Abstract
The horseshoe crab is often referred to as a “living fossil,” representative of the oldest classes of arthropods, almost identical to species in existence more than 500 million years ago. Comparative analyses of the defense mechanisms used by the horseshoe crab that allowed it to survive mostly unchanged throughout the millennia reveal a common ancestry of the coagulation and innate immune systems that are totally integrated—indeed, almost inseparable. In human biology, we traditionally view the hemostatic pathways and those regulating innate immune responses to infections and tissue damage as entirely separate entities. But are they? The last couple of decades have revealed a remarkable degree of interplay between these systems, and the linking cellular and molecular mechanisms are rapidly being delineated. In this review, we present some of the major points of intersection between coagulation and innate immunity. We attempt to highlight the potential impact of these findings by identifying recently established paradigms that will hopefully result in the emergence of new strategies to treat a range of inflammatory and hemostatic disorders.
Introduction
The most menacing challenge to survival that is faced by organisms throughout the animal kingdom is invasion by infections and foreign antigens—events that are frequently accompanied by trauma or a wound. Organisms have thus necessarily developed a variety of effective means to restrict and to fight infections, to contain wounds by limiting bleeding with clot formation, and to rapidly initiate healing. The coexistence of thrombosis with inflammatory responses supports the notion that common molecular mechanisms regulate these complex biologic systems. The last couple of decades have seen major progress in identifying cellular and molecular links between these systems.
In this review, we consider some of the key pathways involved in hemostasis, innate immunity, and inflammation, and provide recent data that demonstrate how they are integrated. The interactions are multiple and complex, and present new challenges to identify those that are clinically relevant, with the promise of discovering novel and more effective therapies for a range of diseases.
Coagulation
The coagulation system is characterized by the sequential, rapid, and highly localized activation of a series of serine proteases, culminating in the generation of thrombin, with subsequent conversion of fibrinogen into a fibrin clot. Tissue factor (TF) is the key initiator of coagulation (reviewed in Mackman1 ), and is expressed primarily by subendothelial mural cells and adventitial fibroblasts in and around the vessel wall. Somewhat controversial, it may also be expressed at low levels by monocytes and neutrophils, and found circulating in microparticles and in a soluble form.2 With vascular endothelial cell damage, TF is exposed to the circulation, and complexes with factor VII/VIIa, initiating activation of factors IX and X. Factor Xa converts prothrombin to thrombin in sufficient quantities to activate factors V and VIII. Factors VIIIa and Va are, respectively, cofactors for factor IXa–mediated activation of factor X and factor Xa–mediated conversion of prothrombin to thrombin. The resultant thrombin-induced transformation of fibrinogen into a fibrin clot is solidified by factor XIa, whereas factor XIIIa is required for cross-linking the fibrin. A hemostatic plug develops as activated platelets adhere to and aggregate on TF-presenting cells, further promoting coagulation and thrombus formation.
Thrombin generation is tightly regulated to ensure that clot formation is not excessive and that it remains highly localized. Three major natural anticoagulant mechanisms have been delineated: TFPI, heparin-antithrombin (AT), and the protein C (PC)–thrombomodulin system (reviewed in Crawley and Lane,3 Rau et al,4 and Van de Wouwer et al5 ). These biochemical pathways are physiologically relevant, since their disruption often predisposes to hypercoagulable disorders. Modulation of the extent of clot formation, and restoration of vascular integrity with healing, is further mediated by the fibrinolytic system (supplemental Table 1 provides a list of abbreviations and is available on the Blood website; see the Supplemental Materials link at the top of the online article).
Innate immunity
As with the coagulation system, the innate immune response to damaged host cells and invading pathogens is immediate and local, thereby limiting damage and promoting healing (reviewed in Mogensen6 ). This is accomplished by rapid recognition of potential injurious agents, and the simultaneous recruitment of cellular, molecular, and chemical mechanisms for their effective deactivation, destruction, and/or removal.
The innate cellular response is mediated primarily by phagocytic macrophages and antigen-presenting cells, which relies upon the recognition of conserved structures on pathogens, pathogen-associated molecular patterns (PAMPs), through pathogen recognition receptors (PRRs). PAMPs include for example, LPS, lipoproteins, peptidoglycans, heat shock proteins, and oligosaccharides. Several families of PRRs exist that may be cell associated (eg, Toll-like receptors [TLRs], complement receptors) or soluble (eg, complement components). TLRs are the best characterized and are expressed on immune cells, including endothelial cells and platelets (reviewed in Beutler7 ). PRRs also recognize host cell–derived factors as “danger signals” (alarmins) that are generated during infection, inflammation, or stress.8 Together, PAMPs and alarmins are referred to as danger-associated molecular patterns (DAMPs). Their engagement with PRRs induces the release of proinflammatory and antimicrobial cytokines, and chemokines and up-regulation of leukocyte adhesion molecules. The host responds either with resolution of the insult before host cellular damage or at the other extreme, with the emergence of an inflammatory illness.
The innate immune system also uses complement to aid in the efficient disposal of DAMPs. The complement system comprises more than 30 soluble and membrane-bound proteins.9 Its activation is achieved via 3 main cascade-like pathways: classical (CP), lectin (LP), and alternative (AP). The CP is initiated via antigen-antibody interactions. Activation of the LP starts with recognition of carbohydrates on the surface of microbes and antigens by circulating mannose-binding lectin, followed by activation of mannose-binding lectin–associated serine proteases (MASP1, MASP2, MASP3). The AP is constitutively active, amplified by the presence of almost any foreign substance. A fourth pathway has been described in which thrombin cleaves and activates C5 to the anaphylatoxin, C5a.10 Except for the latter, all pathways converge with activation of C3, and generation of anaphylatoxins and opsonins (C3a, C5a) and a lytic membrane attack complex (C5b-9), all designed to recruit activated leukocytes and to destroy the invader. Similar to coagulation, to prevent host cell damage,11 the complement system is tightly controlled by membrane-anchored and fluid-phase regulators. Notably, unregulated activation of complement is a common cause of atypical hemolytic-uremic syndrome, a thrombotic microangiopathy.
Evolutionary perspectives
The notion that coagulation and innate immunity are coregulated and intertwined is clearly evident from comparative studies. Invertebrates and vertebrates share several major biologic host defense systems (reviewed in Iwanaga and Lee12 ). These include TLR-mediated antimicrobial production, coagulation, reactive oxygen species production, phagocytosis, and complement activation. The horseshoe crab, a “living fossil” representative of the oldest classes of arthropods, has an open circulatory system. It lacks an adaptive immune system, but has effective innate defense mechanisms to restrict invasion of common pathogens and to promote rapid healing. Ninety-nine percent of its circulating cells are hematocytes that express PRRs that bind to invading pathogens (reviewed in Iwanaga13 ). This causes the cells to degranulate, leading to formation of a localized matrix (clot) that restricts the loss of hemolymph, and simultaneously traps bacteria, preventing invasion of infection into the hemocoel. This coordinated response is possible due to the contents of hematocyte secretory granules, which include clotting factors, clottable coagulogen, antimicrobial factors, lectins that recognize carbohydrates on pathogens, and defensins. Released coagulation factors C and G are serine protease zymogens with opsonic properties and structural motifs similar to those in human complement and coagulation factors.14 Upon release, these are activated and induce a cascade that transforms coagulogen to coagulin. The activation peptide fragments, the coagulin itself, and other hematocyte-derived proteins have bacterial agglutinating and microbicidal activities. Finally, analogous to factor XIIIa in humans, a transglutaminase cross-links the coagulin, improving the seal and promoting wound healing.
The implication from most studies is that the vertebrate blood clotting system is evolutionarily a by-product of the innate immune system (reviewed in Krem and Di Cera15 ), the blood clotting serine proteases having diverged from those comprising the complement system. Differences gradually emerged, evident by the acquisition of unique protein structures, such as kringle domains and gla domains, as the increasingly complex vertebrate systems required more specialized mechanisms to defend against a range of infections and injuries. However, links between the systems remain, and the value of this combinatorial approach is highlighted in the horseshoe crab, which has allowed it to survive for more than 500 million years.
Initiation: common steps via TF
Initiation of the host response to injury and/or pathogen invasion, with simultaneous induction of procoagulant and proinflammatory activities, is centered around TF (Figure 1). Bacterial components activate TLRs on monocytes, inducing the release of proinflammatory cytokines and up-regulation of leukocyte adhesion molecules, while also increasing the expression of TF.16 Many other DAMPs have similar effects, often synergizing to simultaneously promote coagulation and inflammation by up-regulating TF expression and inducing cytokine release from monocytes, neutrophils, and endothelial cells. Whatever the initiating event, a positive feedback loop ensues, whereby the inflammatory system sustains TF expression through the action of cytokines, chemokines, and activated complement components, enhanced by interactions with activated granulocytes and platelets.17
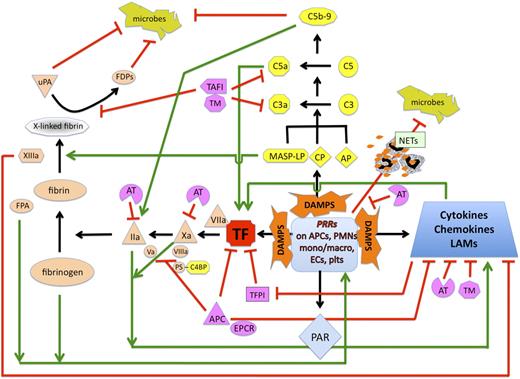
Interplay between coagulation and innate immune pathways in response to DAMPs. Danger-associated molecular patterns (DAMPs) from invading pathogens or damaged host cells are recognized by pathogen recognition receptors (PRRs) on antigen-presenting cells (APCs), neutrophils (PMNs), monocytes, macrophages (mono/macro), endothelial cells (ECs), and platelets (plts). This results in tissue factor (TF) exposure, sustained by cytokines and chemokines with proinflammatory and opsonic properties, and associated with increased expression of leukocyte adhesion molecules (LAMs). In parallel, DAMP-induced complement activation via 1 or more pathways (CP, classical; AP, alternative; LP, lectin) leads to generation of potent active complement factors, C3a and C5a, and the membrane attack complex, C5b-9. MASP2 directly converts prothrombin to thrombin. C5a feeds back to promote expression of more TF. TF-VIIa initiates activation of coagulation, leading to transformation of factor X to Xa with cofactor VIIIa, and prothrombin to thrombin (IIa) with cofactor Va. C5b-9 lyses pathogens, but also supports generation of IIa. Fibrin monomers are formed by thrombin cleavage of fibrinogen, itself considered a DAMP, with release of fibrinopeptide A (FPA), which is also chemotactic and activates leukocytes. Factor XIIIa, generated by both IIa and MASP1, cross-links fibrin monomers and suppresses cytokine release and leukocyte adhesion. Fibrinolysis is facilitated by urokinase (uPA) to generate fibrin degradation products (FDPs), both of which have antimicrobial activity. Both IIa and Xa can activate PARs and usually promote inflammation, although the response varies according to the nature of the stimulus and likely many factors. Several natural mechanisms modulate and localize the response to injury and pathogen invasion. Thus, TFPI suppresses TF activity, but is itself down-regulated by inflammatory cytokines. AT interferes with proinflammatory/procoagulant factors Xa and IIa, and depresses PRR-mediated activation of monocytes and cytokine release. APC interferes with TF release and generation of Xa and IIa, and via EPCR and PAR signaling, suppresses cytokine release, provides cytoprotection, and promotes EC integrity. Protein S (PS) enhances the anticoagulant activity of APC, and aided by C4BP, promotes phagocytosis of apoptotic cells. Thrombomodulin (TM) is a critical cofactor for thrombin-mediated activation of PC and, by binding to thrombin, neutralizes its proinflammatory and procoagulant activities (not shown). TM also suppresses leukocyte adhesion and activation, interferes with complement activation, and supports thrombin-mediated generation of TAFIa, the latter which inactivates C3a and C5a. TLR-mediated activation of platelets induces binding to neutrophils with formation of neutrophil extracellular traps (NETs) that trap and kill bacteria. Black arrows represent long-established coagulation and innate immune/complement pathways. Green arrows indicate increase in response; red lines indicate suppression.
Interplay between coagulation and innate immune pathways in response to DAMPs. Danger-associated molecular patterns (DAMPs) from invading pathogens or damaged host cells are recognized by pathogen recognition receptors (PRRs) on antigen-presenting cells (APCs), neutrophils (PMNs), monocytes, macrophages (mono/macro), endothelial cells (ECs), and platelets (plts). This results in tissue factor (TF) exposure, sustained by cytokines and chemokines with proinflammatory and opsonic properties, and associated with increased expression of leukocyte adhesion molecules (LAMs). In parallel, DAMP-induced complement activation via 1 or more pathways (CP, classical; AP, alternative; LP, lectin) leads to generation of potent active complement factors, C3a and C5a, and the membrane attack complex, C5b-9. MASP2 directly converts prothrombin to thrombin. C5a feeds back to promote expression of more TF. TF-VIIa initiates activation of coagulation, leading to transformation of factor X to Xa with cofactor VIIIa, and prothrombin to thrombin (IIa) with cofactor Va. C5b-9 lyses pathogens, but also supports generation of IIa. Fibrin monomers are formed by thrombin cleavage of fibrinogen, itself considered a DAMP, with release of fibrinopeptide A (FPA), which is also chemotactic and activates leukocytes. Factor XIIIa, generated by both IIa and MASP1, cross-links fibrin monomers and suppresses cytokine release and leukocyte adhesion. Fibrinolysis is facilitated by urokinase (uPA) to generate fibrin degradation products (FDPs), both of which have antimicrobial activity. Both IIa and Xa can activate PARs and usually promote inflammation, although the response varies according to the nature of the stimulus and likely many factors. Several natural mechanisms modulate and localize the response to injury and pathogen invasion. Thus, TFPI suppresses TF activity, but is itself down-regulated by inflammatory cytokines. AT interferes with proinflammatory/procoagulant factors Xa and IIa, and depresses PRR-mediated activation of monocytes and cytokine release. APC interferes with TF release and generation of Xa and IIa, and via EPCR and PAR signaling, suppresses cytokine release, provides cytoprotection, and promotes EC integrity. Protein S (PS) enhances the anticoagulant activity of APC, and aided by C4BP, promotes phagocytosis of apoptotic cells. Thrombomodulin (TM) is a critical cofactor for thrombin-mediated activation of PC and, by binding to thrombin, neutralizes its proinflammatory and procoagulant activities (not shown). TM also suppresses leukocyte adhesion and activation, interferes with complement activation, and supports thrombin-mediated generation of TAFIa, the latter which inactivates C3a and C5a. TLR-mediated activation of platelets induces binding to neutrophils with formation of neutrophil extracellular traps (NETs) that trap and kill bacteria. Black arrows represent long-established coagulation and innate immune/complement pathways. Green arrows indicate increase in response; red lines indicate suppression.
Although there is a lack of a clear view of the cellular and tissue source(s) of TF in the setting of infections and inflammation, loss-of-function and blocking studies in animal models confirm the pivotal role of TF in modulating inflammation and coagulation, and mediating their cross-talk. Mice with genetically reduced levels of TF exhibit less coagulation, inflammation, end-organ damage, and mortality in response to endotoxin.18 Interestingly, the cytoplasmic domain of TF regulates its endotoxin-TLR–mediated expression, and lack of this structure in a murine model of endotoxemia transiently increases hemostatic system activity and significantly dampens the inflammatory response.19 Because TF is the centerpiece for initiation and propagation of coagulation and inflammation, elucidating its cellular sites of expression, and the mechanisms by which it is regulated, is key for the development of TF-targeted therapies.
The main inhibitor of TF-initiated coagulation is TFPI. With inflammation-induced TLR-dependent activation of neutrophils, TFPI is degraded and/or suppressed, leaving TF available to further promote coagulation and inflammation. These imbalances are associated with a poor outcome in patients with sepsis and acute respiratory distress syndrome, and further demonstrate the interplay between innate immunity and coagulation. Although exogenous administration of TFPI has provided protection in animal models of sepsis, a survival benefit has not been shown in humans with sepsis.20 Further studies are necessary to clarify the mechanisms underlying its failure in that setting, and how it might be used more successfully.
Thrombin, factor Xa, and the protease-activated receptors
In addition to thrombin playing a key role in coagulation, thrombin's proinflammatory properties are well documented. Thrombin induces the release of a host of proinflammatory cytokines from endothelial cells, mural cells, epithelial cells, adipocytes, and immune cells.21-23 It is chemotactic for monocytes and neutrophils, induces expression of adhesion molecules, and promotes release of growth factors and chemokines from platelets. Thrombin directly activates both C3 and C5,10,24 and modulates innate immune responses by altering cytokine secretion and receptor expression by antigen-presenting cells.25 Thrombin at low concentrations may enhance endothelial barrier function, but at higher doses, it increases endothelial permeability, the latter being a feature of inflammation.26
In a similar manner to thrombin, factor Xa exhibits a wide range of proinflammatory properties (reviewed in Krupiczojc et al27 ). Moreover, generation of factor Xa may be facilitated by pathogens, such as viruses28 or immune cells, without requiring TF. For example, neutrophil-derived cathepsin G, and some microbial proteinases directly cleave and activate factor X. Factor Xa inhibitors have thus been used to treat local inflammatory disorders caused by those specific bacteria.29 This underscores the importance of delineating the mechanisms by which specific microbes invade and spread by modulating the coagulation and immune systems.
Most of the aforementioned noncoagulation-related cellular activities of thrombin and factor Xa are mediated by the protease-activated receptors (PARs). The PARs, of which there are 4 (PAR1, 2, 3, 4), are G-coupled 7-pass transmembrane proteins that play a key role in linking coagulation and inflammation (reviewed in Shpacovitch et al30 ). Analogous to PRRs, which are the sentinels of innate immunity, PARs are sensors for extracellular proteases. PARs are expressed by a variety of cells, including leukocytes, platelets, and endothelial cells. PAR signaling is critical for thrombin-mediated platelet activation, and is thus a key player in hemostasis. However, several proteases, involved in both coagulation and inflammation, regulate PAR signaling, implicating these in a spectrum of biologic systems. Thus, PAR1 is activated by thrombin, factor Xa, APC, trypsin, granzyme A, gingipains-R, and matrix metalloprotease-131 ; PAR2, by factor Xa, factor VIIa-TF, and neutrophil proteinase 3 (PR3); and PAR4, by trypsin, cathepsin G, gingipains-R, and relatively high concentrations of thrombin. Activation of PAR2 by factor Xa or VIIa-TF mediates house dust mite allergen responses, and promotes the expression of defensins, proinflammatory cytokines, and chemokines. PAR2 physically interacts with TLR4 in activating NFkB by sharing utilization of intracellular adaptor proteins, and thereby modulates the inflammatory response.32 PAR2 also cooperates with PAR1, but increases endothelial barrier protective properties of factor Xa.26 All may be variably cleaved and inactivated by a variety of inflammatory proteases, including cathepsin G, plasmin, elastase, PR3, and trypsin.30
The in vivo role of the PARs, particularly PAR1 in sepsis, is currently an active area of research, rife with controversy. There are opposing views as to whether PAR1 deficiency confers a survival advantage in endotoxemia, although inconsistencies may be partly ascribed to differences in endotoxin (LPS) dose.33,34 Studies by Kuliopulos and coworkers suggest that during progression of sepsis, PAR1 switches from being a vascular-disruptive receptor to a vascular-protective receptor and that the beneficial effects of PAR1 require transactivation of PAR2 (Kaneider et al35 ). The protective effects of APC in LPS-induced endotoxemia—believed to be mediated via PAR1–sphingosine-1-phosphate pathways36 —were evident only after endotoxin exposure. In contrast, others have observed a beneficial effect of APC (below) even when administered concurrently with endotoxin.37 The complexity is enhanced by the observation that PAR1 signaling may be anti-inflammatory in endothelial cells and proinflammatory in dendritic cells.34 These examples highlight the fact that PAR signaling pathways are important in coagulation and innate immune responses, coregulated, and interdependent. The differential, temporally distinct, and dose-dependent responses of the PARs to a wide range of proteases, and their complex interactions with an array of intracellular signaling pathways, reveal both the challenges and the potential benefits of targeting these for therapeutic purposes.38
Antithrombin
AT plays a key role in protecting against thrombosis, neutralizing thrombin, and factors IXa, Xa, XIa, and XIIa.4 During sepsis, decreased hepatic synthesis, more rapid clearance, and neutrophil elastase degradation cause AT levels to decrease, contributing to a hypercoagulable state. In vitro, AT interferes with endothelial cell and monocyte release of cytokines, and the expression of TF and leukocyte adhesion molecules, and indirectly interferes with platelet aggregation.39 The protective properties of exogenous AT were observed in several bacterial sepsis and acute lung injury studies in animals. Unfortunately, phase 3 studies failed to show that AT could reduce mortality rates in patients with severe sepsis.20 Nonetheless, in concert with other factors or in other diseases, it is likely that AT does have physiologic importance in modulating the inflammatory response.
The protein C–thrombomodulin mechanism
The protein C (PC) pathway decidedly provides strong evidence of the interplay between coagulation and inflammation/innate immunity, and underlines the impact of delineating these interactions for the development of novel therapies (reviewed in Jackson and Xue40 ). The major components—activated PC (APC), endothelial PC receptor (EPCR), protein S (PS), and thrombomodulin (TM)—each are critically involved in regulating coagulation, inflammation, and the innate immune response.
PC is a circulating zymogen that is activated by thrombin when complexed with the endothelial protein TM. The resultant APC inactivates factors Va and VIIIa, enhanced by the cofactor protein S (PS), and suppresses further thrombin generation (reviewed in Van de Wouwer et al5 ). Thrombotic disorders are associated with defects in PC and PS, and resistance of factor Va to APC. APC has several means by which it dampens inflammation. It inhibits expression of TF and the release of proinflammatory cytokines by monocytes, blocks expression of leukocyte adhesion molecules, inhibits neutrophil chemotaxis, and is cytoprotective. APC also is endothelial-barrier protective, an effect that is believed to be mediated via EPCR-PAR1 signaling that induces release of sphingosine-1-phosphate.41 Indeed, many of the protective effects of APC are mediated by binding to EPCR, which positions it for activation of PAR1, although other receptors, such as ApoER2, likely also contribute.42 When dissociated from EPCR, APC binds to PS on activated platelets and endothelial cells where it interferes with coagulation. When bound to EPCR, APC transmits its anti-inflammatory and vasculoprotective signals. Thus, EPCR is a molecular switch for APC in determining whether the latter will function as an anticoagulant or an anti-inflammatory molecule. EPCR itself is down-regulated during inflammation by activated neutrophil PR3-mediated proteolytic degradation,43 again demonstrating bidirectional pathways between coagulation and inflammation.
APC has entered the clinic, not primarily as an anticoagulant, but rather to treat patients with severe sepsis where it provides protection against multiorgan failure.44 Indeed, APC appears to have a broad range of activities against alarmin-mediated organ damage, as it has been shown recently in animal models to suppress hypoxia-induced brain injury, renal ischemia, acute lung injury, and arthritis.40 Nonetheless, there is considerable controversy as to whether the beneficial effects of APC, particularly in the setting of sepsis, are due to its anti-inflammatory or its anticoagulant properties.45 Moreover, its efficacy in treating severe sepsis in pediatric populations, or in patients with severe sepsis and a low risk of death or single organ failure, has not been demonstrated,46 raising questions as to the mechanisms underlying its apparent restricted utility. The design of mutant forms of APC that dissociate its anticoagulant and immune-modulating effects, coupled with greater mechanistic insights into the relevant intracellular signaling pathways, will hopefully help to clarify some of these issues, and lead to the development of safer therapies for both hemostatic and immune disorders.47
A cofactor for the anticoagulant function of APC, deficiency of PS is associated with a thrombotic diathesis. PS provides a direct connection between coagulation and innate immunity. Approximately 60% of PS circulates in complex with the complement component, C4b-binding protein (C4bBP), a cofactor that negatively regulates complement activation. When bound to the C4bBPβ form, PS loses its anticoagulation cofactor function. In the early stages of inflammation, free PS also binds to apoptotic cells providing a platform for a PS-C4bBP complex that promotes phagocytosis of apoptotic cells. Interestingly, autoantibody-induced PS deficiency has been observed in postinfectious purpura fulminans,48 as well as in patients with systemic lupus erythematosis with excess inflammation.49
TM is expressed by endothelial cells throughout the vasculature and plays a key role in simultaneously regulating coagulation and innate immunity. During sepsis or inflammation, TM levels are down-regulated by cytokines and C5a, and are rendered anticoagulantly inactive by the products of activated neutrophils that oxidize and degrade the protein. This decreases the generation of the anticoagulant/anti-inflammatory/cytoprotective APC. Mutations in mice that diminish the expression and anticoagulant function of TM result in both a hypercoagulable state and a poor immune response to endotoxemia.50 Mice lacking the N-terminal lectin-like domain of TM, which does not regulate coagulation, exhibit increased sensitivity to tissue damage in models of arthritis, acute lung injury, sepsis, and ischemia-reperfusion injury, with higher levels of inflammatory cytokines, increased neutrophil tissue infiltration, and activation of complement.51 The lectin-like domain interferes with leukocyte adhesion to endothelial cells, promotes endothelial cell survival, and suppresses complement activation. It binds to and agglutinates bacteria, promotes their phagocytosis,52 and interferes with DAMP-PRR interactions.53
TM additionally regulates innate immune responses by acting as a cofactor for thrombin-mediated generation of the plasma carboxypeptidase, TAFIa, which inactivates C3a and C5a.54 Finally, TM is expressed by monocytes and dendritic cells, and may therefore play a role in the pathogenesis of atopy and asthma.55 In preclinical models, recombinant forms of TM confer protection against inflammation, ischemia-reperfusion injury, and endotoxemia.51,53,56-58 Sepsis-associated disseminated intravascular coagulation in patients responded beneficially to treatment with recombinant soluble TM, effects that may be attributed to its combined effects on inflammation and coagulation.59
In view of the wide spectrum of activities of APC, TM, EPCR, and PS, characterization of the structure-function correlates of each molecule, their complex interactions with components of the immune system, and the signaling mechanisms by which they are regulated are crucial for the development of therapies with greater specificity and safety.
Fibrin/ogen and plasmin/ogen
Critical for generation of fibrin clots, fibrinogen also plays a role in innate immunity and may be viewed as a DAMP, as it stimulates monocyte secretion of several cytokines after engagement with TLR4.60 Fibrin/ogen interacts directly with integrins on monocytes, macrophages, neutrophils, and dendritic cells, thereby promoting diverse proinflammatory responses. Mice deficient in fibrinogen, or expressing mutant forms, exhibit a dampened inflammatory response in different mouse models (reviewed in Adams et al61 ). Fibrin/ogen activation peptides are chemotactic for phagocytic leukocytes and promote adhesion of immune cells.62,63 The observation that MASP2 converts prothrombin to thrombin on the surface of bacteria is proposed to be a host immune response to locally generate immunoreactive, protective fibrin/ogen peptides.64 The potential value of targeting these pathways in disease requires further study in vivo.
Cross-linking fibrin requires factor XIIIa, generated by thrombin activation of factor XIII, but notably also activated by MASP1.65 Factor XIIIa also plays a role in innate immune responses, although the pathways are not fully delineated. It increases endothelial barrier function, but also enhances leukocyte adhesion and migration. Factor XIII is also activated intracellularly in monocytes, where it enhances their phagocytic properties by altering the cytoskeleton.66 Elastases from activated neutrophils degrade factor XIIIa, thereby interfering with its functions in coagulation and inflammation. In vivo studies using a rat sepsis model support its protective properties in that administration of factor XIII improves capillary perfusion and reduces leukocyte adhesion and cytokine release.67
The fibrinolytic system modulates the immune system, is itself regulated by inflammatory mediators, and is also recruited by invading pathogens, often at the expense of the host. Indeed, the finding that several bacteria activate plasminogen (eg, Staphylococcus aureus, Streptococcus, Yersinia pestis) to help invade and/or propagate in the host (reviewed in Degen et al68 ) may lead to the design of specific strategies to disarm these harmful organisms.
The 2 major activators of fibrinolysis are urokinase plasminogen activator (uPA), and tissue-type plasminogen activator (tPA). uPA and its GPI-linked receptor uPAR are expressed by several blood-borne cells and are up-regulated during infections. uPA promotes activation of neutrophils, induces discharge of proinflammatory mediators from monocytes,69 and has direct cytolytic effects on some bacteria. uPAR facilitates leukocyte adhesion and migration via uPA-dependent and -independent pathways, and activated neutrophils release a chemotactically active form of soluble uPAR, thereby amplifying the inflammatory response. Deficiency of either uPAR or uPA in mice results in reduced leukocyte recruitment and a dampened inflammatory response in models of pneumonia and arthritis, respectively.70
tPA also displays properties that extend beyond its well-recognized role in cleaving plasminogen to plasmin. This is most evident in the brain, where active tPA is found in parenchymal cells and interacts with the N-methyl-d-aspartate receptor, low-density lipoprotein receptor-related protein, and annexin-II receptor, inducing mostly proinflammatory and neurotoxic effects. Increased tPA activity levels have been linked to neural damage associated with prion disease. Yet, tPA is also believed to be important for normal learning and synaptic plasticity, and low levels have been found in models of neuroinflammation and neurodegeneration (reviewed in Yepes et al71 ), underscoring the need for a critical balance to avoid disease.
Plasmin, like thrombin, has multiple substrates, and thus a wide spectrum of biologic effects beyond clot lysis, some of which are mediated via activation of PAR1. The zymogen for plasmin, plasminogen, binds directly to the extracellular matrix, positioning plasmin to readily degrade extracellular matrix proteins and activate matrix metalloproteases, thereby facilitating leukocyte activation and migration. It is therefore not surprising that plasminogen deficiency in mice is characterized by resistance to inflammatory stimuli, with diminished macrophage migration across extracellular matrix.72
The overwhelming evidence of interplay between innate immune defense mechanisms and essentially all components of the coagulation/fibrinolytic system compels us to view these as entirely integrated.
Platelets
Platelets are well recognized for their vital role in maintaining hemostasis and promoting thrombus growth. They are also critical players in immune surveillance, a key cellular interface between coagulation and inflammation. In response to injury and infection, platelets are rapidly activated and localize to sites of injury and infection, releasing prothrombotic, proinflammatory, and antimicrobial mediators and bidirectionally interacting with other innate immune cells (reviewed in Weyrich and Zimmerman73 and Ma and Kubes74 ).
Platelets are activated by a range of agonists, PAMPS, and alarmins, and release factors that target cells of the coagulation, innate, and adaptive immune systems. Indeed, the list of inflammatory and immune-modulating factors that are secreted by activated platelets is long, underscoring the vital role of the platelet in hemostasis and immunity. This includes, among others, chemokines, histamine, serotonin, PDGF, TGFβ, IL-1β, HMGB1, ADP, and RANTES, as well as peptides with direct microbicidal properties.75 For example, the chemokine platelet factor 4 binds to receptors on neutrophils, triggering their adhesion to immobilized platelets and enhancing phagocytosis of invading pathogens. Platelets also display chemokine receptors on their surface, which allows for simultaneous amplification of the platelet release response, promoting inflammation and thrombosis.
CD40 ligand (CD40L) is a transmembrane glycoprotein member of the tumor necrosis factor superfamily, expressed by endothelial cells, monocytes, T cells, and most prominently by platelets. Upon activation, CD40L is translocated to the platelet surface within seconds, where it remains cell associated, or is cleaved to yield soluble CD40L. Membrane-bound and soluble forms exert diverse proinflammatory and prothrombotic effects by binding mainly to CD40 on the surface of vascular wall and circulating cells (reviewed in Rizvi et al76 ). For example, CD40L-CD40 interactions trigger endothelial cells to acquire a prothrombotic phenotype, with enhanced expression of adhesion molecules and the release of IL-8, MCP-1, matrix metalloproteases, and reactive oxygen species. Interactions of platelets with neutrophils and monocytes may also be mediated by CD40L-CD40 binding, promoting leukocyte activation, migration, extravasation, and propagation of the inflammatory reaction. Soluble CD40L also binds to platelet integrin glycoprotein IIb/IIIa, amplifying platelet activation in an autocrine fashion. Moreover, via expression of CD40L, T cells directly contact and activate platelets, inducing platelet release of RANTES, which further promotes T-cell recruitment.77 CD40L-CD40 signaling has additional well-established roles in adaptive immunity, being required for immunoglobulin class switching. Dysregulation of CD40L-CD40 signaling pathways has been implicated in contributing to the development of autoimmune, inflammatory and thrombotic diseases, and efforts are under way to further unravel the molecular mechanisms, to identify potential therapeutic targets, and to assess the role of CD40L as a biologic marker of disease.76
As with other immune cells, platelets express several PRRs, including TLRs and complement receptors.78 They store complement regulatory and activating factors,79 and provide a surface for generation of C3a and the C5b-9 complex. C3a may activate other platelets, and C5b-9 can catalyze prothrombin conversion to thrombin.80 It is likely that platelets and procoagulant platelet-derived microparticles focus complement and TF to sites of injury, recruiting immune cells to that region, for a rapid and highly localized inflammatory and procoagulant response.81
TLR2 expression on platelets can be activated by bacteria, which promotes platelet adhesion/aggregation with release of reactive oxygen species and expression of P-selectin.82 P-selectin has several procoagulant and proinflammatory properties. It induces monocyte release of TF-containing microparticles. It acts as a receptor for C3b,79 further targeting complement activation to sites of thrombosis and inflammation. Platelet-associated P-selectin binds to the receptor PSGL-1 on leukocytes, promoting their adhesion to endothelial cells and aggregation of platelets on leukocytes. Soluble P-selectin, in murine ischemia-reperfusion injury models, reduces complement activation, and protects against organ damage.83 Inhibition of either P-selectin or PSGL-1 dampens fibrin deposition and reduces leukocyte adhesion (reviewed in Polgar et al84 ).
TLR4 activation on platelets also induces platelet binding to adherent neutrophils, followed by robust neutrophil activation and the formation of so-called neutrophil extracellular traps,85,86 weblike DNA structures that trap bacteria and yeast, killing them via release of neutrophil constituents. These fascinating findings, which provide novel insights into the pathogenesis of acute respiratory distress syndrome, emphasize the role of platelets—traditionally viewed as “cells for hemostasis”—in enhancing the first-line innate immune defense by neutrophils.
As the complex interplay between hemostasis and the innate immune system as modulated by platelets is further elucidated, it will impact on our understanding of often serious diseases, raising the hopes for the development of better therapies. Identifying which molecular mechanism(s) is more or less important during the course of a range of diseases will continue to be a challenge, requiring excellent preclinical models, and carefully designed clinical trials.
The antiphospholipid syndrome
The interplay between innate immunity and coagulation is evident in many disease states. The antiphospholipid (aPL) syndrome (APS) represents one example of a serious disorder, in which recent elegant studies have provided important new insights into its pathogenesis that will impact on therapeutic strategies. APS is characterized by the presence of aPL antibodies in patients with associated arterial and/or venous thromboses and/or recurrent fetal loss. One recently proposed paradigm to explain aPL-dependent fetal loss suggests that the aPL antibody activates complement on trophoblasts, leading to generation of C5a, which binds to C5aR on neutrophils. The C5a-activated neutrophils up-regulate TF expression, which itself potentiates inflammation in the decidua with release of reactive oxygen species, trophoblast injury, and ultimately fetal loss.87 Autoantibodies to EPCR, known to be expressed on the trophoblast surface,88 have also been identified as a risk factor for APS-associated fetal loss.89 Although not demonstrated, it is possible that these autoantibodies not only interfere with generation of APC, but also enhance complement activation on the trophoblast surface. Further studies are necessary to determine how these findings relate to aPL-associated vascular thrombosis, as well as the relevance to human disease.
Overall, the observations in this one example of a difficult to manage, clinical disorder demonstrate the importance of viewing all thrombotic and inflammatory diseases in the context of both coagulation and inflammation, the end point of which may impact on therapy selection.
Conclusion
We have focused on only a few of the many biochemical and cellular pathways that link hemostasis and the innate immune system. Many others exist. For example, we have not explored the role of the endothelial cell, which maintains hemostatic balance by variably expressing anticoagulant and procoagulant molecules, yet is also an active immune cell, expressing PRRs, mediating leukocyte trafficking, and regulating complement activation. Several shared cell surface and intracellular signaling pathways have not been mentioned. Despite major advances in our understanding of the numerous interactions between the systems, patients continue to lack therapies for serious illnesses in which thrombosis and inflammation coexist. Characterization of APC as more than an anticoagulant molecule, and its introduction into the clinic to treat patients with serious sepsis, is only a first step. Viewing the coagulation and innate immune system pathways as wholly integrated will provide new directions for the development of many more effective treatment strategies.
The online version of this article contains a data supplement.
Acknowledgments
We thank Dr Marc Hoylaerts for providing valuable suggestions to improve the quality of this paper.
M.D. was supported in part by the Association for International Cancer Research (AICR).
Authorship
Contribution: M.D. and E.M.C. equally researched and wrote this review.
Conflict-of-interest disclosure: E.M.C. holds a patent for use of the lectin-like domain of thrombomodulin as an anti-inflammatory agent. M.D. declares no competing financial interests.
Correspondence: Edward M. Conway, Centre for Blood Research, University of British Columbia, Life Sciences Centre, No. 4303, 2350 Health Sciences Mall, Vancouver BC V6T 1Z3, Canada; e-mail: emconway@interchange.ubc.ca.
References
Author notes
*M.D. and E.M.C. contributed equally in preparing and writing this review.