvMhuyA~p5J-W9P7vftpvqPd~XyLwCsEawztVJwmNlkRA9qq3bkmBInEB~Yu-zT0KcWY0QvqEWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal