Abstract
The mouse Lupo (I282N) mutation in proline-serine-threonine phosphatase–interacting protein 2 (PSTPIP2) leads to reduced expression of PSTPIP2 that is associated with a macrophage-mediated autoinflammatory disease. Another mutation in PSTPIP2, L98P, termed chronic multifocal osteomyelits (cmo), leads to a disease in mice that resembles chronic recurrent multifocal osteomyelits in humans. The cellular basis of cmo disease was investigated. cmo disease develops independently of lymphocytes and is cured by bone marrow transplantation. Macrophages, mast cells, and osteoclasts from cmo mice fail to express detectable PSTPIP2 protein. Asymptomatic Pstpip2cmo/cmo mice have increased circulating levels of macrophage inflammatory protein 1-α and interleukin-6, and their macrophages exhibit increased production of these inflammatory mediators, which is normalized by retroviral expression of wild-type PSTPIP2. Spleens of asymptomatic cmo mice contain increased numbers of macrophage precursors, and cmo mice mobilize more macrophage precursors in response to a sterile inflammatory stimulus. Signal transducer and activator of transcription 1 is elevated in cmo splenic macrophages, which also exhibit increased colony-stimulating factor-1–stimulated proliferation and increased extracellular signal-regulated kinase 1/2 phosphorylation. PSTPIP2 overexpression in macrophages leads to the opposite phenotype. Thus, PSTPIP2 deficiency causes both an expansion of macrophage progenitors and increased responsiveness of mature macrophages to activating stimuli, which together prime the organism for exaggerated and sustained responses leading to autoinflammatory disease.
Introduction
Proline-serine-threonine phosphatase–interacting protein 2 (PSTPIP2),1 also known as macrophage actin-associated and tyrosine phosphorylated protein (ie, MAYP),2 belongs to the Pombe Cdc15 homology (PCH) family of proteins that has recently been shown to coordinate membrane and cytoskeletal dynamics.3,4 Several PCH proteins play important roles in immunity by regulating neutrophil migration,5 T-cell activation,6-8 cell-surface expression of Fas ligand,9,10 and cytokine production.11,12 Mutations in PSTPIP1, the PCH family member most similar to PSTPIP2, lead to pyogenic arthritis, pyoderma gangrenosum, and acne (ie, PAPA) syndrome in humans by promoting interleukin-1β (IL-1β) processing.11,13
PSTPIP2 is selectively expressed in macrophages and macrophage precursors2,12 and is an actin-bundling protein that regulates filopodia formation and macrophage motililty.14 We have previously described the Lupo Pstpip2 mutation in mice. This I282N missense mutation leads to a macrophage-mediated autoinflammatory disease characterized by skin necrosis, inflammation of paws and ears, and inflammatory bone resorption.12 PSTPIP2 expression in Pstpip2Lupo/Lupo bone marrow–derived macrophages (BMMs) was reduced 3-fold resulting from the instability of the mutant protein. In addition, Lupo macrophages exhibited increased constitutive production of markers of macrophage activation, monocyte chemoattractant protein-1 (MCP-1) and soluble tumor necrosis factor-α receptor type I (sTNFR I),12 suggesting that PSTPIP2 negatively regulates macrophage activation. Consistent with this conclusion, compared with wild-type (wt) mice, Lupo mice had elevated levels of circulating MCP-1 and other cytokines (IL-4, regulated upon activation, normal T cell expressed and secreted [RANTES], transforming growth factor-β), whereas the levels of interferon-γ (IFN-γ), leptin, TNF-α, and collagen VI were normal.12
Another mutation in PSTPIP2, L98P, was described in the chronic multifocal osteomyelitis (cmo) mouse.15,16 The cmo mouse initially causes inflammation in the caudal vertebrae and phalanges with mixed inflammatory infiltrate of polymorphonuclear leukocytes, macrophages, lymphocytes, plasma cells, and osteoclasts. Later, the inflammatory infiltrate is replaced by new bone and fibrous tissue, leading to tail kinks and hind-foot deformities. Subsequently, the mouse develops inflammation of the ears involving the dermis, epidermis, and cartilage.15,16 Here we demonstrate that cmo disease, like Lupo disease, is autoinflammatory. We further show that the PSTPIP2 deficiency causes enhanced colony-stimulating factor-1 (CSF-1) signaling, the expansion of early macrophage precursors, and increased proinflammatory cytokine release by activated macrophages. These characteristics of cmo mice are expected to engender hyperresponsiveness to trauma and infection and to contribute to the onset and relapses characteristic of this type of inflammatory disease.
Methods
Materials
Unless otherwise specified, all reagents were purchased from Sigma.
Mice and genotyping
BALB/cAnPt-cmo, BALB/cByJ, and Rag1−/− mice were obtained from The Jackson Laboratory and maintained under specific pathogen–free conditions in a barrier facility at Albert Einstein College of Medicine. Mouse breeding and the study protocols were approved by the Animal Institute at Albert Einstein College of Medicine. Pstpip2 mutation genotyping was performed by polymerase chain reaction amplification and sequencing by the use of oligonucleotides sequences 5′-CATGTCAAGGTGACAATGAAATC-3′ and 5′-ACACCTGAGGCTTCTCTGTAGAA-3′.
For bone marrow (BM) transfers, 4-week-old BALB/cAnPt-cmo or BALB/cByJ mice were irradiated with 1100 cGy in a split dose and transplanted with approximately 5 × 106 Ficoll-separated, T cell–depleted (anti-Thy1.2), mononuclear BM cells intravenously 2 hours after irradiation as previously described.17 Mice were housed in specific pathogen–free barrier cages after transplant, and Baytril (Bayer HealthCare) was added in their drinking water. Mice were inspected twice each week for 12 weeks after irradiation for evidence of the cmo phenotype, including the presence of tail kinks, paw deformities, or ear inflammation.
To examine the effect of Rag1 deficiency, cmo mice were crossed with C57BL6 Rag 1−/− mice. The resulting F1 (cmo+/−, Rag1+/−) mice were crossed to yield F2 mice. Those homozygous for the cmo mutation were genotyped at the Rag1 locus and inspected twice weekly for 12 weeks for evidence of the cmo phenotype.
Histology
Mice were euthanized by CO2 inhalation, then perfused with periodate-lysine-paraformaldehyde-glutaraldehyde fixative by injection of the fixative in the heart.18 Tissues were dissected, further fixed by immersion in periodate-lysine-paraformaldehyde-glutaraldehyde, and embedded in paraffin. Sections, stained with F4/80 antibody (a gift from Dr David A. Hume, University of Edinburgh) and counterstained with hematoxylin, were analyzed by light microscopy as previously described.12
Thioglycollate challenge
Mice (96-106 days old) were injected intraperitoneally with 1 mL of 3.8% Brewers thioglycollate broth (Becton Dickinson). After 3 days, mice were killed by exposure to CO2 and their peritoneal cells harvested by lavage with 10 mL phosphate-buffered saline (PBS). Red blood cells were lysed, and the leukocytes were counted by the use of a cell counter (Beckman Coulter). Aliquots of 106 cells were stained with F4/80 (Caltag), CD11b (Mac1), and Ly6G (Gr1) antibodies (BD Pharmingen), fixed, and analyzed by flow cytometry.
Hematopoietic progenitor assays
Asymptomatic 6- to 9-week-old cmo mice and wt control mice of matched sex and age were euthanized by CO2 inhalation. BM cells and splenocytes were counted in 3% acetic acid–containing trypan blue by use of a hemocytometer. High proliferative potential colony-forming cells (HPP-CFC) and colony-forming unit macrophages (CFU-Ms) in BM and splenic-cell suspensions were determined by the use of agar cultures as described.19 HPP-CFC cultures contained stem cell factor (SCF; 50 ng/mL) and IL-3 (20 ng/mL; StemCell Technologies). In some cultures, CSF-1 (18 ng/mL; a gift from Chiron Corporation) and/or IL-6 (20 ng/mL) were added. CFU-M cultures contained CSF-1 (18 ng/mL) with or without IL-6 (20 ng/mL; StemCell Technologies). Colony number and morphology were determined by light microscopy by the use of an Olympus CK2 inverted microscope and a 4× objective (Nikon). Images of CFU-M colonies were acquired with a Kodak DC290 digital camera equipped with an adaptor for microscope. HPP-CFC colonies were scanned by the use of an Epson Perfection 4990 scanner. Colony size was measured on digital images by the use of ImageJ software.20
Analysis of monocyte and macrophage populations by flow cytometry
Cell suspensions of BM cells were diluted in staining buffer (5% newborn calf serum [Hyclone Laboratories Inc] in balanced salt solution containing 0.1% NaN3) were stained by incubation (20 minutes at 4°C) with Cy5-15.1.4.1–labeled21 rat IgG2a anti–mouse Ly-6C monoclonal antibody, phycoerythrin (PE)–M1/70 rat IgG2b anti–mouse CD11b (Mac-1)22 antibody, and Texas Red–wheat germ agglutinin (WGA). The mAbs were ammonium sulfate precipitated from serum-free (HB101) culture supernatants and conjugated with PE (Molecular Probes) or cyanine 5.18 (Cy5; Amersham Pharmacia Biotech) by the use of standard protocols. WGA (Vector Laboratories) was conjugated with Texas Red by the use of standard protocols. Polyclonal purified rat IgG (Jackson Immunoresearch) was used for control mice. A total of 10 μL anti-CD16/32 (FcγRIII/II) mAb 2.4G2 and 10 μL rat serum (PelFreez) were added in the first incubation to eliminate background staining caused by nonspecific FcγR binding. The cells (≥ 50 000 cells/sample) were analyzed on a FACSVantage SE flow cytometer (Becton Dickinson). Spectral overlaps between FITC and PE, Cy7PE and PE, and Cy5 and Texas Red were corrected by electronic compensation. FACS data were analyzed by the use of FlowJo software (TreeStar Inc).
BMM preparation, immortalization, and retroviral infection
BMM from PSTPIP2cmo/cmo mice and wt control mice were derived23 and cultured as previously described.12 Day 10 BMM were immortalized with SV-U19.5 retrovirus encoding large T antigen,24 and cells from representative cloned cell lines were subsequently infected with an MSCV-IRES-GFP retroviral vector encoding myc-tagged PSTPIP2 as described.14 Clones expressing myc-PSTPIP2 at levels comparable with those of wt BMM or greater were selected to assess the capacity of retrovirally expressed PSTPIP2 to restore normal cytokine production.
Measurement of cytokines
Cell-culture supernatants and mouse sera initially were screened for cytokine production by the use of mouse inflammation antibody arrays (RayBiotech) or multiplexed beadlyte mouse cytokine kits (Millipore). Subsequent evaluation of macrophage inflammatory protein–1α (MIP-1α) and IL-6 production by immortalized BMM was performed by the use of enzyme-linked immunosorbent assay kits purchased from R&D Systems and eBioscience, respectively. For lipopolysaccharide (LPS) stimulation, primary or immortalized BMM were incubated with 1 μg/mL LPS as described12 and the supernatants assayed as described above.
Proliferation assays
Either day 3 BMM23 and splenocyte-derived macrophages (SDM; prepared as described for BMM23 ) or the nonadherent fraction of BM or splenic cells obtained after incubation on tissue-culture plastic (2 hours in α-minimum essential medium (MEM)–containing 10% fetal calf serum [FCS]) were used. Proliferation assays were performed by plating the nonadherent cells (104/mL) in triplicate in 30-mm diameter tissue culture dishes in 10% FCS-α-MEM and CSF-1 (36 ng/mL) alone or in combination with IL-6 (10 ng/mL). From day 3, the medium was changed daily. At daily intervals, cells were detached with 0.005% Zwittergent (Calbiochem) in PBS and counted with a cell counter. Doubling times were determined from the regions of the growth curves exhibiting logarithmic-phase growth.
CSF-1 stimulation of SDMs, immunoprecipitation, and Western blots
SDMs were prepared as described previously except that SCF (10 ng/mL) was added during the first 3 days of culture to promote the expansion of early myeloid precursors. Day 3 nonadherent cells were plated in 6-well tissue culture dishes in 15% FCS-α-MEM with 120 ng/mL CSF-1. After 7 days of culture, the adherent cells were rendered quiescent by incubation for 6 hours in 0.1% FCS-α-MEM then stimulated with CSF-1 or IL-6 in the same medium for the indicated times, rinsed with ice-cold PBS, and lysed in 2% sodium dodecyl sulfate. BAC1.2F5 macrophages25 were propagated in 10% FCS-α-MEM plus 36 ng/mL CSF-1, stimulated, and lysed as described previously.14 Proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to Immobilon membranes (Whatman), and blotted with antibodies against PSTPIP2,14 CSF-1R,24 phospho-CSF-1R Y708 (Cell Signaling Technology), phospho–extracellular signal-regulated kinase 1/2 (Erk 1/2; BioLabs), phospho-STAT1 Y701 (BD Transduction Laboratories), phospho-STAT3 (Cell Signaling Technology), STAT1 (BD Transduction Laboratories), and STAT3 (Santa Cruz). Chemiluminescence was measured by the use of a Fujifilm LAS3000 imager (Fuji) and quantified by the use of MultiGauge software (Fuji) from the manufacturer.
Results
Absence of PSTPIP2 in cmo myeloid cells
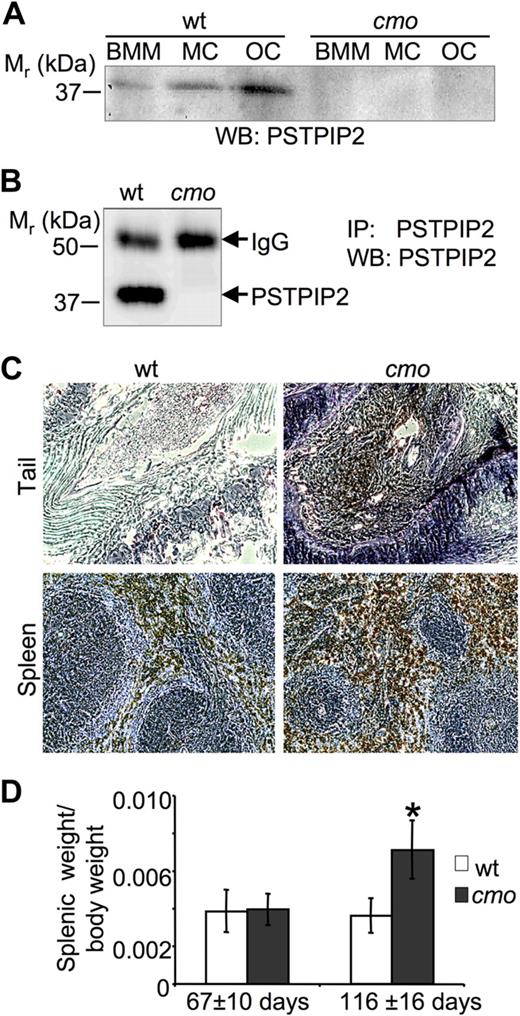
The L98P cmo mutation resides in a predicted alpha helical coiled-coil domain of PSTPIP2 (amino acids 93-121) and is expected to drastically alter the folding of PSTPIP2 by destabilizing the α helix.4,15 Indeed, we failed to detect PSTPIP2 protein expression by Western blot analysis of whole-cell lysates from cmo BMM, BM-derived mast cells, or osteoclasts, whereas wt PSTPIP2 was readily detectable (Figure 1A). To increase the sensitivity of detection of PSTPIP2, it was immunoprecipitated from NP-40 soluble lysates of BMM by the use of an anti-PSTPIP2 antibody to a C-terminal peptide located approximately 200 amino acids from the cmo mutation.14 PSTPIP2 was not detectable in immunoprecipitates from cmo BMM despite its abundance in immunoprecipitates from wt BMM (Figure 1B).
PSTPIP2 deficiency in macrophages, mast cells, and osteoclasts of cmo mice is associated with splenomegaly and increased numbers of macrophages in the spleen and tails of affected cmo mice. (A) Western blots (WB) of whole-cell lysates of bone marrow–derived macrophages (BMM), mast cells (MC), and osteoclasts (OC) obtained from wt and cmo mice probed with an antibody against PSTPIP2. (B) Absence of PSTPIP2 in immunoprecipitates (IP) from cmo BMM. IgG indicates immunoglobulin G. (C) Sections of tails and spleens of cmo mice stained for the macrophage marker F4/80 (brown) and counterstained with hematoxylin (blue) showing extensive macrophage infiltration. (D) Development of splenomegaly in cmo mice more than 13 weeks old. Data ± SD, *P < .01, Student t test; n ≥ 10.
PSTPIP2 deficiency in macrophages, mast cells, and osteoclasts of cmo mice is associated with splenomegaly and increased numbers of macrophages in the spleen and tails of affected cmo mice. (A) Western blots (WB) of whole-cell lysates of bone marrow–derived macrophages (BMM), mast cells (MC), and osteoclasts (OC) obtained from wt and cmo mice probed with an antibody against PSTPIP2. (B) Absence of PSTPIP2 in immunoprecipitates (IP) from cmo BMM. IgG indicates immunoglobulin G. (C) Sections of tails and spleens of cmo mice stained for the macrophage marker F4/80 (brown) and counterstained with hematoxylin (blue) showing extensive macrophage infiltration. (D) Development of splenomegaly in cmo mice more than 13 weeks old. Data ± SD, *P < .01, Student t test; n ≥ 10.
cmo disease is autoinflammatory and involves extramedullary hematopoiesis and macrophage accumulation
We have previously shown that the Lupo inflammatory disease is associated with macrophage accumulation in affected tissues and can be cured by macrophage depletion.12 Immunohistological examination of the kinked tails of cmo mice indicated that they similarly accumulate macrophages at sites of inflammation (Figure 1C). Also, associated with extensive splenic accumulation of F4/80-positive macrophages, from 3 months of age, cmo mice developed a significant splenomegaly (Figure 1D).
To investigate whether lymphocytes and autoantibodies are necessary for the development of disease in cmo mice, we crossed the cmo mutation onto the lymphocyte-deficient Rag1−/− background. Paw inflammation and tail kinks developed in the double-homozygous Pstpip2cmo/cmo/Rag1−/− mice with the same penetrance (100%) and age of onset (47 ± 7 vs 42 ± 9 days, n = 10, P = .22, Student t test) as in Pstpip2cmo/cmo/Rag1+/− mice, demonstrating the autoinflammatory nature of cmo disease. In addition, the disease was transferable by BM transplantation (n = 7, 100% penetrance at 3 months after transplantation) and could be cured by transplantation of wt BM cells (n = 5, 100% cure at 3 months after transplantation).
PSTPIP2-deficient cmo macrophages exhibit increased constitutive and LPS-induced proinflammatory mediator production that is corrected by re-expression of PSTPIP2
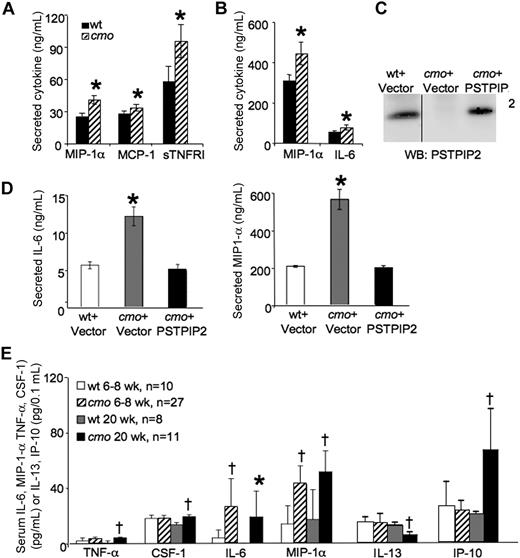
PSTPIP2 is highly expressed in macrophages and up-regulated during the late phase of the LPS response.12 To determine whether the cmo disease is associated with aberrant cytokine production by macrophages, we tested the constitutive and LPS-induced cytokine production by wt and cmo BMM against a panel of 40 inflammatory mediators (Figure 2). cmo BMM grown in the presence of CSF-1 released significantly more MIP-1α, MCP-1, and sTNFR I than wt macrophages (Figure 2A). In addition, LPS induced a greater increase in IL-6 and MIP-1α production in cmo than in wt macrophages (Figure 2B). Similar results were obtained with SDMs (data not shown). Importantly, retroviral expression of PSTPIP2 in immortalized cmo macrophages (Figure 2C) restored normal LPS-induced cytokine production (Figure 2D), demonstrating that PSTPIP2 is a negative regulator of classical macrophage activation.
Increased levels of inflammatory mediators are produced by macrophages in cmo mice before the onset of overt disease. (A) Secretion of cytokines and chemokines by cultured BMM from wt and cmo mice (n ≥ 5). (B) LPS-stimulated inflammatory mediator release by BMM of wt and cmo mice (n ≥ 5). (C) Reconstitution of PSTPIP2 expression in cmo macrophages by retroviral transduction. The vertical line indicates a repositioned gel lane from the same blot. (D) Reconstitution of PSTPIP2 expression in immortalized cmo BMM restores normal LPS-stimulated production of IL-6 and MIP-1α. (E) Screening of cytokine and chemokine levels in mouse sera from asymptomatic (6-8 weeks of age, n = 10-27) and diseased (20 weeks of age, n = 8-11) cmo mice and wt control mice. Data ± SD. *P < .05, †P < .01, Student t test. No significant differences in serum levels or BMM secretion were found for the other inflammatory mediators tested (BLC, D30L, Ltaxin, Eotaxin-2, Fas ligand, Fractalkine, G-CSF, GM-CSF, M-CSF, IFN-γ, IL-2, IL-3, IL-4, IL-9, IL-10, IL-12p40p70, IL-12p70, IL-13, IL-17, I-TAC, KC, Leptin, LIX, Lymphotactin, MIG, MIP-1γ, RANTES, SDF-1, TCA-3, TECK, TIMP-1, TIMP-2, sTNFRI, and sTNFRII).
Increased levels of inflammatory mediators are produced by macrophages in cmo mice before the onset of overt disease. (A) Secretion of cytokines and chemokines by cultured BMM from wt and cmo mice (n ≥ 5). (B) LPS-stimulated inflammatory mediator release by BMM of wt and cmo mice (n ≥ 5). (C) Reconstitution of PSTPIP2 expression in cmo macrophages by retroviral transduction. The vertical line indicates a repositioned gel lane from the same blot. (D) Reconstitution of PSTPIP2 expression in immortalized cmo BMM restores normal LPS-stimulated production of IL-6 and MIP-1α. (E) Screening of cytokine and chemokine levels in mouse sera from asymptomatic (6-8 weeks of age, n = 10-27) and diseased (20 weeks of age, n = 8-11) cmo mice and wt control mice. Data ± SD. *P < .05, †P < .01, Student t test. No significant differences in serum levels or BMM secretion were found for the other inflammatory mediators tested (BLC, D30L, Ltaxin, Eotaxin-2, Fas ligand, Fractalkine, G-CSF, GM-CSF, M-CSF, IFN-γ, IL-2, IL-3, IL-4, IL-9, IL-10, IL-12p40p70, IL-12p70, IL-13, IL-17, I-TAC, KC, Leptin, LIX, Lymphotactin, MIG, MIP-1γ, RANTES, SDF-1, TCA-3, TECK, TIMP-1, TIMP-2, sTNFRI, and sTNFRII).
PSTPIP2 deficiency leads to elevated levels of circulating inflammatory mediators in vivo
To address the significance of the increased inflammatory mediator production by macrophages for disease development in vivo, we examined the levels of circulating cytokines and chemokines in asymptomatic 6- to 8-week-old and symptomatic 20-week-old cmo mice. The 17 inflammatory mediators measured were those increased in cmo macrophages (IL-6, MIP-1α, MCP-1, and sTNFRI) as well as others (TNF-α, CSF-1, IL-1α, IL-1β, IL-4, IL-7, IL-10, IL-12, IL-13, IL-15, IP-10, KC, and RANTES) that are considered important for the development of autoinflammatory disease. In asymptomatic mice, only 2 inflammatory mediators, MIP-1α and IL-6, were elevated (Figure 2E) and, significantly, only those 2 mediators exhibited increased LPS-stimulated release in cultured cmo macrophages compared with wt macrophages (Figure 2B). Symptomatic PSTPIP2-deficient mice exhibited elevated levels of circulating IL-6, MIP-1α TNF-α, CSF-1, and IP-10 and decreased levels of IL-13, a T-cell cytokine that induces alternative macrophage activation (Figure 2E).26-28 These results suggest that increased production of MIP-1α and IL-6 play an important role in the initiation of cmo disease, whereas the increased levels of TNF-α, CSF-1, and IP-10 in diseased mice could be the consequence of ongoing inflammation.
Increased numbers of monocyte/macrophage progenitors with greater proliferative potential precedes the onset of disease
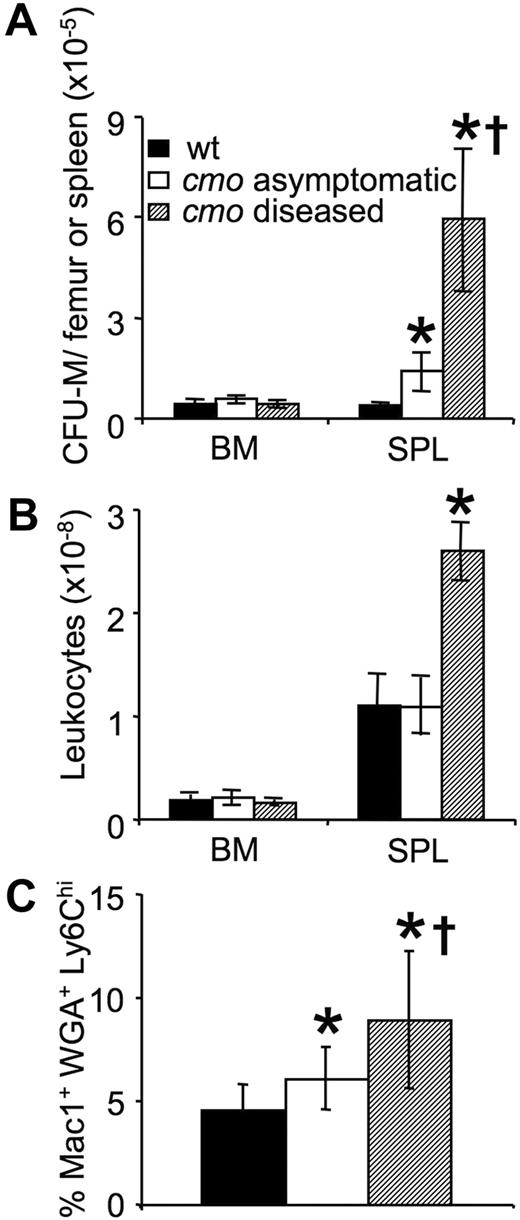
Macrophage accumulation in the inflamed tissues and spleens of cmo mice in the absence of a corresponding increase in macrophage chemotaxis to CSF-1 (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article) suggested that macrophage production or proliferation may be altered in PSTPIP2-deficient mice. To address this possibility, we first compared the numbers of CSF-1–responsive CFU-M in the BM and spleens of asymptomatic cmo, diseased cmo, and wt mice (Figure 3). There was an approximately 3-fold increase in the numbers of CFU-M in the spleens of asymptomatic cmo compared with wt mice and a further approximately 12-fold increase in symptomatic mice (Figure 3A). There was no effect of disease on BM cellularity; however, consistent with the increase in their splenic mass (Figure 1D) and CFU-M numbers (Figure 3A), symptomatic cmo mice had increased splenic cellularity (Figure 3B). These data indicate that there is an early appearance and progressive accumulation of CSF-1–responsive macrophage progenitors in the spleens of cmo mice. In contrast, there was no change in the number of CFU-M in BM with increasing severity of disease. However, the frequency of Mac1+WGA+Ly6Chi late monocyte precursors29 was significantly elevated in the BM of asymptomatic cmo mice and increased further in diseased mice (Figure 3C). These data suggest that increased monopoiesis in BM and extramedullar monopoiesis in spleen contribute to disease development in cmo mice.
Expansion of myeloid progenitors in cmo mice precedes disease onset. (A) Increased frequency of CFU-M in spleens of asymptomatic and diseased cmo mice. (B) Splenic cellularity increases after disease onset. (C) Increased frequency of Mac1+ WGA+ Ly6Chi late monocyte precursors in the BM of cmo mice. Data ± SD, n ≥ 3 (A-B), n ≥ 10 (C); *P < .05 versus wt, †P < .05 versus cmo asymptomatic.
Expansion of myeloid progenitors in cmo mice precedes disease onset. (A) Increased frequency of CFU-M in spleens of asymptomatic and diseased cmo mice. (B) Splenic cellularity increases after disease onset. (C) Increased frequency of Mac1+ WGA+ Ly6Chi late monocyte precursors in the BM of cmo mice. Data ± SD, n ≥ 3 (A-B), n ≥ 10 (C); *P < .05 versus wt, †P < .05 versus cmo asymptomatic.
To determine whether the splenic CFU-M were derived from more primitive progenitors in the BM or spleen, we compared the numbers of early (HPP-CFC) and late (CFU-M) macrophage precursors in the BM and spleens of asymptomatic cmo mice (Table 1). HPP-CFCs were measured by the culture of cells in the presence of IL-3 and SCF with or without CSF-1. For any combination of these cytokines, there was no difference in the number of HPP-CFCs in the BM of cmo compared with wt mice, and CSF-1 increased the number of HPP-CFCs to a similar extent in both wt and cmo cultures. However, in the cmo cultures, the size of CSF-1–stimulated colonies was larger than their size in wt cultures, suggesting an increased CSF-1–driven proliferation of these cells and/or their progeny as they matured within colonies. Regardless of the cytokine combination and consistent with the development of splenomegaly, cmo spleens contained more HPP-CFCs than wt spleens, and these HPP-CFCs possessed greater proliferative potential. As in the case of HPP-CFCs, the number of CFU-Ms in BM was similar in wt and cmo mice, whereas the cmo spleens contained more CFU-Ms of greater proliferative potential than the wt spleens. These studies indicate that both CSF-1–responsive primitive and late macrophage precursors possessing increased proliferative capacity are increased in PSTPIP2-deficient mice.
Hematopoietic parameters in wt and asymptomatic cmo mice
| Assay/parameter . | NA* . | IL-6 . | CSF-1 . | IL-6 and CSF-1 . | ||||
|---|---|---|---|---|---|---|---|---|
| wt . | cmo . | wt . | cmo . | wt . | cmo . | wt . | cmo . | |
| HPP-CFC (BM) | ||||||||
| Colony no., ×10−4 | 6 ± 1.4 | 8 ± 1.9 | 8 ± 3.5 | 9 ± 1.8 | 17 ± 5.1† | 24 ± 6.3† | 14 ± 4.9† | 19 ± 6.0† |
| Colony size, mm2 | 1.0 ± 0.4 | 1.0 ± 0.2 | 0.8 ± 0.3 | 1.2 ± 0.4 | 1.1 ± 0.1 | 1.5 ± 0.2§ | 0.8 ± 0.1‡ | 1.3 ± 0.1§ |
| HPP-CFC (SPL) | ||||||||
| Colony no., ×10−4 | 2 ± 0.6 | 5 ± 0.3§ | 4 ± 0.4† | 7 ± 0.9§ | 3 ± 1.2 | 8 ± 0.3†§ | 4 ± 0.9 | 10 ± 0.3†‡§ |
| Colony size, mm2 | 0.6 ± 0.14 | 1.2 ± 0.16§ | 1.2 ± 0.21† | 1.6 ± 0.04†§ | 1.4 ± 0.06† | 2.4 ± 0.03†§ | 1.5 ± 0.12† | 2.7 ± 0.32†§ |
| CFU-M (BM) | ||||||||
| Colony no., ×10−3 | 150 ± 0.6 | 130 ± 18 | 30 ± 4‡ | 50 ± 5†‡ | ||||
| Colony size, μm2 | 2661 ± 647 | 3057 ± 255 | 2590 ± 1139 | 3597 ± 579 | ||||
| CFU-M (SPL) | ||||||||
| Colony no., ×10−3 | 7 ± 1.7 | 13 ± 1.3§ | 3 ± 1.1‡ | 14 ± 1.0§ | ||||
| Colony size, μm2 | 1910 ± 405 | 6134 ± 1167§ | 9387 ± 1084‡ | 11 033 ± 1867‡ | ||||
| Macrophage proliferation (BM) doubling time, hours | 26.9 ± 0.1 | 22.2 ± 0.2§ | ||||||
| Macrophage proliferation (SPL) doubling time, hours | 42.4 ± 2.8 | 35.3 ± 1.9§ | ||||||
| Assay/parameter . | NA* . | IL-6 . | CSF-1 . | IL-6 and CSF-1 . | ||||
|---|---|---|---|---|---|---|---|---|
| wt . | cmo . | wt . | cmo . | wt . | cmo . | wt . | cmo . | |
| HPP-CFC (BM) | ||||||||
| Colony no., ×10−4 | 6 ± 1.4 | 8 ± 1.9 | 8 ± 3.5 | 9 ± 1.8 | 17 ± 5.1† | 24 ± 6.3† | 14 ± 4.9† | 19 ± 6.0† |
| Colony size, mm2 | 1.0 ± 0.4 | 1.0 ± 0.2 | 0.8 ± 0.3 | 1.2 ± 0.4 | 1.1 ± 0.1 | 1.5 ± 0.2§ | 0.8 ± 0.1‡ | 1.3 ± 0.1§ |
| HPP-CFC (SPL) | ||||||||
| Colony no., ×10−4 | 2 ± 0.6 | 5 ± 0.3§ | 4 ± 0.4† | 7 ± 0.9§ | 3 ± 1.2 | 8 ± 0.3†§ | 4 ± 0.9 | 10 ± 0.3†‡§ |
| Colony size, mm2 | 0.6 ± 0.14 | 1.2 ± 0.16§ | 1.2 ± 0.21† | 1.6 ± 0.04†§ | 1.4 ± 0.06† | 2.4 ± 0.03†§ | 1.5 ± 0.12† | 2.7 ± 0.32†§ |
| CFU-M (BM) | ||||||||
| Colony no., ×10−3 | 150 ± 0.6 | 130 ± 18 | 30 ± 4‡ | 50 ± 5†‡ | ||||
| Colony size, μm2 | 2661 ± 647 | 3057 ± 255 | 2590 ± 1139 | 3597 ± 579 | ||||
| CFU-M (SPL) | ||||||||
| Colony no., ×10−3 | 7 ± 1.7 | 13 ± 1.3§ | 3 ± 1.1‡ | 14 ± 1.0§ | ||||
| Colony size, μm2 | 1910 ± 405 | 6134 ± 1167§ | 9387 ± 1084‡ | 11 033 ± 1867‡ | ||||
| Macrophage proliferation (BM) doubling time, hours | 26.9 ± 0.1 | 22.2 ± 0.2§ | ||||||
| Macrophage proliferation (SPL) doubling time, hours | 42.4 ± 2.8 | 35.3 ± 1.9§ | ||||||
BM indicates bone marrow; CFU-M, colony-forming unit megakaryocytes; cmo, chronic multifocal osteomyelitis; CSF, colony-stimulating factor; HPP-CFC, high proliferative potential colony-forming cells; IL, interleukin; NA, no addition (absence); SPL, spleen; and wt, wild-type.
HPP-CFC assays were performed in media containing 60 ng/mL SCF and 20 ng/mL IL-3 in the absence (NA) or presence of 50 ng/mL IL-6 and/or 18 ng/mL CSF-1. CFU-M assay was performed in media containing 18 ng/mL CSF-1 ± 10 ng/mL IL-6. For proliferation assays, day 3 myeloid precursors were grown in media containing 36 ng/mL CSF-1. Doubling times were calculated exclusively for the exponential growth phase.
Different from NA; P < .05, Student t test.
Different from CSF-1; P < .05, Student t test.
Different from wt; P < .05, Student t test.
Because IL-6 was elevated in the circulation of asymptomatic mice (Figure 2E) and monocytes have been reported to produce IL-6 constitutively,30 we also examined the involvement of IL-6 in the proliferation of myeloid precursors in wt and cmo mice (Table 1). IL-6 has been shown to enhance the effect of other cytokines on HPP colony formation.31 Consistent with this finding, in splenocyte cultures HPP-CFC number and colony size were increased for both wt and cmo. In contrast, there was no effect of IL-6 on HPP-CFC number or colony size in either wt or cmo BM cultures. As previously reported,32 exogenous IL-6 decreased CFU-M numbers in wt BM and splenocyte cultures (Table 1). In contrast, the effects of IL-6 on CFU-M numbers as well as colony size were attenuated in cmo cultures. The effect of IL-6 on colony size was less dramatic in cmo cultures as a result of an approximately 3-fold larger colony size in the absence of IL-6. The development of larger colonies in cmo cultures in the absence of IL-6 was not caused by endogenous production of IL-6 in the cmo cultures because neutralizing antibodies to IL-6 or the IL-6 receptor (gp130) failed to reduce cmo colony size (data not shown). These findings are consistent with the existence of greater numbers of more primitive macrophage progenitors in the spleens of cmo compared with wt mice.
PSTPIP2 negatively regulates macrophage proliferation
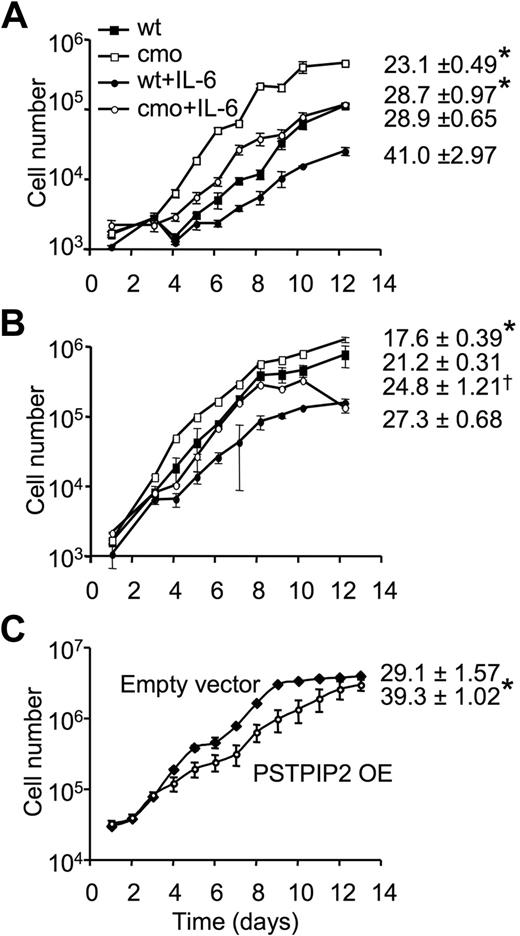
To determine whether the increase in colony size observed in the macrophage progenitor assays reflected significant changes in the proliferative responses of PSTPIP2-deficient macrophage precursors in BM and spleens of cmo mice, we examined the proliferation of purified BM and splenic promonocytes in the presence of CSF-1. The rate of CSF-1–stimulated macrophage proliferation was significantly increased in both splenic (Figure 4A) and BM (Figure 4B) macrophages of cmo mice (Table 1). In contrast, there was no difference in the proliferation rate of wt and cmo promonocytes grown in the presence of GM-CSF or IL-3 (data not shown). The increased CSF-1–induced proliferation by cmo macrophages was maintained when nonadherent BM (doubling times, wt 21 hours vs cmo 18 hours, in the presence of CSF-1 alone, or wt 27 hours vs cmo 25 hours, in the presence of CSF-1 and IL-6) or splenic cells (doubling times, wt 29 hours vs cmo 23 hours, in the presence of CSF-1 alone, or wt 41 hours vs cmo 29 hours, in the presence of CSF-1 and IL-6) were cultured in the presence of CSF-1 and IL-6, although IL-6 suppressed proliferation (Figure 4A-B). Interestingly, splenocyte macrophages exhibited greater differences between cmo and wt proliferation rates than BM-derived macrophages. In contrast, overexpression of PSTPIP2 in the splenic macrophage cell-line BAC1.2F525 significantly decreased the rate of cell proliferation (39 ± 1 hour vs 29 ± 1.6 hours in wt; Figure 4C). These data indicate that PSTPIP2 negatively regulates CSF-1–induced macrophage proliferation.
PSTPIP2 attenuates macrophage proliferation. Proliferation of nonadherent splenocytes (A) or nonadherent BM cells (B) from wt and asymptomatic cmo mice. Triplicate samples with cells harvested from 2 mice/genotype. (C) Growth curves of BAC1.2F5 macrophages retrovirally transduced with MSCV-IRES-GFP vector (empty vector) or MSCV-IRES-GFP-PSTPIP2 (PSTPIP2 OE). The doubling times are presented on the right side of each curve. Data ± SD, n = 3; *P < .01 versus wt, †P < .05 versus wt.
PSTPIP2 attenuates macrophage proliferation. Proliferation of nonadherent splenocytes (A) or nonadherent BM cells (B) from wt and asymptomatic cmo mice. Triplicate samples with cells harvested from 2 mice/genotype. (C) Growth curves of BAC1.2F5 macrophages retrovirally transduced with MSCV-IRES-GFP vector (empty vector) or MSCV-IRES-GFP-PSTPIP2 (PSTPIP2 OE). The doubling times are presented on the right side of each curve. Data ± SD, n = 3; *P < .01 versus wt, †P < .05 versus wt.
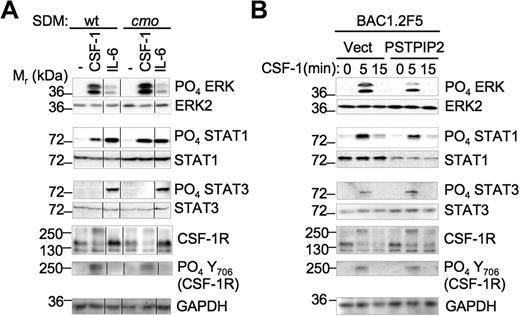
Enhanced activation of Erk1/2 and STAT1 by CSF-1 signaling in cmo macrophages lacking PSTPIP2
To determine how PSTPIP2 attenuates macrophage proliferation, we stimulated day 10 SDM with CSF-1 or IL-6 and analyzed the activation of downstream pathways that regulate macrophage proliferation and activation. There was no difference in the level of cell-surface CSF-1R expression between wt and cmo macrophages (data not shown). However, PSTPIP2 deficiency in splenic macrophages led to increased expression of STAT1 (1.8- ± 0.2-fold vs wt, n = 5) but not STAT3 levels (Figure 5A). In addition, PSTPIP2 deficiency enhanced the ability of CSF-1 to stimulate Erk1/2 phosphorylation (1.5- ± 0.2-fold vs wt, n = 5) and STAT1 phosphorylation (2.2- ± 0.6-fold vs wt, n = 5; Figure 5A). These responses were specific for CSF-1 because the IL-6–induced phosphorylation of Erk1/2 and STAT1 was not affected by PSTPIP2 deficiency (Figure 5A). The increase in Erk1/2 phosphorylation occurred without any alteration in the kinetics (data not shown). Despite the negative regulation of STAT1 phosphorylation by PSTPIP2, we could not detect significant differences between wt and cmo splenic macrophages in their phosphorylation of tyrosine 706 of CSF-1R, a site previously reported to selectively enhance STAT1 activation (Figure 5).33 In contrast, overexpression of PSTPIP2 in BAC1.2F5 macrophages reduced both the expression of STAT1 (0.4- ± 0.05-fold vs wt, n = 3) and the CSF-1–induced phosphorylation of Erk1/2 (0.6- ± 0.1-fold vs wt, n = 3; Figure 5B). These data are consistent with negative regulation of STAT1 expression and Erk1/2 activation by PSTPIP2.
PSTPIP2 negatively regulates STAT1 expression and the activation of Erk1/2 and STAT1 by c-fms in splenic macrophages. (A) CSF-1 and IL-6 signaling in primary SDM from wt and cmo mice. The vertical lines indicate repositioned gel lanes from the same blot. (B) PSTPIP2 overexpression in BAC1.2F5 macrophages inhibits Erk1/2 activation and STAT1 expression. These results were reproduced in 3 to 5 experiments.
PSTPIP2 negatively regulates STAT1 expression and the activation of Erk1/2 and STAT1 by c-fms in splenic macrophages. (A) CSF-1 and IL-6 signaling in primary SDM from wt and cmo mice. The vertical lines indicate repositioned gel lanes from the same blot. (B) PSTPIP2 overexpression in BAC1.2F5 macrophages inhibits Erk1/2 activation and STAT1 expression. These results were reproduced in 3 to 5 experiments.
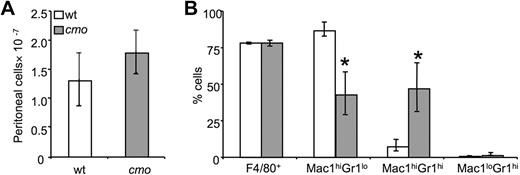
Increased recruitment of immature macrophages in cmo mice in response to a local inflammatory stimulus
The expansion of early macrophage progenitors could prime cmo mice to mount exaggerated local inflammatory responses. Although we have found that cmo macrophages exhibit decreased chemotactic responses to CSF-1, they produce more MIP-1α, a chemoattractant for monocytes and granulocytes. To address the role of PSTPIP2 in an acute inflammatory response, we induced sterile peritonitis in wt and cmo mice by using thioglycollate broth. Monocyte recruitment to the peritoneum was not affected by PSTPIP2 deficiency (Figure 6A). However, cmo mice recruited significantly fewer Mac1hiGr1lo mature macrophages and more Mac1hi Gr1hi immature macrophages in response to thioglycollate (Figure 6B). Thus, the PSTPIP2-deficient mice recruited more primitive macrophages that presumably have a greater potential to expand by local proliferation34 than wt mice.
Increased thioglycollate-induced recruitment of macrophage precursors in peritoneal exudates of cmo mice. (A) Both wt and cmo mice recruit comparable numbers of leukocytes in the inflamed peritoneum. (B) Phenotypic analysis of the leukocytes in peritoneal exudates. Data ± SD, n = 7; *P < .01 versus wt.
Increased thioglycollate-induced recruitment of macrophage precursors in peritoneal exudates of cmo mice. (A) Both wt and cmo mice recruit comparable numbers of leukocytes in the inflamed peritoneum. (B) Phenotypic analysis of the leukocytes in peritoneal exudates. Data ± SD, n = 7; *P < .01 versus wt.
Discussion
cmo and Lupo diseases share phenotypic similarities with chronic recurrent multifocal osteomyelitis (CRMO). CRMO is an autoinflammatory disorder that presents in childhood with seemingly unprovoked episodes of bone pain caused by sterile multifocal osteomyelitis35 ; it is often accompanied by psoriasis or inflammatory bowel disease. What triggers each episode remains unknown. Cultures from these patients are negative, and bacterial ribosomal DNA are not detected in bone biopsies.36 Trauma has been reported as an inciting factor for bone lesions in 25% of CRMO patients.37 Evidence for a genetic component derives from familial studies,35,38 which established a susceptibility region on chromosome 18q, where pstpip2 is located, and from an autosomal-recessive form of the disease called Majeed syndrome, which is caused by mutations in LPIN2.39 However, in the limited number of cases studied, mutations in PSTPIP2 have not yet been implicated.40 Irrespective of the connection between PSTPIP2 and CRMO, studies of mice bearing mutations in PSTPIP2 promise to reveal novel cellular processes and pathways that are dysregulated in autoinflammatory disease.
We previously showed that the I282N mutation (Lupo) of PSTPIP2 was associated with a 3-fold reduction in PSTPIP2 expression and led to autoinflammatory disease.12 Lupo mice exhibited increased inflammatory mediator production by macrophages and extensive macrophage infiltration in the affected tissues, suggesting that PSTPIP2 is antiinflammatory.12 Like Lupo mice, the inflamed tissues of cmo mice contain large numbers of F4/80-positive macrophages (Figure 1D). In addition, macrophage precursors infiltrate the spleen and cause splenomegaly in the cmo mice (Figure 3). Consistent with the predicted disruption of PSTPIP2 protein folding and stability by the L98P mutation causing cmo disease,15,35,41 we could not detect PSTPIP2 protein expression in cmo myeloid cells (Figure 1). This absence of PSTPIP2 leads to increased constitutive and LPS-stimulated production of several inflammatory mediators by cmo macrophages (Figure 2). Importantly, circulating levels of MIP-1α and IL-6 are elevated in cmo mice before the development of overt disease, suggesting that the activation of macrophages contributes to disease development. That activated macrophages, rather than mast cells or granulocytes, play a central role in disease development is emphasized by the observations that IgE receptor–induced mast-cell degranulation is reduced in cmo BM-derived mast cells (data not shown) and that PSTPIP2 is not expressed in Mac1lowGr1hi polymorphonuclear cells.12
The extensive macrophage accumulation in inflamed tissues of cmo mice could be caused by increased production of monocyte chemotactic factors, such as MIP-1α, by macrophages at these sites. However, the splenomegaly in cmo mice raised the possibility that a cell-autonomous effect of the loss of PSTPIP2 on the myeloid lineage leading to increased monopoiesis and/or increased macrophage proliferation, could also contribute, by systemically “priming” the mice for responses to local inflammatory stimuli. BM of asymptomatic cmo mice contained early myeloid precursors (HPP-CFC) that hyperproliferated in the presence of CSF-1. In addition, the spleens of asymptomatic cmo mice contained greater numbers of HPP-CFCs and CFU-Ms that also hyperproliferated in the presence of CSF-1 (Table 1). Consistent with this finding, nonadherent BM and splenic cells proliferated faster than wt counterparts in the presence of CSF-1 (Figure 4; Table 1). Other investigators42 have shown that PSTPIP2 mRNA levels are significantly up-regulated during the differentiation of stem cells to late hematopoietic progenitors and during granulocyte monocyte-CSF–induced differentiation of hematopoietic cells in vitro.43 Together, these data suggest that the up-regulation of PSTPIP2 expression during myeloid differentiation promotes the differentiation and/or suppresses the proliferation of early macrophage progenitors. CSF-1 and receptor activator of NF-kappaB ligand regulate osteoclastogenesis,44,45 and osteoclasts have been noted at sites of inflammation in the bones of both cmo and Lupo mice.12,16 The up-regulation of monocytopoiesis in cmo mice provides a source of progenitors for the osteoclasts, the recruitment and generation of which would be enhanced by MIP-1α,46 which is produced in increased amounts by cmo macrophages. In addition, IL-6, a cytokine that promotes inflammation-induced osteclastogenesis47 is also produced in increased amounts by cmo macrophages (Figure 2). Apart from the suppression of CSF-1–regulated progenitor cell proliferation by PSTPIP2, our comparison of the proliferation rates of purified wt and cmo BM and splenic promonocytes in the presence of CSF-1 indicated that PSTPIP2 also negatively regulates macrophage proliferation.
Thus, it appears that the myeloid system of cmo mice is primed to provide monocytes for recruitment to sites of local inflammation and in addition, the macrophages at those sites are able to release elevated levels of proinflammatory cytokines, including chemotactic factors, which can further increase recruitment. However, because PSTPIP2 promotes macrophage motililty (supplemental Figure 1),14 its absence in cmo macrophages may counterbalance the effect of increased chemotactic factors. Indeed, in the inflammatory response to thioglycollate challenge, cmo mice recruited comparable numbers of F4/80-positive macrophages to the peritoneum but, consistent with their primed monocytopoiesis, the proportion of immature macrophages was greater than in wt mice (Figure 6).
The increased response of cmo macrophages to activation by LPS, together with the hematopoietic phenotype of cmo mice, was reminiscent of the hematopoietic phenotype of STAT3-deficient mice, in which hyperproliferation of myeloid cells and increased responsiveness to LPS48-50 also were observed. Furthermore, failure of IL-6 activation of STAT3, attributable to an IL-6 receptor (gp130) mutation, also induced a cmo-like expansion of macrophage progenitor cells and increased macrophage proliferation in association with increased Erk1/2 activation and overexpression of c-fms.32 However, our analysis of downstream signaling pathways leading to cell proliferation indicated that STAT3 levels and phosphorylation as well as c-fms expression were normal in cmo macrophages. In contrast, STAT1 was significantly up-regulated, and CSF-1–stimulated phosphorylation of STAT1 and Erk1/2 was significantly increased. Overexpression of PSTPIP2 induced the opposite phenotype, suggesting that PSTPIP2 normally suppresses STAT1 expression and the activation of Erk1/2 and STAT1 by CSF-1. Because the Erk1/2 pathway regulates macrophage proliferation downstream of CSF-1,32,51,52 our data suggest that hyperactivation of Erk1/2 by CSF-1 may be responsible for the increased proliferation of PSTPIP2-deficient macrophages. Interestingly, overexpression of the closely related PSTPIP1 in T lymphocytes also attenuates Erk1/2 phosphorylation.7
In addition to promoting macrophage development, survival, and proliferation, CSF-1 also stimulates MIP-1α and TNF-α production by macrophages and increases IL-6 production by monocytes44 (data not shown). Although the mechanism is not known, STAT1 could play an important role because STAT-1– and Akt-mediated macrophage priming by IFN-β is necessary for optimal LPS-stimulated production of several inflammatory mediators, including IL-653 and for poly (I:C)–induced MIP-1α mRNA expression in keratinocytes.54 In contrast, STAT3 is dispensable for CSF-1–induced macrophage proliferation51 and MIP-1α expression54 and has antiproliferative and antiinflammatory effects in macrophages.32,50,55,56 Thus, our data suggest that up-regulation of STAT1 in cmo macrophages could contribute to their increased production of IL-6 and MIP-1α.
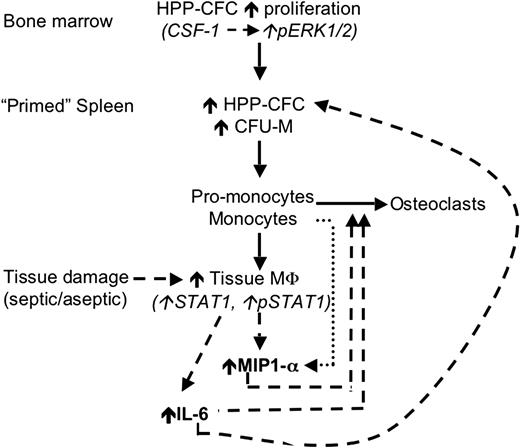
In summary, the loss of macrophage regulation by PSTPIP2 promotes cmo disease development at 2 levels. At one level, there is overproduction of macrophage and osteoclast progenitors and, at the other level, increased macrophage production of inflammatory cytokines, which act both to enhance monopoiesis and to recruit macrophages and osteoclast precursors to sites of inflammation (Figure 7). Analysis of PSTPIP2 function should provide insight into novel pathways involved in these processes.
A hypothetic model of the mechanism by which PSTPIP2 deficiency in cmo mice primes the innate immune system for exaggerated and prolonged inflammatory responses. Before disease onset (solid lines), cmo mice produce increased numbers of primitive myeloid progenitors (HPP-CFC) in the BM that migrate to the spleen, where they either become resident HPP-CFCs or differentiate into CFU-M. Tissue damage (dashed lines) induces increased production of MIP-1α and IL-6 by tissue macrophages. IL-6 promotes the proliferation of splenic HPP-CFC, and MIP-1α increases the recruitment of circulating (pro-) monocytes to peripheral tissues ( ), thus establishing a positive feedback loop. MIP-1α and IL-6 also promote the recruitment of osteoclast precursors and osteoclastogenesis, leading to inflammatory bone resorption.
), thus establishing a positive feedback loop. MIP-1α and IL-6 also promote the recruitment of osteoclast precursors and osteoclastogenesis, leading to inflammatory bone resorption.
A hypothetic model of the mechanism by which PSTPIP2 deficiency in cmo mice primes the innate immune system for exaggerated and prolonged inflammatory responses. Before disease onset (solid lines), cmo mice produce increased numbers of primitive myeloid progenitors (HPP-CFC) in the BM that migrate to the spleen, where they either become resident HPP-CFCs or differentiate into CFU-M. Tissue damage (dashed lines) induces increased production of MIP-1α and IL-6 by tissue macrophages. IL-6 promotes the proliferation of splenic HPP-CFC, and MIP-1α increases the recruitment of circulating (pro-) monocytes to peripheral tissues ( ), thus establishing a positive feedback loop. MIP-1α and IL-6 also promote the recruitment of osteoclast precursors and osteoclastogenesis, leading to inflammatory bone resorption.
), thus establishing a positive feedback loop. MIP-1α and IL-6 also promote the recruitment of osteoclast precursors and osteoclastogenesis, leading to inflammatory bone resorption.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ranu Basu, Xiao-Hua Zong, Halley Ketchum (Albert Einstein College of Medicine), and Xinyu Bing (University of Iowa) for technical support and Werner Wilke (University of Iowa) for his technical expertise with the flow cytometry experiments.
This work was supported by a New York Community Trust Blood Diseases Grant (V.C.) and National Institutes of Health grants RO1 CA25604 (E.R.S.) and K01AR 054486 (V.C.).
National Institutes of Health
Authorship
Contribution: V.C., P.J.F., and E.R.S designed the research; P.J.F. and E.R.S. supervised the research; V.C. performed histology, cytokine, colony formation, and proliferation assays; V.C. and Y.-G.Y. performed signaling studies; V.C. and E.R.S. performed the thiogllycolate challenge; P.J.F. provided cmo mouse and Rag-deficient cmo; R.d.B. generated BMM cell lines and performed FACS analysis; A.J.S. evaluated monocyte precursors in bone marrow by FACS; L.A.O. performed bone marrow transplant and evaluated cmo phenotype on Rag1+/− and Rag1−/− background; T.J.W. supplied reagents and technical advice for bone marrow transplants; E.R.S. performed colony assays; and V.C., P.J.F., and E.R.S. performed data analysis and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Violeta Chitu or E. Richard Stanley, Department of Developmental and Molecular Biology, Albert Einstein College of Medicine, Bronx, NY 10461; e-mail: vchitu@aecom.yu.edu or rstanley@aecom.yu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal