Abstract
Acute lymphoblastic leukemia (ALL) is the most common childhood cancer and remains a major cause of mortality in children with recurrent disease and in adults. Despite observed graft-versus-leukemia effects after stem cell transplantation, successful immune therapies for ALL have proven elusive. We previously reported immunostimulatory oligodeoxynucleotides containing CpG motifs (CpG ODN) enhance allogeneic Th1 responses and reduce leukemic burden of primary human ALL xenografts. To further the development of CpG ODN as a novel ALL therapy, we investigated the antileukemia activity induced by CpG ODN in a transplantable syngeneic pre-B ALL model. CpG ODN induced early killing of leukemia by innate immune effectors both in vitro and in vivo. Mice were treated with CpG ODN starting 7 days after injection with leukemia to mimic a minimal residual disease state and achieved T cell–dependent remissions of more than 6 months. In addition, mice in remission after CpG ODN treatment were protected from leukemia rechallenge, and adoptive transfer of T cells from mice in remission conferred protection against leukemia growth. To our knowledge, this is the first demonstration that CpG ODN induce a durable remission and ongoing immune-mediated protection in ALL, suggesting this treatment may have clinical utility in patients with minimal residual disease.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common form of cancer in children, accounting for one-third of all childhood malignancies and nearly 80% of all pediatric leukemias.1 Although current therapies have resulted in event-free survival approaching 75%,2 relapsed ALL comprises nearly 6% of all childhood cancers1,3 and has a much lower event-free survival of 47%.4 Furthermore, nearly half of all ALL cases occur in adults, who have a much poorer prognosis, with the majority dying of the disease.5 New treatment strategies are urgently needed for this condition.
The strategy of pursuing immune therapy as a novel therapeutic approach for ALL is supported by the observed graft-versus-leukemia effect after hematopoietic stem cell transplantation. Although transplantations and donor lymphocyte infusions have lower efficacy in ALL than in other leukemias, the consistently higher relapse rates associated with autologous and especially syngeneic transplantations compared with allogeneic transplantations are supportive of significant therapeutic immune activity.6-9 In pediatric transplantations, the development of chronic graft-versus-host disease correlates strongly with decreased relapse.8 Furthermore, posttransplantation immune suppression is associated with an increased risk of relapse.10
Despite evidence of a graft-versus-leukemia effect against ALL, enhancing the anti-ALL immune response has proven difficult to achieve. Several features of ALL may undermine the immune system's ability to mount a targeted response, including a lack of costimulatory molecule expression,11,12 the impairment of dendritic cell (DC) antigen presentation,13 and a resistance to immune cytotoxic mechanisms.14,15 Coupled with the characteristic rapid cell proliferation of ALL and the immune-compromised condition of patients receiving chemotherapy, these evasion strategies render ALL a challenging target for immune therapy. Although several strategies have been used in an attempt to overcome these obstacles, the generation of early anti-ALL immune activity, together with protective memory T-cell responses, remains an elusive goal.
We have previously reported the ability of immunostimulatory DNA-containing unmethylated CpG dinucleotides (CpG ODN) to enhance anti-ALL immune responses.16,17 These synthetic nucleic acid oligomers bind to Toll-like receptor 9 (TLR9) and act by mimicking the “danger” signal provided to the immune system by infection. We have shown that they directly alter the immunogenicity of primary human ALL blasts and generate strong anti-ALL innate immune activity in xenograft models of human ALL.16,17 To eliminate any xenograft immune influence, we wanted to evaluate whether CpG ODN stimulate a similar magnitude of antileukemia activity in a syngeneic model. Furthermore, observations in our previous NOD-scid model were limited to the innate immune system, and we sought to evaluate whether any innate immune activity in our syngeneic model would trigger more durable protection provided by the adaptive immune system in a fully immunocompetent mouse. As such, we have investigated the ability of CpG ODN to induce long-term control of established leukemia in a syngeneic setting using a transplantable model of pediatric ALL derived from the Eμ-Ret transgenic mouse.18,19
Methods
Cell lines and tissue culture
Cell lines 83, 289, t309, and 420.2 were all previously derived from primary leukemias developing in Eμ-Ret fusion protein (RFP+) transgenic mice on a BALB/cJ background.18,19 In addition to the wild-type lines cited in the previous sentence, stably transduced green fluorescent protein (GFP)-expressing cell lines 289 and t309 were used in some experiments. These cell lines were generated by brief 2-hour exposure to a high-titer, GFP-expressing, third-generation, self-inactivating vector,20 in vitro passage, and subsequent cell sorting for GFP-bright (GFP+) cells. Cell lines were cultured in RPMI medium with 20% fetal bovine serum and 3% interleukin-7 supernatant, as previously described.21
For evaluation of direct effects of CpG ODN, RFP+ cell lines (2 × 105 cells per well in a 96-well plate) were cultured with either CpG ODN 1826, 6 μg/mL (class B: sequence TCC ATG ACG TTC CTG ACG TT on a phosphorothioate backbone; Coley Pharmaceuticals) or phosphate-buffered saline (PBS). To assess cell proliferation, methylthiazol tetrazolium bromide assays were performed after 72 hours of culture, as described previously.22 For direct toxicity assays, cell lines were plated and treated as described in the first sentence of this paragraph for 48 hours, harvested, stained with the vital dye 7-amino-actinomycin D (7-AAD; BD Biosciences), and analyzed by flow cytometry (fluorescence-activated cell sorter [FACS]) on a FACSCalibur cytometer (BD Biosciences) using CellQuest software (BD Biosciences). Treated cells were also stained with rat anti–mouse fluorescein isothiocyanate–conjugated anti-CD40 and phycoerythrin-conjugated anti-CD86 (BD Biosciences) for assessment of costimulatory molecule expression by FACS.
Mice
Wild-type BALB/cJ, Rag-1-deficient BALB/cJ (RAG1−/−), interferon-γ (IFN-γ)–deficient BALB/cJ (IFN−/−), and common γ chain–deficient NOD-scid mice (NSG) mice were obtained from The Jackson Laboratory. RFP-transgenic mice on a BALB/cJ background were maintained as a breeding colony at the Children's Hospital of Philadelphia. All strains were housed under specific pathogen-free conditions in micro-isolators. Male and female mice more than 5 weeks of age were used for the experiments in this study. All experiments were conducted under the supervision of the Children's Hospital of Philadelphia Institutional Animal Care and Use Committee according to an Institutional Animal Care and Use Committee-approved protocol.
Reverse-transcriptase polymerase chain reaction assay
Total cellular RNA was isolated from each cell line using the RNeasy Mini Kit (QIAGEN) and cDNA generated by oligo dT–primed reverse-transcription (Invitrogen). cDNA (100 ng) was used in each polymerase chain reaction (PCR). TLR9 (forward 5′-AGA TTA GTC AGC GGC AGG AA-3′; reverse 5′-ACT GAG CAC CCC TGC TTC TA-3′) and HPRT1 (forward 5′-CCT GCT GGA TTA CAT TAA AGC ACT G-3′; reverse 5′-CCT GAA GTA CTC ATT ATA GTC AAG G-3′) primers were synthesized by Integrated DNA Technologies. Thirty-five cycles of polymerase chain reaction amplification were performed, and amplification products were then analyzed by agarose gel electrophoresis and visualized by ethidium bromide staining.
In vitro cell-mediated cytotoxicity assay
To assess CpG ODN-induced killing of ALL in a syngeneic setting, we adapted a flow cytometry–based killing assay in which target cells are stained with PKH-26 (Sigma-Aldrich), a lipophilic fluorescent membrane dye, before culturing with splenocytes.23 Single-cell suspensions of splenocytes and bone marrow cells from syngeneic mice were prepared by passage through 40-μm filters. Red blood cells were lysed by incubation in sterile Tris-ammonium chloride solution at 37°C for 10 minutes. Depletion of NK (CD49b+), T (CD3+), and B (B220+) cells from effector cell populations was achieved using EasySep positive selection kits (StemCell Technologies). For depletion of DCs and macrophages, splenocytes and bone marrow cells were incubated for 6 hours at 37°C in CO2 5% with silicon dioxide 600 μg/mL (Sigma-Aldrich).24,25 Cells were stained with phycoerythrin-conjugated F4/80 antibody (eBioscience) to confirm depletion of macrophages by FACS. Antibody-mediated cytokine blockade was achieved by adding 4 μg/mL anti–interleukin-12 (IL-12; BD Biosciences), anti–IFN-α (Abcam Inc), and anti–IFN-γ (BD Biosciences) at the commencement of the killing assays. Supernatants from CpG ODN-treated cell lines were assessed for cytokine production using a Mouse Inflammation Cytokine Bead Array kit (BD Biosciences) as per the manufacturer's instructions.
Splenocyte- or bone marrow–derived effector cells were cocultured in triplicate at increasing effector/target (E:T) ratios from 0:1 to 100:1 with 6000 PKH-stained RFP+ leukemia target cells in a 96-well U-bottom plate, with CpG ODN 6 μg/mL or PBS, as a control. In addition to the class B (B cell–activating) CpG ODN 1826, we performed some in vitro assays with the class C (combined B cell– and DC-activating) CpG ODN 239526 (Coley Pharmaceuticals, sequence TCG TCG TTT TCG GCG CGC GCC G) for comparison. After 24 hours, cells were stained with 7-AAD and allophycocyanin-conjugated rat anti–mouse B220 (B220-APC; Beckman Coulter). CountBright absolute counting beads (Invitrogen) were added to each tube to ensure equal sampling during flow cytometry acquisition. PKH+/B220-intermediate (B220int) cells were then analyzed for 7-AAD uptake using WinList analysis software (Verity Software House).
In vivo assays
To generate cohorts of leukemia-bearing mice, we adoptively transferred 106 GFP+ 289 (289-GFP) cells into syngeneic mice by tail vein injection. Mice were randomized on day 7 after injection to receive CpG ODN 1826, 100 μg in 100 μL, or PBS, 100 μL intraperitoneally every 4 days for 3 doses. In this model, there is a 0% to 20% nonengraftment rate; and by beginning treatment before peripheral detection of blasts, there is a risk of including some nonengrafters on each treatment arm; however, this risk should be randomly distributed among the arms and would not introduce bias. Three mice from each arm were killed on day 28, and blood, bone marrow, and spleen were analyzed for the presence of GFP+/B220int cells. The remaining mice were assessed weekly for GFP+/B220int cells in peripheral blood. In studies involving wild-type t309 cells, lymphoblasts were detected by staining with anti–B220-APC and anti–BP-1-phycoerythrin antibodies (Beckman Coulter). For the evaluation of costimulatory molecule expression changes on leukemia cells in vivo, 289-GFP–bearing mice were killed 48 hours after CpG ODN treatment, and leukemia cells in blood, bone marrow, and spleen were analyzed for CD40 and CD86 expression. For in vivo cell-depletion assays, mice were treated with asialo-GM1 (Cedarlane), anti-CD4 or anti-CD8 antibodies 200 μg (BioExpress), 1 day before each CpG ODN treatment. Cell depletion was confirmed by flow cytometric analysis of cell populations in peripheral blood. The FACS analyses were made using either WinList (Verity Software House) or FlowJo analysis software (TreeStar Inc). T cell adoptive transfer was performed using cells from naive mice or leukemia-bearing mice treated with CpG ODN as described. Spleens were harvested and negatively selected for T cells using an EasySep mouse T cell enrichment kit (StemCell Technologies). T cell–enriched splenocytes were admixed with 105 289-GFP cells and adoptively transferred via tail vein injection into NSG mice with 1 donor spleen per recipient mouse.
Statistical analyses
Statistical analyses were performed using Prism 4 for Windows (GraphPad Software). Data points for the in vitro assays represent means of triplicates. Statistics for comparisons of means were made using the Mann-Whitney test, a nonparametric test that assumes a non-Gaussian distribution and is the most conservative choice for a small sample size. Similarly, for comparisons among multiple groups, all experiments included at least one arm in which the sample size was less than 12; therefore, to be most conservative, Kruskal-Wallis analyses were performed with Dunn Multiple Comparison Tests to compare individual groups. In experiments with multiple independent factors, 2-way analyses of variance (ANOVAs) were performed to determine row, column, and interaction statistics. In all experiments, the interaction term was significant (P < .001) and as such renders the individual row and column statistics difficult to interpret; all statistics presented for factorial ANOVAs state the P value for the interaction terms. Kaplan-Meier statistics were generated for survival curves. A Bonferroni correction was used when multiple survival comparisons were made.
Results
CpG ODN have a minimal direct effect on cell lines
We hypothesize that the CpG ODN effect is primarily through activation of immune effector cells against ALL cells rather than a direct effect on the ALL cells; however, previous studies show stimulation of some human leukemic blasts by CpG ODN, an effect that varies widely among different leukemias.17,27,28 Therefore, we evaluated 4 RFP+ cell lines for direct responses to CpG ODN. All 4 lines expressed TLR9, as detected by reverse-transcriptase polymerase chain reaction (Figure 1A). We noted minimal to moderate differences between CpG ODN- versus PBS-treated cell lines when we evaluated by flow cytometry for direct cytotoxicity (Figure 1B). Similarly, mild decreases in proliferation were observed using methylthiazol tetrazolium bromide assays (data not shown). In addition, cell lines exposed to CpG ODN in vitro (Figure 1C) or in vivo (data not shown) showed negligible increases in cell surface expression of the costimulatory molecules CD40 and CD86. Supernatant taken from cell cultures treated with CpG ODN showed no increases in proinflammatory cytokine production by cytokine bead analysis compared with control (data not shown).
Direct responses of ALL cell lines to CpG ODN. (A) Reverse-transcriptase polymerase chain reaction expression of TLR9 by RFP-ALL cell lines. (B) Direct cytotoxicity of CpG ODN to RFP cell lines. RFP-ALL cell lines were incubated in the presence of CpG ODN 6 μg/mL or PBS for 48 hours, then stained with the viability marker 7-AAD and analyzed by FACS. Percentages of ALL cells that are 7-AAD+ are shown. (C) Expression of costimulatory molecules. ALL cell lines were treated in vitro with CpG ODN 6 μg/mL or PBS and incubated for 24 hours. Cells were stained for the costimulatory molecules CD40 and CD86 and assessed by FACS.
Direct responses of ALL cell lines to CpG ODN. (A) Reverse-transcriptase polymerase chain reaction expression of TLR9 by RFP-ALL cell lines. (B) Direct cytotoxicity of CpG ODN to RFP cell lines. RFP-ALL cell lines were incubated in the presence of CpG ODN 6 μg/mL or PBS for 48 hours, then stained with the viability marker 7-AAD and analyzed by FACS. Percentages of ALL cells that are 7-AAD+ are shown. (C) Expression of costimulatory molecules. ALL cell lines were treated in vitro with CpG ODN 6 μg/mL or PBS and incubated for 24 hours. Cells were stained for the costimulatory molecules CD40 and CD86 and assessed by FACS.
The primary goal of this study was to measure CpG ODN-induced anti-ALL immune activity by effector cells rather than its direct effects on lymphoblasts; therefore, for the subsequent studies, we chose cell lines 289 and t309 because they had a minimal direct response to CpG ODN treatment. No differences were observed in the direct response to CpG ODN between parental 289 cells and the GFP-labeled 289 clone used in the ensuing in vivo studies (data not shown).
CpG ODN stimulate syngeneic antileukemia activity in vitro
To investigate early CpG ODN-induced innate antileukemia activity, we adapted a 24-hour cytotoxicity assay culturing ALL cell lines with syngeneic lymphocytes and assessing for viability of target cells by 7-AAD staining using FACS. We observed no significant cell death in ALL cells cocultured with PBS-treated splenocytes; however, with CpG ODN stimulation, there was a significant increase in leukemic cell death with increasing E:T ratio (P for interaction < .001; Figure 2A). A similar pattern of CpG ODN-induced killing of leukemia cells was obtained using bone marrow cells as the source of effectors (P for interaction < .001; Figure 2B). Cell lines t309 and 420.2 also showed increased killing by CpG ODN-treated splenocytes (data not shown). No additional killing was detected after a second 24-hour incubation after the addition of fresh CpG ODN.
CpG ODN-induced cytotoxicity against syngeneic ALL. RFP-ALL cell lines were stained with PKH and cocultured in triplicate with either syngeneic murine splenocytes (A) or bone marrow (B) in the presence of CpG ODN or PBS. After 24 hours, cells were harvested, stained for B220 and 7-AAD, and analyzed by FACS. Each graph shows a single representative experiment. Percentages of 7-AAD+ ALL cells (PKH+/B220+) are depicted when cultured with PBS- or CpG ODN-stimulated effector cells at increasing E:T ratios (2-way ANOVA, P < .001 for each graph).
CpG ODN-induced cytotoxicity against syngeneic ALL. RFP-ALL cell lines were stained with PKH and cocultured in triplicate with either syngeneic murine splenocytes (A) or bone marrow (B) in the presence of CpG ODN or PBS. After 24 hours, cells were harvested, stained for B220 and 7-AAD, and analyzed by FACS. Each graph shows a single representative experiment. Percentages of 7-AAD+ ALL cells (PKH+/B220+) are depicted when cultured with PBS- or CpG ODN-stimulated effector cells at increasing E:T ratios (2-way ANOVA, P < .001 for each graph).
To measure the contribution of individual innate immune cell types to the observed CpG ODN-induced antileukemia activity, we performed the cytotoxicity assays using splenocytes depleted of various cell populations of interest. NK-cell depletion resulted in a partial but statistically significant decrease in ALL cell death in cytotoxicity assays (2-way ANOVA, P < .001). A similar reduction in killing was observed when macrophages were depleted from the splenocyte population by incubation with silica. Furthermore, the effect of NK-cell and macrophage depletion was additive, with double-depletion resulting in further decreased killing. However, detectable killing was obtained even with the double-depleted effector cells. Bone marrow effector cells showed similar abrogation of antileukemia activity with NK cell, macrophage, and combined depletions (data not shown). Depletion of neither CD3+ nor B220+ cells decreased cytotoxicity (data not shown). The combined blockade of IFN-α, IFN-γ, and IL-12 during the cytotoxicity assay also attenuated killing, albeit modestly (P < .001 for interaction and P < .001 for comparisons of CpG ODN vs CpG ODN + blocking antibodies at E:T ratios of ≥ 25:1).
CpG ODN stimulate syngeneic antileukemia activity in vivo
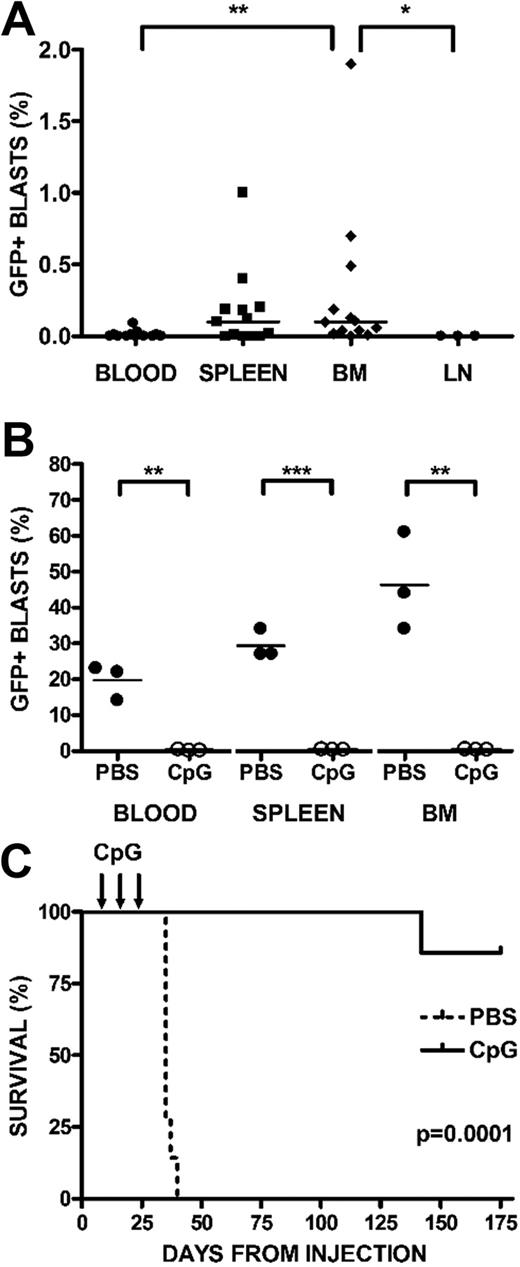
BALB/cJ mice were injected with syngeneic 289-GFP. Starting at day 7 after injection, mice were randomized to receive CpG ODN 100 μg or PBS intraperitoneally every 4 days for 3 doses. Treatment began before detection of 289-GFP in the peripheral blood; to confirm that this time point was consistent with a minimal residual disease (MRD) status, we killed several mice at day 7 and demonstrated that GFP-expressing B220int leukemia cells were primarily detectable in bone marrow (11 of 13 mice, median, 0.1%; range, 0%-1.9%) and spleen (8 of 13 mice, median, 0.1%; range, 0%-1%) but not in blood (2 of 13 mice, median, 0%; range, 0%-0.09%) or lymph nodes (0 of 3 mice, 0%; Figure 3A). The only statistically significant differences in these compartments were between bone marrow and blood (P < .01) and bone marrow and lymph node (P < .05).
CpG ODN stimulate syngeneic antileukemia activity in vivo. BALB/cJ mice were injected with syngeneic, GFP+ ALL via tail vein. On day 7 after injection, mice were randomized to receive either CpG ODN or PBS via intraperitoneal injection every 4 days for 3 doses. A subset of mice was killed at day 7, and blood, bone marrow (BM), spleens, and lymph nodes (LN) were harvested, stained for B220, and analyzed by FACS. (A) Day 7 engraftment. Peripheral blood, spleen, BM, and LN are depicted at day 7, when treatment begins. Percentages of GFP+/B220+ cells are shown, with significant differences noted only between BM and blood (**P < .01) and BM and LN (*P < .05). (B) Day 28 engraftment. Percentages of GFP+/B220+ cells in peripheral blood, BM, and spleen after treatment with PBS or CpG ODN in mice killed at day 28. CpG ODN treatment conferred significant protection in blood P < .003, marrow P < .005, and spleen (P < .001). (C) Survival after CpG treatment. The remaining mice were followed with weekly peripheral blood assessments, analyzed by FACS for B220 and GFP, and killed when peripheral blasts were more than 70% or with signs of clinical illness. Kaplan-Meier analysis of mice receiving CpG ODN versus PBS controls followed for 6 months after treatment is depicted (P < .001).
CpG ODN stimulate syngeneic antileukemia activity in vivo. BALB/cJ mice were injected with syngeneic, GFP+ ALL via tail vein. On day 7 after injection, mice were randomized to receive either CpG ODN or PBS via intraperitoneal injection every 4 days for 3 doses. A subset of mice was killed at day 7, and blood, bone marrow (BM), spleens, and lymph nodes (LN) were harvested, stained for B220, and analyzed by FACS. (A) Day 7 engraftment. Peripheral blood, spleen, BM, and LN are depicted at day 7, when treatment begins. Percentages of GFP+/B220+ cells are shown, with significant differences noted only between BM and blood (**P < .01) and BM and LN (*P < .05). (B) Day 28 engraftment. Percentages of GFP+/B220+ cells in peripheral blood, BM, and spleen after treatment with PBS or CpG ODN in mice killed at day 28. CpG ODN treatment conferred significant protection in blood P < .003, marrow P < .005, and spleen (P < .001). (C) Survival after CpG treatment. The remaining mice were followed with weekly peripheral blood assessments, analyzed by FACS for B220 and GFP, and killed when peripheral blasts were more than 70% or with signs of clinical illness. Kaplan-Meier analysis of mice receiving CpG ODN versus PBS controls followed for 6 months after treatment is depicted (P < .001).
Three mice from each group were killed on day 28, 2 weeks after the last treatment was administered, and blood, bone marrow, and spleens were analyzed by flow cytometry for GFP+ ALL. Although PBS-treated mice had a significant proportion of GFP+ cells in all 3 compartments, ALL cells were undetectable in the blood (P < .003), marrow (P < .005), and spleens (P < .001) of CpG ODN-treated mice (Figure 3B). In every experiment, PBS-treated mice generally died of disease within 6 weeks, whereas CpG ODN-treated mice remained disease-free beyond day 175 after injection (P < .001; Figure 3C).
CpG ODN induce long-term protection from ALL
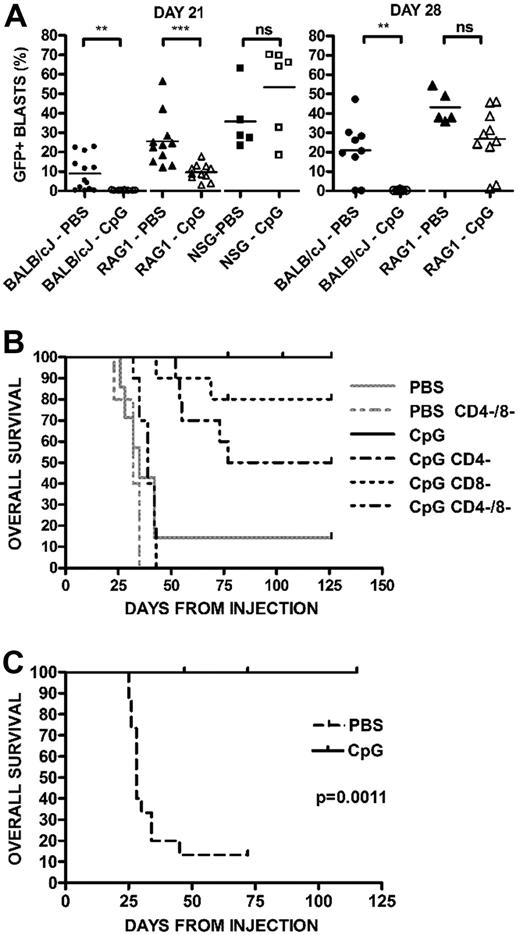
To assess the role of innate and adaptive immune responses induced by CpG ODN against syngeneic ALL, we administered CpG ODN therapy in BALB/cJ, RAG1−/− (T and B cell–deficient, but intact innate immunity) and NSG (deficient in both adaptive and innate immunity) mice. Mice were injected with 289-GFP cells and began CpG ODN treatment on day 7. An early role for innate responses is revealed at day 21 when the RAG1−/− mice treated with CpG ODN had a significantly lower disease burden than PBS-treated RAG−/− mice (P < .001; Figure 4A left panel), in contrast to the NSG mice, which showed no protective effect with CpG ODN. However, by day 28, the CpG ODN-treated RAG1−/− mice lost their protection from ALL (Figure 4A right panel), suggesting that an adaptive response is required for prolonged therapeutic benefit.
Evidence of innate and adaptive immune responses. (A) CpG ODN activity in wild-type, RAG1−/− BALB/cJ, and NSG mice. Mice were injected with GFP+ ALL and treated with CpG ODN. Percentages of GFP+/B220+ cells in peripheral blood samples on day 21 after injection from PBS- and CpG-treated BALB/cJ wild-type (N = 13 PBS, 10 CpG), RAG−/− (N = 11 PBS, 11 CpG), and NSG (N = 5 PBS, 6 CpG) mice are shown. ***P < .001. **P < .01. NS indicates not significant. The right panel depicts the same mice at day 28, at which point the RAG1−/− mice have no difference between treated and untreated arms. NSG mice in both arms were dead of disease and are not depicted. (B) Survival of PBS- and CpG-treated BALB/cJ with and without T depletion. Wild-type BALB/cJ mice were given syngeneic GFP+ ALL and were treated with CD4- and CD8-depleting antibodies before each CpG ODN treatment. Kaplan-Meier analysis of mice receiving CpG ODN versus PBS controls followed for 4 months. Combined depletion of both CD4+ and CD8+ cells (CD4−/8−) results in a marked decrease in survival from nondepleted, CpG ODN-treated mice (P < .001). (C) Survival in IFN-γ−/− mice. IFN-γ−/− mice were given ALL cells and treated with CpG ODN or PBS, as in panel A, beginning on day 7. CpG ODN-treated mice have significantly prolonged survival compared with PBS-treated control (P < .002).
Evidence of innate and adaptive immune responses. (A) CpG ODN activity in wild-type, RAG1−/− BALB/cJ, and NSG mice. Mice were injected with GFP+ ALL and treated with CpG ODN. Percentages of GFP+/B220+ cells in peripheral blood samples on day 21 after injection from PBS- and CpG-treated BALB/cJ wild-type (N = 13 PBS, 10 CpG), RAG−/− (N = 11 PBS, 11 CpG), and NSG (N = 5 PBS, 6 CpG) mice are shown. ***P < .001. **P < .01. NS indicates not significant. The right panel depicts the same mice at day 28, at which point the RAG1−/− mice have no difference between treated and untreated arms. NSG mice in both arms were dead of disease and are not depicted. (B) Survival of PBS- and CpG-treated BALB/cJ with and without T depletion. Wild-type BALB/cJ mice were given syngeneic GFP+ ALL and were treated with CD4- and CD8-depleting antibodies before each CpG ODN treatment. Kaplan-Meier analysis of mice receiving CpG ODN versus PBS controls followed for 4 months. Combined depletion of both CD4+ and CD8+ cells (CD4−/8−) results in a marked decrease in survival from nondepleted, CpG ODN-treated mice (P < .001). (C) Survival in IFN-γ−/− mice. IFN-γ−/− mice were given ALL cells and treated with CpG ODN or PBS, as in panel A, beginning on day 7. CpG ODN-treated mice have significantly prolonged survival compared with PBS-treated control (P < .002).
The role of T cells in the adaptive response was directly evaluated by cell depletion analyses. Combined CD4 and CD8 depletion had a significant effect on survival in wild-type BALB/cJ mice (P < .001 vs CpG ODN treatment without T depletion; Figure 4B). Consistent with previous results, none of the CpG ODN-treated nondepleted BALB/cJ mice had detectable peripheral blasts. Individual depletion of either CD4 or CD8 resulted in some breakthrough leukemia: CD4-depletion results in an incomplete but statistically significant incidence of breakthrough disease at a delayed time point (P < .003; Bonferroni correction requires P < .01 for significance) versus nondepleted mice, whereas CD8 depletion results in some breakthrough events but without statistical significance compared with either the nondepleted or singly CD4-depleted arms. We also noted a significant diminution of CD4+ cells after administration of CpG ODN itself with a median peripheral CD4+ percentage of 19.1% (range, 16.6%-26.6%) in PBS-treated mice versus 4.76% (range, 3.94%-5.71%; P < .03) in CpG ODN-treated mice. Depletion of NK cells did not affect the generation of protective responses (data not shown). In addition, this effect was independent of IFN-γ, as IFN−/− BALB/cJ CpG-treated mice achieved durable remission whereas untreated mice quickly died of disease (P > .001; Figure 4C).
CpG ODN treatment generates ongoing T cell–mediated protection from leukemia
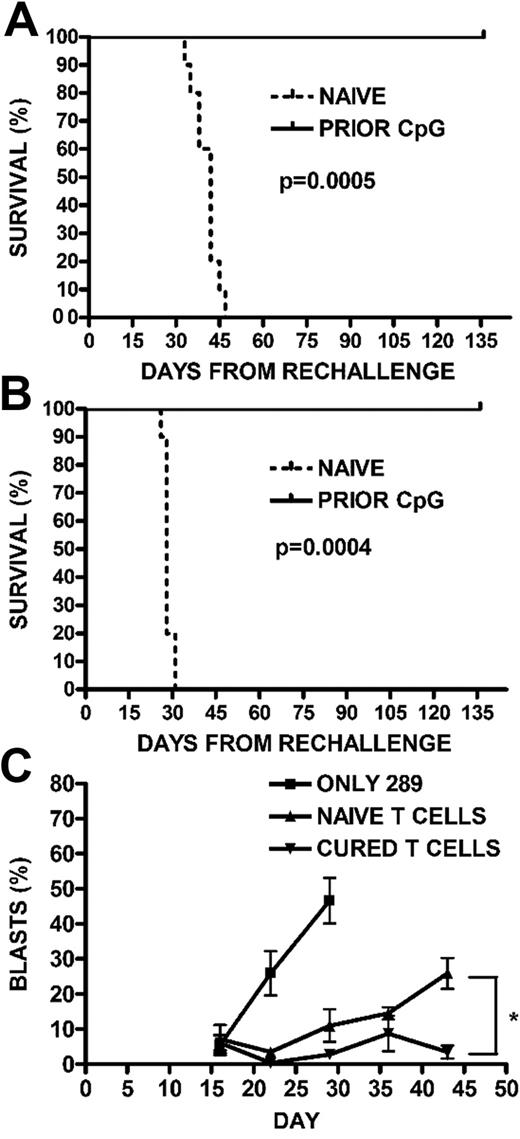
We next investigated whether long-term disease-free survival achieved with early CpG ODN treatment correlated with ongoing protection from leukemia. CpG ODN-treated mice that were in remission from their 289-GFP ALL were rechallenged with 105 289-GFP cells 5 weeks after their initial leukemia injection, that is, after all of the PBS-treated controls had died of disease. Controls for the rechallenge arm of this experiment included both leukemia-naive untreated mice and leukemia-naive but CpG ODN-treated mice. All groups were then followed for disease progression. By day 47, all of the leukemia-naive mice, both untreated and CpG ODN-treated, had died of disease, whereas none of the previously CpG ODN-treated mice had peripheral GFP+ ALL detectable by flow cytometry (P < .001; Figure 5A). An additional cohort of mice was engrafted with wild-type t309 cells, treated with CpG ODN, and rechallenged as in the previous experiment. Mice with t309 who were previously CpG ODN-treated and then rechallenged with wild-type t309 also failed to develop leukemia, whereas the leukemia-naive arms died of disease by day 31 after rechallenge (P < .001; Figure 5B), suggesting that these observations are not limited to one cell line or to the presence of GFP as an antigen. Finally, to evaluate the ability of T cells to inhibit leukemia cell expansion in the absence of other adaptive immune mechanisms, we adoptively transferred T cells from CpG-treated leukemic mice or CpG-treated leukemia-naive mice into NSG mice. Engraftment with T cells from the leukemia-bearing treated mice controls development of peripheral blasts (P < .02; Figure 5C).
Generation of protective memory responses. (A) Survival after rechallenge with 289-GFP ALL. Previously, CpG ODN-treated mice in remission were rechallenged with 105 GFP+ ALL cells, along with a leukemia-naive cohort. The survival curve for mice given GFP+ 289 is shown (P < .001). (B) Survival after rechallenge with wild-type t309 ALL. To demonstrate that the observed protection from ALL was not limited to a single cell line or to the presence of GFP as an antigen, we repeated the rechallenge experiment in mice who had been cured of wild-type t309, as in panel A. The survival curve for mice followed for 4 months is shown (P < .001). (C) Adoptive transfer of T cells into NSG mice. BALB/cJ mice were given CpG ODN 100 μg every 4 days for 3 doses, as in panels A and B, beginning on day 7 after injection with 106 GFP+ ALL cells. A group of nonleukemic mice were also injected with CpG ODN. One month after injection, mice in both groups were killed, and T cells were magnetically selected from their splenocytes to be injected into NSG mice along with 105 GFP+ ALL cells. Mice were followed for development of peripheral blasts by FACS, with mice receiving ALL-targeted T cells showing lower percentages than stimulated but naive T cells (*P < .02).
Generation of protective memory responses. (A) Survival after rechallenge with 289-GFP ALL. Previously, CpG ODN-treated mice in remission were rechallenged with 105 GFP+ ALL cells, along with a leukemia-naive cohort. The survival curve for mice given GFP+ 289 is shown (P < .001). (B) Survival after rechallenge with wild-type t309 ALL. To demonstrate that the observed protection from ALL was not limited to a single cell line or to the presence of GFP as an antigen, we repeated the rechallenge experiment in mice who had been cured of wild-type t309, as in panel A. The survival curve for mice followed for 4 months is shown (P < .001). (C) Adoptive transfer of T cells into NSG mice. BALB/cJ mice were given CpG ODN 100 μg every 4 days for 3 doses, as in panels A and B, beginning on day 7 after injection with 106 GFP+ ALL cells. A group of nonleukemic mice were also injected with CpG ODN. One month after injection, mice in both groups were killed, and T cells were magnetically selected from their splenocytes to be injected into NSG mice along with 105 GFP+ ALL cells. Mice were followed for development of peripheral blasts by FACS, with mice receiving ALL-targeted T cells showing lower percentages than stimulated but naive T cells (*P < .02).
Discussion
The results of this study show that CpG ODN treatment induces significant immune activity against preexisting ALL in a syngeneic setting, resulting in long-term control of disease. These results confirm and extend our previous findings of in vivo control of ALL by CpG ODN-induced innate immune responses and demonstrate the ability of CpG ODN to generate durable remissions in mice with established bone marrow disease. These results argue strongly for the further development of this agent for clinical application in the treatment of ALL.
The generation of T-cell responses by ALL is inefficient and indeed often leads to anergy rather than activation. This is thought to be the result of the lack of costimulatory molecule expression by the majority of ALL cells, which contributes to the generation of anergic T cells,12,29 and the dysfunction of DCs in pediatric ALL patients.13 The manipulation of either leukemic blasts or DCs ex vivo to enhance leukemia antigen presentation and costimulation has been shown to be sufficient for inducing Th1 T-cell responses.12,29 Interestingly, a block in the differentiation of CD4+ Th1 T cells in vivo has recently been reported to prevent the generation of protective responses in a mouse model of BCR-ABL+ ALL.30 The results presented here demonstrate that the administration of CpG ODN to leukemia-bearing mice is sufficient to overcome the endogenous deficiencies in anti-ALL immune responses and generates long-term protection against disease in the absence of cell manipulation or vaccination. The promising vaccine responses achieved recently using leukemia cells combined with CD40 ligand and IL-2 indicate that antigen-presenting cell maturation in the presence of leukemia antigens can lead to the generation of detectable anti-ALL T-cell responses in patients.31 Our study suggests that the use of CpG ODN may provide a simpler strategy to achieve both the killing of the residual leukemia and coincident maturation of antigen-presenting cells necessary to efficiently prime protective T-cell responses in vivo.
Whereas the activity observed in our model could be the result of direct stimulation of leukemia cells, the results are consistent with our earlier reports; the CpG ODN-generated control of ALL in vivo is independent of the ability of the leukemia cells to respond directly.16 Rather than CpG ODN-mediated improvement in antigen-presentation by the blasts themselves, our results indicate that the observed antitumor effect is the result of stimulation of the immune system, which results in antigens from leukemia cells killed by innate immune responses being taken up and presented by activated antigen-presenting cells. Our observation that CpG ODN treatment in the absence of leukemia does not lead to protection from rechallenge supports a model in which killed leukemic cells are the source of antigen for the protective adaptive response. Whereas all mouse ALL cell lines used in this study expressed TLR9, the cells showed little response to CpG ODN treatment as measured by changes in cell viability, proliferation, cytokine production, and costimulatory molecule expression. For our in vivo assays, we expressly chose cell lines with minimal TLR9 responses to bias experiments against a requirement for TLR9 expression by the target cells. The lack of a direct response and the near-complete cure rates of ALL with low TLR9 expression argue strongly that the observed antileukemia immune activity generated by CpG ODN treatment is the result of changes triggered by TLR9-expressing immune effector cells rather than an effect on the targets themselves. This finding suggests that CpG ODN treatment may be applicable even to patients with ALL blasts that do not express TLR9.
Others have reported CpG ODN activity that may be detrimental to the leukemia-bearing host.27,28 However, these results were found primarily in mature B-cell malignancies; and more importantly, we have seen no evidence of responses to CpG ODN that would contribute to ALL progression. We have previously reported that immature and mature B cells respond differently to CpG ODN stimulation,17 which may be responsible for the lack of detrimental effects of CpG ODN in precursor B-cell ALL. Nevertheless, any potential pro-leukemic activity may be masked by the strong anti-ALL immune responses generated by CpG ODN treatment in our model.
The activity against preexisting disease achieved by early intervention with CpG ODN is similar to results reported using exogenous IL-12 administration.32,33 The comparable immune activity generated by these 2 interventions is not surprising given the role of IL-12 in mediating downstream effects of CpG ODN. However, the ability of IL-12 to induce protective memory T-cell responses has been mixed and appears to be profoundly influenced by the timing and dosage used. Recently, IL-12 has been shown to generate long-term protection against intraperitoneally administered leukemia rechallenge.32 This long-term protection was restricted to protection by CD4+ T cells. IFN-γ is a key mediator of IL-12–induced antitumor activity. However, this antitumor activity may ultimately be undermined by the antagonistic effects of elevated IFN-γ, either via up-regulation of inflammatory cytokines, such as IL-10,33 or by induction of apoptosis in CD4+ T cells, which are required for memory induction.34 We observed a reduction in CD4+ cells after CpG ODN treatment, yet our in vivo model supports a durable protective effect for 6 months of observation despite this diminution in a memory cell compartment. Furthermore, single depletion of CD4+ cells results in breakthrough leukemia compared with T-replete mice, implicating CD4+ T cells as important mediators of this effect; however, the decreased survival in CD4-depleted mice is incomplete and delayed compared with depletion of both CD4+ and CD8+ T cells, suggesting that both cell types are necessary for complete long-term protection from ALL.
CpG ODN have been shown to induce production of IL-12, as well as nitric oxide, IFN-γ, TNF-α, and IL-6, each of which has been demonstrated to have a role in tumor control in various models.35,36 By stimulating a broader set of immune activation pathways, CpG ODN may provide a more effective and physiologic strategy to achieve the required long-term protection against ALL progression than an isolated cytokine, such as IL-12. Our model supports the early involvement of both macrophages and NK cells and shows efficacy in an IFN-γ−/− model, suggesting that CpG ODN stimulates the immune system upstream of multiple redundant pathways, resulting an immune response that is resistant to abrogation by knockout of individual immune effector cells or cytokines. Our finding of an effect in the IFN-γ−/− mice is consistent with reports that these mice are capable of generating functional tumor-targeted cytotoxic T cells in lymphoid tissues after immunization with tumor.37 This lack of dependence on IFN-γ may have clinical relevance as low IFN-γ producers are overrepresented in the high-risk pediatric ALL population.38
The cell depletion and cytokine blockade studies described here indicate that the generation of syngeneic anti-ALL activity requires cytokine production, involves early activity of NK cells, DCs, and macrophages, and generates a T cell–mediated adaptive response for long-term disease control. This activation pathway is consistent with those reported to mediate CpG ODN effects in many other model systems.26 Given that our previous studies with CpG ODN in xenografts with primary human ALL strongly implicated NK cells in the control of disease progression, as well as the observed diminution of in vitro killing after NK depletion and lack of early therapeutic effect in NOD-scid/γc mice in the current study, we were surprised that asialo-GM1-depletion in vivo did not affect survival after CpG ODN. A clear role for NK cells in the CpG ODN-mediated generation of tumor-specific T-cell responses has been observed in mice bearing subcutaneous B16 melanoma tumors.39 Our in vitro studies indicate a role for macrophages in the killing of leukemia cells, a finding consistent with previous reports using a weakly immunogenic murine melanoma model,35 and this cytotoxic activity may be sufficient to prime the downstream T-cell responses. Consistent with our findings, antileukemia T-cell responses generated by exogenous IL-12 can be achieved in the absence of NK cells.32 The site of leukemia killing in other tumor models is primarily in the spleen after intraperitoneal injection or subcutaneously in the case of the melanoma studies, whereas our intravenous approach was demonstrated to have primary engraftment in the bone marrow as well as spleen. The observed differences in the contributions of innate cell populations may reflect the distinct tumor sites in the different models. Although we cannot state the site of antigen priming in our model definitively, it is clear from the durable remissions achieved that the necessary effector cells are capable of trafficking to the relevant sites of MRD.
Houot and Levy recently demonstrated a CpG ODN-mediated tumor vaccine using the A20 lymphoma cell line.40 They found that intratumoral injection of high-dose CpG ODN for 5 days induced resolution of subcutaneous tumors composed of this cell line. With antibody-mediated activation of effector and inhibition of regulatory T cells, they were able to achieve systemic remission of large subcutaneous masses and protection from subcutaneous rechallenge 100 days after treatment, an effect that was obviated by either CD4 or CD8 depletion. In comparison, our model achieves a similar outcome across multiple cell lines without the requirement for external modulation of either effector or regulatory T cells. Determining whether this difference is the result of the CpG ODN regimen used, the site of disease, or the characteristics of the leukemia will be important for the development of optimal clinical protocols using this agent.
We chose an early intervention point (day 7 after injection) in an attempt to model MRD. In children, ALL therapy is unique in the duration of standard therapy, requiring low-dose maintenance chemotherapy for 2 years in girls and for as long as 3 years in boys. The historical data supporting prolonged treatment even for children who are in apparent remissions argue that there is a persistent MRD burden that requires protracted therapy to keep under control and eventually eliminate. One current MRD definition is a bone marrow blast percentage of less than 0.01% by flow cytometry. Approximately 22% of patients with B-cell ALL and up to 89% of patients with high-risk ALL subsets overall have MRD greater than 0.01% at the end of induction.41 We observed effective CpG ODN-induced elimination of disease progression at blast levels in this range, which is encouraging for the development of immune-based therapies for the treatment of children with high-risk ALL. Whereas the application of immune therapy to patients on standard therapy regimens is thought to be limited by poor immune function and need for reconstitution,42 the report of significant immune responses by high-risk ALL patients to an autologous vaccine indicates that sufficient immune cell populations are present during this treatment window. The ability of CpG ODN to induce both strong innate and adaptive antileukemia immune activity may render it an appropriate agent for therapeutic application in ALL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cecilia Sheen, Junior Hall, Theresa Ryan, Jessica Hulitt, and Yueh Chang for excellent technical assistance.
This work was supported in part by National Cancer Institute grant R03-CA123554 (G.S.D.R.) and by an Alex's Lemonade Stand Foundation Young Investigator Award and National Institutes of Health K12 award 2K12CA076931-11 (A.E.S.).
National Institutes of Health
Authorship
Contribution: A.E.S. and G.S.D.R. designed and performed research, analyzed data, and wrote the paper; D.M.B. performed research and analyzed data; M.M. and V.I.B. provided critical reagents; and S.A.G. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gregor S. D. Reid, ARC 904G, Children's Hospital of Philadelphia, Joseph Stokes Jr Research Institute, Abramson Research Center, 34th St & Civic Center Blvd, Philadelphia, PA 19104-4318; e-mail: reidg@email.chop.edu.