Abstract
Homozygous deletion of a 84-kb genomic fragment in human chromosome 1 that encompasses the CFHR1 and CFHR3 genes represents a risk factor for hemolytic uremic syndrome (HUS) but has a protective effect in age-related macular degeneration (AMD). Here we identify CFHR1 as a novel inhibitor of the complement pathway that blocks C5 convertase activity and interferes with C5b surface deposition and MAC formation. This activity is distinct from complement factor H, and apparently factor H and CFHR1 control complement activation in a sequential manner. As both proteins bind to the same or similar sites at the cellular surfaces, the gain of CFHR1 activity presumably is at the expense of CFH-mediated function (inhibition of the C3 convertase). In HUS, the absence of CFHR1 may result in reduced inhibition of terminal complex formation and in reduced protection of endothelial cells upon complement attack. These findings provide new insights into complement regulation on the cell surface and biosurfaces and likely define the role of CFHR1 in human diseases.
Introduction
The complement system is important for host innate and adaptive immunity and mounts a protective immune response to invading microbes.1 The alternative complement pathway is spontaneously activated, and generates C3 convertases (C3bBb) that cleave the central component C3 to the anaphylactic peptide C3a and C3b.2,3 C3b attached to a foreign surface binds factor B and generates the C3 convertase (C3bBb), which enhances further complement activation resulting in opsonization and phagocytosis of particles. Binding of an additional C3b molecule to the C3 convertase forms the C5 convertase (C3bBbC3b) of the alternative pathway. This convertase cleaves C5 and generates the potent chemoattractant C5a as well as C5b, which initiates the terminal complement pathway assembly.4 C5b immediately undergoes conformational changes and binds C6 and C7 in a nonenzymatic manner. The assembled C5b67 complex is released from the convertase and attaches to lipid bilayers. Upon binding of C8 and C9, the lytic membrane attack complex (MAC) is formed.3,5
Once activated, this powerful defense system is tightly controlled on host cell surfaces by both membrane-anchored and surface-attached soluble regulators. Proper and coordinated function of these regulators is essential for tissue integrity. Single gene mutations predispose to severe renal and retinal diseases, that is, hemolytic uremic syndrome (HUS; OMIM no. 235400), membranoproliferative glomerulonephritis type II (MPGN II; OMIM no. 609814), or age-related macular degeneration (AMD; OMIM no. 603075).6,7
HUS is caused by occlusion of arterioles and capillaries in the kidney, due to endothelial cell and platelet damage.8 MPGN II is a rare renal disease, with formation of dense deposits at the glomerular basement membrane and thickening of the peripheral capillary walls.9 Similarly, the retinal disease AMD, which causes visual impairment of elderly people, is caused by deposits (drusen) that form on the Bruch membrane and lead to atrophy of the retinal pigment epithelium and chorodial neovascularization under the macular area.10
These diverse diseases are caused by defective local complement regulation and are associated with gene variations and mutations coding for complement components and regulators, such as complement factor H (CFH).8,11 In addition deletion of a 84-kb genomic fragment on human chromosome 1, which leads to the loss of the complement factor H–related genes 1 and 3 (CFHR1 and CFHR3), is associated with both HUS12 and AMD.13 However, this chromosomal deletion has opposite effects, for HUS the deletion increases the risk for the disease and for AMD the deletion has a protective effect.
In atypical HUS (aHUS), the absence of CFHR1 and CFHR3 in plasma correlates with the presence of autoantibodies to CFH.14 The autoantibodies bind to the C-terminal surface binding region of CFH15 and inhibit CFH surface attachment resulting in damage of endothelial cells as well as platelets.16 The CFHR1 plasma protein is composed of 5 short consensus repeats and is identified in 2 glycosylated forms. CFHR1β (42 kDa) has 2 and CFHR1α (37 kDa) has one attached carbohydrate side chain.17-19 The high sequence identity of the 3 C-terminal SCRs of CFHR1 and CFH (100%, 100%, and 98%) suggests related functions. CFHR1 lacks cofactor and decay-accelerating activity,20 and the function is currently unknown. Given the opposing effects of CFHR1/CFHR3 deficiency in human diseases, we were interested to identify the function of CFHR1 protein. Here we identify CFHR1 as a regulator of the complement pathway that inhibits C5 convertase activity and MAC assembly.
Methods
Proteins and antibodies
Recombinant CFHR1 and deletion mutants CFHR1 SCR1-2 (CFHR1/1-2) and CFHR1 SCR3-5 (CFHR1/3-5) were expressed as described.21 CFHR1/1-2 cDNA was cloned into vector pPICZαB using specific primers CFHR11-2 forward, 5′TTTCTGCAGCCGAAGCAACATTTTGTGATTTTCAA3′ and CFHR11-2 reverse, 5′TTTTCTAGAGCAGTGGACCTGCATTTGGGAGGGGT3′. CFH/SCR11-15 was expressed in insect cells, as previously described.14,15 All proteins were expressed in Picha pastoris and purified by nickel chelate affinity chromatography.14 Vitronectin was purchased from BD Biosciences; C3b, C5, C5b6, factor H, and factor I, from Merck Biosciences; C7, C8, C9, from Comptech. CFHR1 was purified from human plasma by HiTrap Heparin HP column (GE Healthcare) affinity chromatography. Pooled elution fractions were concentrated (Superdex 200; GE Healthcare) and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE); CFHR1 was dissected from the gel, concentrated, and dialyzed (1× PBS, pH 4.7). Mouse monoclonal antibody (mAb) C1822 (Alexis) was used to detect the C-terminus of CFH and CFHR1. A novel CFHR1 mAb JHD10 was generated by immunizing mice with purified CFHR1/SCR1-2 fragments.
Serum probes
Normal human plasma (HP) was obtained from healthy volunteers (Jena, Germany) upon informed consent obtained in accordance with the Declaration of Helsinki. CFH depletion was performed by immunadsorbance as described.12 For C8 depletion, goat polyclonal anti-C8 (Comptech) was used as described for CFH depletion. The study is approved by the ethics board of the Friedrich Schiller University of Jena. The board approved study of patient material (DNA and blood) for genetic and molecular characterization of complement factor H and complement factor H family members in HUS, MPGN2, and AMD.
Cell culture and confocal microscopy
Human umbilical vein endothelial cells (HUVECs; ATCC CRL-1730) were cultivated as described.23 For confocal microscopy (LSM 510 META; Zeiss) HUVECs were grown on chamber slides (Nunc) and incubated for 60 minutes with CFHR1 or CFH (100 μg/mL), and binding was visualized using mAb JHD10 (CFHR1) or polyclonal anti–SCR1-4 (CFH).24 C5 deposition on erythrocytes incubated in HPΔCFHΔC8 was detected with C5 mAb (Comptech).
Immunohistochemistry
Immunohistochemistry was performed on tissues derived from 2 human donor eyes (2 female patients, 79 and 81 years of age) with history neither of clinically documented early AMD nor of morphologic evidence of ocular disease. The donor eyes were obtained at autopsy and were processed fewer than 15 hours after death. In addition, normal kidney tissue was obtained from 2 human adult donor kidneys that were not used for transplantation. Posterior eye poles and portions of decapsulated kidneys were embedded in optimal cutting temperature compound and frozen in isopentane-cooled liquid nitrogen. Cryostat-cut sections (6 μm) were fixed in cold acetone, blocked with 10% normal goat serum, and incubated in a monoclonal mouse antibody to CFHR1 (JHD10) diluted 1:100 in phosphate-buffered saline (PBS) overnight at 4°C. Antibody binding was detected by Alexa 488–conjugated secondary antibodies (Molecular Probes). Nuclear counterstaining was performed with propidium iodide. For preabsorption experiments, the primary antibody was treated for 1 hour with either CFHR1 or CFH.
Binding of CFHR1 to heparin, C3b, C5, and C5b6
MaxiSorp plastic plates (Nunc) were coated with heparin (500 units/well) or C3b (10 μg/mL), C5, or C5bC6 (5 μg/mL) and incubated with CFHR1 (50 μg/mL) or CFH (75 μg/mL) dissolved in binding buffer B (10 nM Na2HPO4, 27 mM KCl, 1.4 M NaCl, 2% BSA, pH 7.4). Bound CFHR1 was detected with mAb C18 and bound CFH with SCR1-4 antiserum. In control experiments, buffer B was added without proteins.
For identification of C5 binding site in CFHR1, mAb JHD10 or mAb C18 (15 μg/mL) was immobilized to a microtiter plate and used to catch CFHR1 (30 μg/mL). After washing, C5 or C5b6 (5 μg/mL) was added in gelatin veronal buffer (Sigma) and bound proteins were identified using a C5 mAb. CFHR1-specific antiserum was used to confirm binding of CFHR1 to the immobilized mAbs. C5 binding to CFH or CFHR1 was measured by immobilizing equimolar concentrations of CFH or CFHR1 to a microtiter plate. C5 binding to immobilized CFHR1 and CFH was assayed by incubation of increasing concentrations of C5 (1.5-50 μg/mL) for 1 hour, and bound C5 was identified with mouse monoclonal antibodies (1:2000; Quidel). In addition, binding of C5 (50, 150, 200, and 400 nM) to immobilized CFHR1 or CFH/SCR10-13 (carboxylated dextran chip CM5; Biacore AB) was assayed by surface plasmon resonance in 75 mM PBS at a flow rate of 5 μL/minute at 25°C. Controls (binding of the fluid phase ligand to the uncoated surface) were subtracted.
Cofactor assay
Cofactor activity of heparin-bound CFH was analyzed by measuring factor-I–mediated degradation of C3b after SDS-PAGE and Western blot analysis. For competition CFH immobilized to heparin-coated microtiter plates (EpranEx; Plasso) was incubated with CFHR1 (0.13 μg-13.3 μg). Then C3b (2 μg) and CFI (0.28 μg) were added and cofactor activity was determined.24 Degradation products (β chain band and α'43 band) were analyzed by densitometry.
ELISA
To investigate CFHR1 regulation of the C3 convertase, the C3 convertase was generated by incubation of C3b (2 μg/mL) and C3 (80 μg/mL) with factor D (4 μg/mL) and factor B (40 μg/mL) in activation buffer C (20 mM Hepes, 144 mM NaCl, 7 mM MgCl2, 10 mM EGTA, pH 7.4). Activity of C3 convertase was determined after incubation of constant amounts of C3 (80 μg/mL) and increasing amounts of CFHR1 (25 and 50 μg/mL), CFH (50 g/mL), or 25 μg/mL human serum albumin (HSA) by C3a generation. C3a concentrations were determined by enzyme-linked immunosorbent assay (ELISA; Quidel). Sheep erythrocytes (109) were incubated with C3b (10 μg/mL) in veronal buffer overnight at 4°C. C3 and C5 convertase was generated by incubation of erythrocytes for 40 minutes at 30°C with C3 (10 μg/mL), factor D (5 μg/mL), and factor B (10 μg/mL) in veronal buffer supplemented with Ni2+ and properdin (5 μg/mL). C5 convertase–loaded erythrocytes were preincubated with recombinant or plasma-derived CFHR1 or CFH (50 μg/mL) or with BSA (50 μg/mL) before the addition of C5 (50 μg/mL). C5a generation was analyzed after 15 minutes by ELISA (DRG Diagnostics).
Erythrocyte lysis assay
Increasing concentrations of CFHR1 (5-160 μg/mL) or CFH, vitronectin, or HSA were added to CFH- and CFHR1-depleted plasma (30%) and incubated at 37°C for 15 minutes with approximately 2 × 107 sheep erythrocytes in activation buffer C (see above). Supernatants were recorded at 415 nm. In similar experiments, generation of complement activation products C3a and C5a was followed by ELISA (Quidel; DRG Diagnostics).
Hemolysis of chicken erythrocytes, which are more sensitive for MAC-induced hemolysis, was investigated in complement-inactivated (20 mM HEPES, 144 mM NaCl, 10 mM EDTA, pH 7.4) defHP with constant concentrations of C5b6 (5 ng/mL), increasing amounts of CFHR1 (25-100 μg/mL), CFH, or BSA. In addition C5b6 complexes (5 ng/mL) were preincubated with either recombinant CFHR1 (50 μg/mL), purified CFHR1 (12.5 μg/mL), vitronectin (12.5 μg/mL), or BSA (12.5 μg/mL) in 20 mM HEPES, 144 mM NaCl, 10 mM EDTA, pH 7.4, for 5 minutes at 20°C. Lysis of sheep erythrocytes (2 × 107) was followed after addition of C7 (final concentration, 1 μg/mL) C8 (0.2 μg/mL), and C9 (0.2 μg/mL) by absorbance at 415 nm. To compare similar molar amounts of the plasma-purified CFHR1 with vitronectin, C5b6 complexes (5 ng/mL) were preincubated with increasing concentrations (1-300 nM) of either plasma-purified CFHR1, vitronectin, or BSA for 5 minutes at 20°C. After addition of C7 (final concentration, 1 μg/mL), C8 (0.2 μg/mL), and C9 (0.2 μg/mL) the mixture was added to sheep erythrocytes (2 × 107) and incubated for 30 minutes at 37°C. Percentage of cell lysis (absorbance at 415 nm) was calculated by the formula: [(sample − background)/(100% lysis − background)] × 100, where background hemoglobin release was obtained from sheep erythrocytes incubated in buffer only, and 100% lysis was achieved using distilled water.
Flow cytometry
CFHR1/CFHR3-deficient plasma was depleted of CFH and C8 (HPΔCFHΔC8) by immunoaffinity chromatography and added to sheep erythrocytes in the presence or absence of 50 μg/mL CFHR1 in 20 mM HEPES, 250 mM mannitol, 8 mM MgCl2, 10 mM EGTA, pH 7.4. At each time point, the sample was transferred to ice-cold buffer with 1% wt/vol BSA with protease inhibitor mix (Complete Inhibitor Mix; Roche). C5 was detected using C5 mAb (Comptech).
Binding of serum-derived CFHR1 to HUVECs was investigated by incubating HUVECs, which have been grown serum free for 3 days, in 25% normal human serum with mAb JHD10.
Immunoprecipitation
Monoclonal C5 antibodies (Comptech) were immobilized to protein A sepharose beads (GE Healthcare) by incubation overnight at 4°C. Antibody-loaded beads were washed 3 times in PBS (1×) and incubated for 2 hours at 4°C with 50% NHP. The beads were washed 5 times in PBS (1×) and bound antibodies and proteins were eluted with 0.1 M glycine/0.5 M NaCl, pH 2.7. Eluates were separated by SDS-PAGE and transferred to a membrane, and CFHR1 was detected with a polyclonal antiserum and C5, with a rabbit polyclonal antiserum in combination with a secondary horseradish peroxidase–conjugated antiserum.
Statistical analysis
Statistics were analyzed with the Student t test; P values less than .05 were considered significant. Statistical analysis of defined groups was performed with the Jonckheere-Terpstra trend test25 ; P values were calculated for the 2-sided test of no trend.
Results
CFHR1 binds to C3b, C3d, heparin, and human cells
The 3 C-terminal SCRs of CFHR1 display almost sequence identity to the central C3b and surface binding region of CFH (ie, SCR18-20; Figure 1A). This homology suggests related functions. Therefore binding of CFHR1 to the ligand C3b was investigated. CFHR1 binds to C3b, and CFH, which harbors 3 interaction sites for C3b, showed more pronounced binding (Figure 1B).
CFHR1 binds to C3b and cells and competes with CFH. (A) CFHR1 is composed of 5 SCRs domains. SCR1 and SCR2 show 42% and 34% sequence identity to SCR6 and SCR7 of factor H (CFH), respectively. The 3 C-terminal SCRs show 100%, 100%, and 98% sequence identity to SCR18, SCR19, and SCR20 of CFH, respectively, which comprise the C-terminal surface binding region. (B) Equimolar concentrations of CFHR1 (25 μg/mL) and CFH (75 μg/mL) bind to immobilized C3b. Data represent mean values ± SDs from 3 separate experiments. Control represents binding of antibodies to immobilized BSA. (C) Plasma-derived CFHR1 (red curve) binds to HUVECs. Cells were incubated in human plasma, and bound CFHR1 was visualized with specific mAb (JHD10) by flow cytometry. Control: cells were treated with the secondary antibody alone (black curve). (D) Immunofluorescence staining of CFHR1 (green fluorescence) in renal and retinal human tissue. CFHR1 is detected at the lining of renal (i) and ocular (iv) blood vessels including large arteries, afferent and efferent arterioles associated with glomeruli (i), or the choriocapillaries (iv) as well as the Bruch membrane (nuclear counterstain: propidium iodide; original magnification, ×100). Preabsorption of mAb JHD10 with CFHR1 blocks reactivity (ii,v). In contrast, preabsorption with CFH (iii,vi) does not affect reactivity and demonstrates specificity of signals in panels i and iv. Thus, CFHR1 is present at the surface of endothelial cells and at the Bruch membrane. Inlays represent magnifications. Autofluorescence of lipofuscin-containing cells appears yellow. (E) HUVECs were incubated with a combination of CFHR1 (100 μg/mL) and CFH (100 μg/mL). After addition of the appropriate secondary antibodies, bound proteins were identified by confocal laser scanning microscopy. CFHR1 binding was detected with the CFHR1-specific mAb JHD10 in combination with a secondary anti–mouse antibody labeled with Alexa 647 (red fluorescence; i) and binding of CFH with a polyclonal antiserum specific for the N-terminal domains of CFH (anti–SCR1-4) together with a secondary goat anti–rabbit antibody labeled with Alexa 488 (ii; green fluorescence). An overlay of subpanels i and ii (iii) reveals colocalization of the 2 regulators as indicated by the yellow signal. Binding of primary (JHD10) and secondary antibodies showed no signal (control; iv). All cells were stained with DAPI to visualize the cell nucleus (bar represents 20 μm). (F) CFHR1 competes with factor H for heparin binding and thus affects the regulatory activity at surfaces. Constant amounts of factor H (10 μg/mL) were bound to immobilized heparin, and CFHR1 was used at increasing concentrations (0.1-20 μg/mL) as competitor. After competition, C3b and factor I were added. After incubation for 30 minutes the supernatant was removed, separated by SDS-PAGE, and transferred to a membrane, and C3b and degradation fragments were visualized with C3 antiserum (top panel). The mobility of the α' and β chain as well as the degradation fragments are indicated. A densitometric analysis as determined by the ratio of the α' 43 band and the β chain is shown in the bottom panel. A representative experiment of 3 separate experiments is shown.
CFHR1 binds to C3b and cells and competes with CFH. (A) CFHR1 is composed of 5 SCRs domains. SCR1 and SCR2 show 42% and 34% sequence identity to SCR6 and SCR7 of factor H (CFH), respectively. The 3 C-terminal SCRs show 100%, 100%, and 98% sequence identity to SCR18, SCR19, and SCR20 of CFH, respectively, which comprise the C-terminal surface binding region. (B) Equimolar concentrations of CFHR1 (25 μg/mL) and CFH (75 μg/mL) bind to immobilized C3b. Data represent mean values ± SDs from 3 separate experiments. Control represents binding of antibodies to immobilized BSA. (C) Plasma-derived CFHR1 (red curve) binds to HUVECs. Cells were incubated in human plasma, and bound CFHR1 was visualized with specific mAb (JHD10) by flow cytometry. Control: cells were treated with the secondary antibody alone (black curve). (D) Immunofluorescence staining of CFHR1 (green fluorescence) in renal and retinal human tissue. CFHR1 is detected at the lining of renal (i) and ocular (iv) blood vessels including large arteries, afferent and efferent arterioles associated with glomeruli (i), or the choriocapillaries (iv) as well as the Bruch membrane (nuclear counterstain: propidium iodide; original magnification, ×100). Preabsorption of mAb JHD10 with CFHR1 blocks reactivity (ii,v). In contrast, preabsorption with CFH (iii,vi) does not affect reactivity and demonstrates specificity of signals in panels i and iv. Thus, CFHR1 is present at the surface of endothelial cells and at the Bruch membrane. Inlays represent magnifications. Autofluorescence of lipofuscin-containing cells appears yellow. (E) HUVECs were incubated with a combination of CFHR1 (100 μg/mL) and CFH (100 μg/mL). After addition of the appropriate secondary antibodies, bound proteins were identified by confocal laser scanning microscopy. CFHR1 binding was detected with the CFHR1-specific mAb JHD10 in combination with a secondary anti–mouse antibody labeled with Alexa 647 (red fluorescence; i) and binding of CFH with a polyclonal antiserum specific for the N-terminal domains of CFH (anti–SCR1-4) together with a secondary goat anti–rabbit antibody labeled with Alexa 488 (ii; green fluorescence). An overlay of subpanels i and ii (iii) reveals colocalization of the 2 regulators as indicated by the yellow signal. Binding of primary (JHD10) and secondary antibodies showed no signal (control; iv). All cells were stained with DAPI to visualize the cell nucleus (bar represents 20 μm). (F) CFHR1 competes with factor H for heparin binding and thus affects the regulatory activity at surfaces. Constant amounts of factor H (10 μg/mL) were bound to immobilized heparin, and CFHR1 was used at increasing concentrations (0.1-20 μg/mL) as competitor. After competition, C3b and factor I were added. After incubation for 30 minutes the supernatant was removed, separated by SDS-PAGE, and transferred to a membrane, and C3b and degradation fragments were visualized with C3 antiserum (top panel). The mobility of the α' and β chain as well as the degradation fragments are indicated. A densitometric analysis as determined by the ratio of the α' 43 band and the β chain is shown in the bottom panel. A representative experiment of 3 separate experiments is shown.
CFHR1 uses the C-terminus for both C3b and heparin binding: Surface plasmon resonance showed that deletion mutant CFHR1/3-5 (supplemental Figure 2A blue line, available on the Blood website; see the Supplemental Materials link at the top of the online article), but not CFHR1/1-2 (black line), bound to immobilized C3b and to heparin (supplemental Figure 2A-B). The binding affinity of the C-termini of CFHR1 and of CFH was determined by surface plasmon resonance. The affinity of CFHR1/3-5 to C3b is KD = 6.4 × 10−6 M and that of CFH/18-20 is KD = 2.6 × 10−6 M. Thus, the C-terminus of CFHR1 binds C3b with lower affinity than that of CFH (supplemental Figure 2C-D).
Binding of CFHR1 to cell surfaces
Binding of CFHR1 to human cells was investigated by flow cytometry. HUVECs were incubated in normal human plasma (HP) and binding of native, plasma-derived CFHR1 was detected with the novel CFHR1-specific mAb JHD10 (Figure 1C). Binding of recombinant CFHR1 to HUVECs, as well as to epithelial cells (ARPE-19), was confirmed by confocal microscopy. On the cell surface, CFHR1 showed a patchy distribution (supplemental Figure 3AI-II). In addition CFHR1 bound to C3b-treated rabbit erythrocytes (supplemental Figure 3AIII). The secondary antibodies alone showed no binding (supplemental Figure 3AIa-IIIa). The C-terminus of CFHR1 mediates cell binding, as the deletion mutant CFHR1/3-5, but not CFHR1/1-2, bound to ARPE-19 cells (supplemental Figure 3B). Thus, CFHR1 binds via the C-terminal binding region to C3b and to human cells.
CFHR-1 binding to kidney and retinal tissue
Having demonstrated that CFHR1 binds cell surfaces, we analyzed in vivo CFHR1 expression in human retinal and ocular tissues by immunohistochemistry. In renal tissue, CFHR1 was detected in the lining of blood vessels (green fluorescence), particularly of large arteries and of afferent and efferent arterioles at the vascular poles of glomeruli (Figure 1Di). In the posterior segment of a retina, CFHR1 staining was specifically localized to the Bruch membrane and the choriocapillaries of the choroids (Figure 1Div). Preincubation of the antibodies with CFH did not affect reactivity (Figure 1Diii,vi), but preincubation with CFHR1 did (Figure 1Dii,v). Thus, CFHR1 is present at the surface of endothelial cells and at the Bruch membrane.
CFHR1 and CFH recognize overlapping binding sites at the cell surface
The almost identical C-termini and the conserved binding characteristics of CFHR1 and CFH suggested coordinated as well as competitive binding. Therefore simultaneous binding to cell surfaces was investigated. HUVECs were incubated with CFHR1 and CFH. Surface-bound CFHR1 was visualized by red—and bound CFH by green—fluorescence (Figure 1Ei-ii). A merge of the 2 images revealed overlapping binding as indicated by the yellow fluorescence (Figure 1Eiii).
To investigate whether CFHR1 replaces CFH and reduces local CFH-mediated activity, heparin-bound CFH was competed with increasing concentrations of CFHR1, and cofactor activity was assayed by analyzing the generation of the C3b degradation products α'68 and α'41/43. CFHR1, by replacing CFH, reduced the local regulatory activity as demonstrated by the lower amount of C3b degradation fragments (Figure 1F top panel). Densitometric analyses showed reduction of the α'43 band (as demonstrated by the α'43/β75 ratio) when equal molar amounts of CFHR1 and CFH were used (Figure 1F bottom panel, column 3). This effect demonstrates that CFHR1 may reduce local CFH-mediated complement control.
CFHR1 regulates complement pathway activation
Binding of CFHR1 to C3b suggested a unique regulatory function(s) on the level of C3 convertase. To identify such an activity, a hemolytic assay12,26,27 was used. Sheep erythrocytes representing nonactivator surfaces remain intact when incubated in human plasma (HP). However, these cells are lysed when incubated in complement active HP depleted of CFH and CFHR1 (HPΔCFH). In this system, the absence of CFH results in erythrocyte lysis, and addition of CFH has a dose-dependent protective effect (Figure 2A gray squares). The role of CFHR1 on complement activation was analyzed in this serum. Addition of CFHR1 to HPΔCFH reduced lysis of sheep erythrocytes dose dependently (Figure 2A black triangles) and HSA showed no effect (black squares). The protective effect of CFHR1, used at 80 μg/mL, was 13% and that of CFH was 75%. The inhibitory activity of CFHR1 was comparable with the known terminal pathway inhibitor vitronectin (Figure 2A compare black triangles and gray diamonds).
CFHR1 is a regulator of the alternative pathway of complement. (A) Hemolysis of sheep erythrocytes in the presence of CFHR1- and CFH-depleted normal human plasma (HPΔCFH). Sheep erythrocytes represent nonactivator surfaces when incubated in complement active human plasma. However, when the same cells are incubated in HPΔCFH, these cells represent activator surfaces and are lysed. Factor H acts as a surface protector and reverts the effect ( ). Addition of CFHR1 (5-160 μg/mL) results in a reduction of erythrocytes lysis, which indicates a regulatory effect of this protein in complement control (▲). Similar inhibition of hemolysis is observed with vitronectin (
). Addition of CFHR1 (5-160 μg/mL) results in a reduction of erythrocytes lysis, which indicates a regulatory effect of this protein in complement control (▲). Similar inhibition of hemolysis is observed with vitronectin ( ). HSA has no effect on hemolysis (■). Data show 1 representative of 3 experiments. A415 indicates absorbance at 415 nm. (B) CFHR1 does not affect C3a generation of an in vitro–assembled C3 convertase. C3 was incubated with factor D and factor B, and C3 convertase activity was analyzed by comparing C3a before (column 3) and after (column 4) addition of C3. Addition of CFHR1 (25 or 50 μg/mL) did not significantly effect C3a generation (columns 5-6). CFH (50 μg/mL) strongly effected C3 convertase activity (column 7). Addition of human serum albumin (HSA) did not affect C3a generation (column 8). C3 mAb, which reacts with C3a (1 μg/mL, standard; column 2) did not react with an empty well (co). A representative result of 3 independent experiments is shown and SDs are given. A490 indicates absorbance at 490 nm.
). HSA has no effect on hemolysis (■). Data show 1 representative of 3 experiments. A415 indicates absorbance at 415 nm. (B) CFHR1 does not affect C3a generation of an in vitro–assembled C3 convertase. C3 was incubated with factor D and factor B, and C3 convertase activity was analyzed by comparing C3a before (column 3) and after (column 4) addition of C3. Addition of CFHR1 (25 or 50 μg/mL) did not significantly effect C3a generation (columns 5-6). CFH (50 μg/mL) strongly effected C3 convertase activity (column 7). Addition of human serum albumin (HSA) did not affect C3a generation (column 8). C3 mAb, which reacts with C3a (1 μg/mL, standard; column 2) did not react with an empty well (co). A representative result of 3 independent experiments is shown and SDs are given. A490 indicates absorbance at 490 nm.
CFHR1 is a regulator of the alternative pathway of complement. (A) Hemolysis of sheep erythrocytes in the presence of CFHR1- and CFH-depleted normal human plasma (HPΔCFH). Sheep erythrocytes represent nonactivator surfaces when incubated in complement active human plasma. However, when the same cells are incubated in HPΔCFH, these cells represent activator surfaces and are lysed. Factor H acts as a surface protector and reverts the effect ( ). Addition of CFHR1 (5-160 μg/mL) results in a reduction of erythrocytes lysis, which indicates a regulatory effect of this protein in complement control (▲). Similar inhibition of hemolysis is observed with vitronectin (
). Addition of CFHR1 (5-160 μg/mL) results in a reduction of erythrocytes lysis, which indicates a regulatory effect of this protein in complement control (▲). Similar inhibition of hemolysis is observed with vitronectin ( ). HSA has no effect on hemolysis (■). Data show 1 representative of 3 experiments. A415 indicates absorbance at 415 nm. (B) CFHR1 does not affect C3a generation of an in vitro–assembled C3 convertase. C3 was incubated with factor D and factor B, and C3 convertase activity was analyzed by comparing C3a before (column 3) and after (column 4) addition of C3. Addition of CFHR1 (25 or 50 μg/mL) did not significantly effect C3a generation (columns 5-6). CFH (50 μg/mL) strongly effected C3 convertase activity (column 7). Addition of human serum albumin (HSA) did not affect C3a generation (column 8). C3 mAb, which reacts with C3a (1 μg/mL, standard; column 2) did not react with an empty well (co). A representative result of 3 independent experiments is shown and SDs are given. A490 indicates absorbance at 490 nm.
). HSA has no effect on hemolysis (■). Data show 1 representative of 3 experiments. A415 indicates absorbance at 415 nm. (B) CFHR1 does not affect C3a generation of an in vitro–assembled C3 convertase. C3 was incubated with factor D and factor B, and C3 convertase activity was analyzed by comparing C3a before (column 3) and after (column 4) addition of C3. Addition of CFHR1 (25 or 50 μg/mL) did not significantly effect C3a generation (columns 5-6). CFH (50 μg/mL) strongly effected C3 convertase activity (column 7). Addition of human serum albumin (HSA) did not affect C3a generation (column 8). C3 mAb, which reacts with C3a (1 μg/mL, standard; column 2) did not react with an empty well (co). A representative result of 3 independent experiments is shown and SDs are given. A490 indicates absorbance at 490 nm.
The regulatory role of CFHR1 on the complement pathway was investigated for the C3 convertase. C3 convertases were formed in vitro by incubating C3 with factor B and factor D, and, after addition of C3, convertase activity was monitored by C3a generation. CFHR1 (25 and 50 μg/mL) did not significantly affect C3a levels (Figure 2B columns 5-6), demonstrating that CFHR1 does not affect the C3 convertase. In contrast, CFH (50 μg/mL) strongly inhibited C3a generation (Figure 2B column 7). Thus CFHR1 does not affect the C3 convertase and likely acts downstream of this convertase.
CFHR1 regulates the C5 convertase of the alternative pathway
CFHR1 binds C3b and inhibits alternative complement activation before MAC assembly and insertion. Therefore, we hypothesized that CFHR1 inhibits the C5 convertase of the alternative pathway. To this end, sheep erythrocytes were incubated in complement active HPΔCFH and C3a, and C5a generation was determined. CFHR1 showed a dose-dependent protective effect on erythrocyte lysis, did not affect C3a generation, but reduced C5a generation (Figure 3A). Thus CFHR1 inhibits the C5 convertase of the alternative pathway.
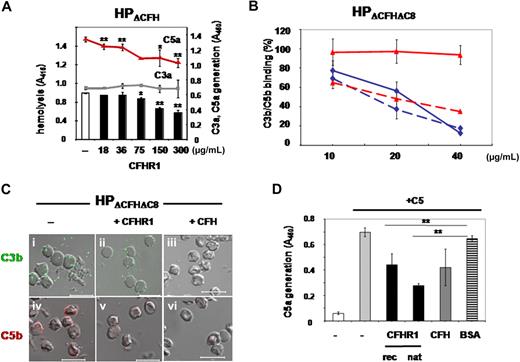
CFHR1 regulates C5 convertase activity, binds to C5 and C5b6, and inhibits binding of C5b6 to the cell surfaces. (A) Sheep erythrocytes were incubated in complement active CFH/CFHR1-depleted HP (HPΔCFH) in the presence or absence of CFHR1 (18-300 μg/mL). Lysis was recorded after 30 minutes. In addition, the concentration of C3a (gray squares) and C5a (red triangles) was assayed in the supernatant. Data represent mean values of 3 separate experiments and SDs are indicated. *P < .05, **P < .005 versus control. (B) Effect of CFHR1 on C3b (solid line) and C5/C5b (stippled line) deposition on the surface of sheep erythrocytes. Sheep erythrocytes were incubated in HPΔCFH-ΔC8 plasma, and deposition of C3b and C5b was assayed in the presence of increasing concentrations of CFHR1 (10, 20, and 40 μg/mL, red solid and stippled lines) and CFH (10, 20, and 40 μg/mL, blue solid and stippled lines) by flow cytometry. Data represent mean values ± SDs of 3 separate experiments. (C) CFHR1 inhibits C5/C5b deposition on sheep erythrocytes (v) that were incubated in human CFH- and C8-depleted plasma (HPΔCFH-ΔC8). CFHR1 does not effect C3b deposition (ii). Factor H (CFH) inhibits both C3b and C5/C5b deposition (iii and vi). Bars represent 10 μm. (D) CFHR1 inhibits C5 convertase activity. Recombinant CFHR1 (column 3) as well as plasma-purified CFHR1 (column 4) inhibit the C5 convertase and cleavage of C5. The C5 convertase was generated on the surface of sheep erythrocytes using purified C3, factor B, and factor D in the presence of Ni2+ and properdin. Cleavage and C5a generation was determined by ELISA. CFH also affected C5a generation (column 5) in contrast to BSA (column 6). Data represent mean values of 3 separate experiments and SDs are indicated. *P < .05, **P < .005 versus BSA.
CFHR1 regulates C5 convertase activity, binds to C5 and C5b6, and inhibits binding of C5b6 to the cell surfaces. (A) Sheep erythrocytes were incubated in complement active CFH/CFHR1-depleted HP (HPΔCFH) in the presence or absence of CFHR1 (18-300 μg/mL). Lysis was recorded after 30 minutes. In addition, the concentration of C3a (gray squares) and C5a (red triangles) was assayed in the supernatant. Data represent mean values of 3 separate experiments and SDs are indicated. *P < .05, **P < .005 versus control. (B) Effect of CFHR1 on C3b (solid line) and C5/C5b (stippled line) deposition on the surface of sheep erythrocytes. Sheep erythrocytes were incubated in HPΔCFH-ΔC8 plasma, and deposition of C3b and C5b was assayed in the presence of increasing concentrations of CFHR1 (10, 20, and 40 μg/mL, red solid and stippled lines) and CFH (10, 20, and 40 μg/mL, blue solid and stippled lines) by flow cytometry. Data represent mean values ± SDs of 3 separate experiments. (C) CFHR1 inhibits C5/C5b deposition on sheep erythrocytes (v) that were incubated in human CFH- and C8-depleted plasma (HPΔCFH-ΔC8). CFHR1 does not effect C3b deposition (ii). Factor H (CFH) inhibits both C3b and C5/C5b deposition (iii and vi). Bars represent 10 μm. (D) CFHR1 inhibits C5 convertase activity. Recombinant CFHR1 (column 3) as well as plasma-purified CFHR1 (column 4) inhibit the C5 convertase and cleavage of C5. The C5 convertase was generated on the surface of sheep erythrocytes using purified C3, factor B, and factor D in the presence of Ni2+ and properdin. Cleavage and C5a generation was determined by ELISA. CFH also affected C5a generation (column 5) in contrast to BSA (column 6). Data represent mean values of 3 separate experiments and SDs are indicated. *P < .05, **P < .005 versus BSA.
Having demonstrated that CFHR1 inhibits the C5 convertase, we next aimed to characterize this inhibitory effect in more detail. To this end, sheep erythrocytes were incubated in CFH-depleted, complement active human plasma, which, in this case, was depleted for CFH and C8 (HPΔCFHΔC8). Surface deposition of C3b and C5b was analyzed by flow cytometry. CFHR1 (10, 20, and 40 μg/mL) did not, but CFH (10, 20, and 40 μg/mL) strongly reduced C3b deposition (compare Figure 3B solid red and blue lines). However, CFHR1 (10, 20, and 40 μg/mL) reduced C5b deposition by 40%, 50%, and 60%, respectively (Figure 3B red stippled line). In the presence of CFH, which acts on the C3 convertase, no further progression to the level of C5 convertase occurs (Figure 3B stippled blue line). These results show that CFHR1 controls complement activation at the level of C5.
The inhibitory effect of CFHR1 on C5b but not C3b generation was further investigated by analyzing surface deposition of C5/C5b and C3b on sheep erythrocytes by microscopy. Again, CFHR1 inhibited C5/C5b but not C3b surface deposition (Figure 3Cii,iv). The C3 convertase inhibitor CFH affected both C3b and C5/C5b deposition (Figure 3Ciii,vi). These results confirm that CFHR1 inhibits the C5 convertase of the alternative pathway and prevents C5 cleavage, as demonstrated by reduction of C5/C5b deposition and C5a generation.
To confirm the CFHR1 inhibitory effects on the C5 convertase, the C5 convertase was generated in vitro using purified complement components. Activity of the convertase was demonstrated by an increase of C5a levels upon incubation of convertase-loaded sheep erythrocytes with C5 (Figure 3D column 2). Both recombinant and plasma-purified CFHR1 (supplemental Figure 1C) reduced C5a generation (Figure 3D columns 3-4). Similarly, C5 cleavage was reduced by CFH (column 5) but not by BSA (Figure 3D column 6). These results confirm that CFHR1 inhibits the C5 convertase and reduces C5 cleavage.
CFHR1 binds C5 and C5b6
CFHR1 inhibits the alternative pathway C5 convertase, therefore direct binding of CFHR1 to C5 and to the activation product C5b6 was studied. CFHR1 was captured with mAbs that bind to either the C-terminus (C18) or the N-terminus (JHD10). C5 and C5b6 bound to CFHR1, which was immobilized via the C-terminus and that has the N-terminal SCRs accessible (Figure 4A columns 1-2). Reduced binding of C5 or C5b6 to CFHR1 was observed when CFHR1 was immobilized via JHD10, which binds the N-terminal epitope and has the C-terminus accessible (Figure 4A columns 5-6). These results indicate that the N-terminal region of CFHR1 binds C5 and C5b6.
CFHR1 but not CFH binds to C5. (A) CFHR1 was captured to the surface of a microtiter plate using the C-terminal (C18) or the N-terminal (JHD10) binding mAbs. C5 or C5b6 was applied with the fluid phase, and bound proteins were detected with mAb to C5. Attachment of CFHR1 to C18 or JHD10 was verified with polyclonal CFHR1 antiserum (columns 4 and 8). In addition, binding of C5b6 to the mAb JHD10 in the absence of CFHR1 was assayed and was at background levels (column 7). Data represent mean values ± SD of 3 separate experiments. **P < .001 versus binding of C5 or C5b6 to C18-mediated CFHR1 immobilization. (B) Formation of native CFHR1/C5 complexes in plasma was identified by immunoprecipitation. C5 antibodies were used to capture C5 complexed with CFHR1 from human plasma as shown by Western blot using polyclonal CFH antibodies (bottom panel, lane 2, arrows) and monoclonal C5 antibodies (top panel, lane 2, arrow). CFHR1 in human plasma (HP, bottom panel, lane 3) or plasma-purified CFHR1 (bottom panel, lane 4) shows similar mobilities. In addition, C5 in human plasma (top panel, lane 3) or purified C5 (top panel, lane 4) shows similar mobilities compared with the immunoprecipitated C5 protein (top panel, lane 2). Eluates derived from noncoated columns incubated with HP contain neither CFHR1 (bottom panel, lane 1) nor C5 (top panel, lane 1). The extra band in the top panel, lane 1, is considered unspecific. (C) Binding of C5 to immobilized CFHR1. CFHR1 (black line) or CFH/SCR10-13 (gray line) was immobilized to the surface of a sensor chip and C5 (50, 150, 200, 400 nM) was added in the fluid phase. (D) Binding of C5 to immobilized CFHR1 and CFH. CFHR1 (10 μg/mL) and CFH (30 μg/mL) were immobilized to a microtiter plate and incubated with increasing concentrations of C5 (1.5-50 μg/mL). Binding of C5 was detected with monoclonal C5 antibodies. Co represents reactivity of the mAb C5 to immobilized CFHR1 in the absence of C5. Representative data from 2 independent experiments are shown.
CFHR1 but not CFH binds to C5. (A) CFHR1 was captured to the surface of a microtiter plate using the C-terminal (C18) or the N-terminal (JHD10) binding mAbs. C5 or C5b6 was applied with the fluid phase, and bound proteins were detected with mAb to C5. Attachment of CFHR1 to C18 or JHD10 was verified with polyclonal CFHR1 antiserum (columns 4 and 8). In addition, binding of C5b6 to the mAb JHD10 in the absence of CFHR1 was assayed and was at background levels (column 7). Data represent mean values ± SD of 3 separate experiments. **P < .001 versus binding of C5 or C5b6 to C18-mediated CFHR1 immobilization. (B) Formation of native CFHR1/C5 complexes in plasma was identified by immunoprecipitation. C5 antibodies were used to capture C5 complexed with CFHR1 from human plasma as shown by Western blot using polyclonal CFH antibodies (bottom panel, lane 2, arrows) and monoclonal C5 antibodies (top panel, lane 2, arrow). CFHR1 in human plasma (HP, bottom panel, lane 3) or plasma-purified CFHR1 (bottom panel, lane 4) shows similar mobilities. In addition, C5 in human plasma (top panel, lane 3) or purified C5 (top panel, lane 4) shows similar mobilities compared with the immunoprecipitated C5 protein (top panel, lane 2). Eluates derived from noncoated columns incubated with HP contain neither CFHR1 (bottom panel, lane 1) nor C5 (top panel, lane 1). The extra band in the top panel, lane 1, is considered unspecific. (C) Binding of C5 to immobilized CFHR1. CFHR1 (black line) or CFH/SCR10-13 (gray line) was immobilized to the surface of a sensor chip and C5 (50, 150, 200, 400 nM) was added in the fluid phase. (D) Binding of C5 to immobilized CFHR1 and CFH. CFHR1 (10 μg/mL) and CFH (30 μg/mL) were immobilized to a microtiter plate and incubated with increasing concentrations of C5 (1.5-50 μg/mL). Binding of C5 was detected with monoclonal C5 antibodies. Co represents reactivity of the mAb C5 to immobilized CFHR1 in the absence of C5. Representative data from 2 independent experiments are shown.
To analyze interaction of CFHR1 with C5 in plasma, CFHR1/C5 complex formation in plasma was analyzed by immunoprecipitation. Normal human plasma was incubated with a C5 monoclonal antibody that was bound to a protein A matrix. Bound complexes were eluted from the matrix, separated by SDS-PAGE, and transferred to a membrane, and C5 and CFHR1 were identified by Western blotting. CFHR1 forms complexes with C5 as demonstrated by the presence of both proteins CFHR1 and C5 in the immunoabsorbed sample (Figure 4B lane 2; compare top panel for C5 and bottom panel for CFHR1). An eluate derived from a CFHR1 or C5 lacking sample showed the absence of either protein (Figure 4B lane 1, Co).
Interaction between CFHR1 and C5 was further confirmed by surface plasmon resonance and by ELISA. CFHR1 showed binding to C5 as indicated by dose-dependent association and reduced dissociation of CFHR1 to C5 (black lines). In contrast to CFHR1, the CFH fragment including SCR10-13 (gray lines) did not interact with C5 (Figure 4C). C5 binding to CFHR1 or CFH was also measured by ELISA and demonstrated dose-dependent binding to CFHR1 but no interaction with CFH (Figure 4D). Thus the interaction with C5 represents a distinct characteristic of CFHR1.
CFHR1 prevents nonenzymatic assembly of MAC
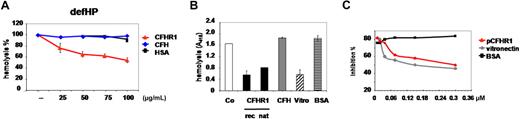
Cleavage of C5 is the last enzymatic reaction of the complement cascade, and MAC complexes are formed by conformational changes and protein assembly. Therefore we asked whether CFHR1 might inhibit MAC assembly and deposition on the lipid bilayer. To this end, chicken erythrocytes were incubated with increasing amounts of CFHR1 together with C5b6 and added to nonlytic, complement inactive HP deficient of CFHR1 and CFHR3, which was used as a source for C7 to C9. MAC formation was followed by assaying lysis of erythrocytes. CFHR1 inhibited lysis in a dose-dependent manner; at 70 μg/mL FHR1 reduced lysis by 38% (Figure 5A red triangles). This inhibitory effect was specific for CFHR1 and was observed neither for CFH nor for BSA (Figure 5A blue diamonds and black squares, respectively). These data identify a second regulatory function of CFHR1: inhibition of MAC assembly or formation.
CFHR1 inhibits nonenzymatic steps of the terminal complement pathway. (A) Chicken erythrocytes were incubated with C5b6 complexes (5 ng/mL) and increasing concentrations of CFHR1, and nonlytic complement inactive defHP was added as a source for terminal complement components. MAC formation was assayed by lyses of erythrocytes. Hemolysis was recorded after 30 minutes by measuring the absorbance at 415 nm. Increasing concentrations of CFHR1 (25-100 μg/mL) affected MAC activity, and CFH or human serum albumin (HSA) showed no effect. Data represent mean values in percentage ± SD derived from 3 independent assays. (B) MAC formation on sheep erythrocytes was induced by incubation with C5b6, C7, C8, and C9 components and detected by hemolysis of cells (column 1). Preincubation of C5b6 with recombinant CFHR1 (50 μg/mL) or plasma-purified CFHR1 (12.5 μg/mL) inhibited hemolysis (columns 2 and 3, respectively). CFH (12.5 μg/mL) showed no effect on MAC formation (column 4), but vitronectin did (12.5 μg/mL; column 5). BSA (12.5 μg/mL) did not induce hemolysis (column 6). Data represent mean values ± SD of 3 separate experiments (except for plasma-purified CFHR1). (C) Inhibitory role of CFHR1 on MAC formation. CFHR1 purified from human plasma (0.1-0.3 μM) inhibited MAC formation on the surface of sheep erythrocytes (red triangles, red line). In addition, the established MAC inhibitor vitronectin was assayed (blue diamond, blue line). CFHR1 and vitronectin had similar activity and BSA did not affect MAC formation (black squares, black line). A representative experiment of 2 is shown.
CFHR1 inhibits nonenzymatic steps of the terminal complement pathway. (A) Chicken erythrocytes were incubated with C5b6 complexes (5 ng/mL) and increasing concentrations of CFHR1, and nonlytic complement inactive defHP was added as a source for terminal complement components. MAC formation was assayed by lyses of erythrocytes. Hemolysis was recorded after 30 minutes by measuring the absorbance at 415 nm. Increasing concentrations of CFHR1 (25-100 μg/mL) affected MAC activity, and CFH or human serum albumin (HSA) showed no effect. Data represent mean values in percentage ± SD derived from 3 independent assays. (B) MAC formation on sheep erythrocytes was induced by incubation with C5b6, C7, C8, and C9 components and detected by hemolysis of cells (column 1). Preincubation of C5b6 with recombinant CFHR1 (50 μg/mL) or plasma-purified CFHR1 (12.5 μg/mL) inhibited hemolysis (columns 2 and 3, respectively). CFH (12.5 μg/mL) showed no effect on MAC formation (column 4), but vitronectin did (12.5 μg/mL; column 5). BSA (12.5 μg/mL) did not induce hemolysis (column 6). Data represent mean values ± SD of 3 separate experiments (except for plasma-purified CFHR1). (C) Inhibitory role of CFHR1 on MAC formation. CFHR1 purified from human plasma (0.1-0.3 μM) inhibited MAC formation on the surface of sheep erythrocytes (red triangles, red line). In addition, the established MAC inhibitor vitronectin was assayed (blue diamond, blue line). CFHR1 and vitronectin had similar activity and BSA did not affect MAC formation (black squares, black line). A representative experiment of 2 is shown.
This complement regulatory activity was confirmed for native plasma-derived CFHR1 (supplemental Figure 1B lanes 3 and 6). Sheep erythrocytes, which are incubated with the terminal complement components C5b6, C7, C8, and C9, are lysed (Figure 5B column 1). Preincubation of C5b6 complexes with recombinant (50 μg/mL) or native (12.5 μg/mL) CFHR1 proteins inhibited hemolysis (Figure 5B columns 2-3). Similarly, preincubation of C5b6 complexes with the MAC inhibitor vitronectin (12.5 μg/mL) inhibited hemolysis (Figure 5B column 5). In this setup, CFH (12.5 μg/mL; column 4) or BSA (12.5 μg/mL; Figure 5D column 6) had no effect. The inhibitory activity of plasma-purified CFHR1 and vitronectin on MAC assembly was compared. Both proteins were added to C5b6 and the terminal components C7, C8, and C9 and then to erythrocytes. Plasma-derived CFHR1 and vitronectin showed similar inhibitory effects on sheep erythrocyte lysis (Figure 5C). These data demonstrate that both recombinant and plasma-derived CFHR1 block MAC formation.
CFHR1- and CFHR3-deficient serum reduces viability of human nucleated cells
As CFHR1 and CFHR3 deficiency increases the risk for aHUS,12 we asked whether the lack of CFHR1 in plasma may result in enhanced complement activation on the surface of nucleated human cells. To this end the metabolic activity of human endothelial cells (HUVECs) upon complement challenge was monitored after uptake of the nonfluorescent substrate resazurin by following the intracellular conversion to the fluorescent dye resorufin. Cellular vitality results in a typical metabolic response that is followed by fluorescence generation (supplemental Figure 4, HP). However, when cells were incubated in complement active, CFHR1/CFHR3-deficient HP (defHP), derived from 3 healthy individuals, cell viability was reduced, as shown by the slower turnover of the substrate (supplemental Figure 4, defHP). When plasma from 3 CFHR1/CFHR3-deficient HUS patients was assayed, cell vitality and metabolic activity were significantly reduced (supplemental Figure 4, defHP, -aAb, HUS). These data indicate that the absence of CFHR1 and CFHR3 results in reduced cell vitality, likely due to inappropriate control of complement activation at the surface of nucleated human endothelial cells.
Discussion
Here we identify CFHR1 as a novel human complement regulator. CFHR1 is a human plasma protein, composed of 5 SCR domains, with 2 functional regions. The N-terminus (ie, SCR1-2) binds C5 and C5b6, and the C-terminus (ie, SCR3-5) binds C3b/C3d and heparin and to host cells. CFHR1 is a complement regulator that controls the activity of the C5 convertase and also assembly and membrane insertion of MAC. This is—to our knowledge—the first description of a regulator of the C5 convertase that does not affect the C3 convertase. CFHR1 and CFH have almost identical C-terminal surface binding regions and the 2 proteins bind to the same ligands (ie, C3b and heparin) and colocalize at the surface of endothelial cells (Figure 1C-D). This simultaneous binding suggests a sequential and coordinated action at the cell surface. As CFHR1 mutations and absence of the protein in plasma are linked to renal and retinal diseases, such as HUS and AMD, the characterization of CFHR1 as a complement regulator deepens our understanding on the molecular mechanisms leading to pathology.
The CFHR1 cDNA identified in 1991 represented the first member of the group of CFH-related plasma proteins.17,18,28 Each of the known 5 CFHR proteins is encoded by a unique gene, which is located adjacent to the Factor H gene on human chromosome 1.29 The 5 CFHR proteins show immune cross-reactivity, and individual domains have significant sequence identity to domains of CTH. The 2 N-terminal SCRs of CFHR1 (ie, SCR1 and SCR2) show 36% and 45% sequence identity to SCR6 and SCR7 of CFH, respectively. The 3 C-terminal SCRs are almost identical to the C-terminus of CFH, except for the residues L290 and A296 in SCR5 of CFHR1 that correspond to S1191 and V1197 in SCR20 of CFH, respectively. The CFHR1 plasma concentration is approximately 70 to 100 μg/mL (1.6 to 2.4 μM; data not shown) and thus comparable with the concentrations of the terminal complement components C5 to C9. Due to its association with high-density lipoproteins,19 the concentration of CFHR1 in the circulation might actually be higher.
CFHR1 is a complement regulator acting in the late alternative and early terminal complement pathway: (1) CFHR1 is—to our knowledge—the first human regulator of the C5 convertase of the alternative pathway that does not inhibit the C3 convertase. CFHR1 at physiologic concentrations inhibits C5 cleavage and prevents C5a generation (Figure 3). This effect is in agreement with C3b and C5 binding, suggesting that CFHR1 binds C3b and C5 simultaneously and thus may contact the C5 convertase (C3bBbC3b) and the substrate C5 at the same time.4,30 CFHR1 regulates C5 convertase activity and inhibits further complement activation. The physiologic effects are inhibition of C5a and C5b generation. Thus, formation of the potent anaphylactic peptide C5a is blocked and also MAC complex formation and cytolysis.30-32
(2) CFHR1, but not CFH, inhibits assembly of C5b6(7) complexes and prevents surface attachment (Figures 3,Figure 4–5). CFHR1 may act in concert with the soluble terminal pathway inhibitors clusterin33 and vitronectin.34 Binding and inhibitory activity on the C5b6 complexes is independent of convertase activity (Figure 5). These complement regulatory functions could also be the reason why pathogens such as Candida albicans, Aspergillus fumigatus, or Pseudomonas aeruginosa bind CFHR1 to their surfaces.35-37
(3) The C-terminus of CFHR1, similar to that of CFH, binds to C3b, heparin, and cell surfaces (Figure 1, supplemental Figure 2). Thus CFHR1 likely has the capacity to discriminate between self- and foreign surfaces. The 3 C-terminal SCRs of CFHR1 bind immobilized C3b with lower affinity compared with the 3 C-terminal SCRs of CFH (KD 6.4 × 10−6 vs 2.6 × 10−6 M; supplemental Figure 2C-D). The lower affinity of CFHR1 to C3b confirms previous results that demonstrate reduced heparin and cell surface binding of a HUS-associated CFH mutant, which encompasses the CFHR1-specific residues at positions 1191 and 1197.21,38 CFHR1 competes with CFH at a heparin surface and thus reduces local CFH-mediated regulatory activity (Figure 1F). CFHR1 may replace CFH at surfaces, and reduction of CFH-mediated C3 convertase inhibition is for the gain of C5 regulatory activity. This balance may explain the opposing effects of CFHR1 deficiency: risk in HUS versus protection in AMD.
CFHR1 and CFHR3 deficiency is a predisposing factor for aHUS,12 correlates with the presence of autoantibodies to CFH,14 and defines a new subgroup of aHUS in children termed DEAP HUS (deficiency of CFHR proteins and CFH autoantibody positive).14 The risk effect of CFHR1 deficiency in aHUS suggests that the coordinated action of the 2 regulators CFHR1 and CFH is required for surface integrity in situations of complement stress. Both CFHR1 and CFH inhibit complement activation, prevent cell lysis of sheep erythrocytes, and protect human endothelial cells (Figure 2 and supplemental Figure 4). Thus the absence of CFHR1 may result in enhanced complement activation on host cell surfaces leading to endothelial cell or platelet damage and to pathology (supplemental Figure 5). This effect is in agreement with the recent detection of CFHR1 in dense deposits of patients with dense deposit disease (MPGNII), suggesting that CFHR1 plays a role in this renal disease.39 However, additional investigations with more patient samples are necessary to define the pathomechanism.
Deficiency of CFHR1 and CFHR3 is a risk factor in HUS, yet has a protective effect in AMD. Currently the reasons for these opposing effects are poorly understood. Based on the presented data, we hypothesize that the unique functions of CFHR1 and CFH are responsible for this difference. CFH is a regulator of the C3 convertase and promotes degradation of C3b and opsonization of a particle with C3b that results in phagocytosis. CFHR1 controls the later steps of the complement activation, and regulates C5 convertase activity and early MAC assembly. Thus CFHR1 blocks C5a formation and consequently inhibits inflammation. Therefore, in the absence of CFHR1 and CFHR3, local CFH binding and activity are increased, resulting in enhanced iC3b deposition and likely phagocytosis of opsonized particles. In the retina, this scenario may be advantageous for the clearance of cellular debris. The prevalence, especially of AMD, is growing, in the background of increasing longevity of the population. Starting to resolve the functional role of the disease-associated protein CFHR1 and of CFHR3 is a further step to define the underlying biologic mechanism of the complement system for the development of disorders such as AMD, HUS, and MPGN.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We especially acknowledge the patients and their family members for participating in this study. We thank Wolfgang Schmidt-Heck (Leibniz Institute for Natural Product Research and Infection Biology) for the statistical analysis of data.
The work of the authors is supported by the German Research Foundation (DFG, Bonn, Germany; Sk/2-1, Zi 432) and Pro Retina (Aachen, Germany).
Authorship
Contribution: S. Heinen, A.H., N.L., U.W., H.-M.D., S.S., K.G., T.E., S. Hälbich, and M.M. performed experiments and discussed results; R.W. generated the monoclonal antibody JHD10; U.S.-S. performed immunohistology; and P.F.Z. and C.S. conceived and directed the study and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christine Skerka, Department of Infection Biology, Leibniz Institute for Natural Product Research and Infection Biology, Hans Knöll Institute, Beutenbergstrasse 11, D-07745 Jena, Germany; e-mail: christine.skerka@hki-jena.de.