Abstract
The development of inflammatory diseases implies inactivation of regulatory T (Treg) cells through mechanisms that still are largely unknown. Here we showed that mast cells (MCs), an early source of inflammatory mediators, are able to counteract Treg inhibition over effector T cells. To gain insight into the molecules involved in their interplay, we set up an in vitro system in which all 3 cellular components were put in contact. Reversal of Treg suppression required T cell–derived interleukin-6 (IL-6) and the OX40/OX40L axis. In the presence of activated MCs, concomitant abundance of IL-6 and paucity of Th1/Th2 cytokines skewed Tregs and effector T cells into IL-17–producing T cells (Th17). In vivo analysis of lymph nodes hosting T-cell priming in experimental autoimmune encephalomyelitis revealed activated MCs, Tregs, and Th17 cells displaying tight spatial interactions, further supporting the occurrence of an MC-mediated inhibition of Treg suppression in the establishment of Th17-mediated inflammatory responses.
Introduction
Traditionally associated with the allergic response, mast cells (MCs) are now viewed as important players in a variety of immune processes, such as pathogen clearance, graft acceptance, tumor immunity, and many inflammatory and autoimmune diseases, where they exhibit either pro- or anti-inflammatory potential depending on the context.1 Interactions of MCs with T cells have a functional role in some T cell–mediated pathologies, such as neutrophilic airway inflammation,2,3 and autoimmune disease of the central nervous system (CNS).4
MC-deficient mouse models show reduced development of both allergic and neutrophilic airway hyperreactivity (AHR).2,3 MC-derived tumor necrosis factor-α (TNF-α) is required for T helper cell (Th17) development in the lung on ovalbumin (OVA) challenge of OTII transgenic mice,3 a neutrophilic AHR model strictly dependent on interleukin-17 (IL-17).5 In multiple sclerosis, MCs colocalize with demyelinating plaques in inflamed brain, and many MC-associated markers have been detected in the affected tissue.6 The MC-deficient strain KitW/W-v shows decreased incidence and severity of experimental autoimmune encephalomyelitis (EAE), the rodent model of multiple sclerosis.7 MCs could be directly activated during EAE by either the specific myelin peptides or locally released agonists, including complement, cytokines, and neuropeptides, but also indirectly stimulated via FcϵRI by specific IgE elicited on activation of Th2 response.1,8 In the CNS, the local release of inflammatory cytokines, proteases, oxygen radicals, and chemokines by MCs directly contributes to myelin degradation, disruption of the blood-brain barrier, and recruitment and activation of other immune cells as T lymphocytes and granulocytes in the brain parenchyma, thus exacerbating the effector phase of autoimmune response.4 MCs are also thought to be involved in initiating EAE, through the interaction with dendritic cells and T cells in secondary lymphoid organs.9
IL-17–secreting Th17 are considered one of the major pathogenic immune components in both EAE10 and neutrophilic AHR.3 Th17 cells differentiate from uncommitted precursors on antigenic activation and costimulation in the concomitant presence of a suppressive and a pro-inflammatory signal delivered by transforming growth factor-β (TGF-β) and IL-6 and/or IL-21, respectively. Once primed in lymph nodes, pathogenic Th17 cells reach the inflamed tissue where they recruit granulocytes and intensify inflammation.11
Regulatory T cells (Tregs) are one of the most relevant sources of TGF-β both in vitro and in vivo. This CD4+ T lymphocyte subset, characterized by the constitutive expression of several costimulatory molecules and the transcription factor Foxp3, is endowed with immune-suppressive properties necessary to induce and maintain tolerance.12 Recent findings support the notion of Treg plasticity in vivo because inflammatory stimuli can counteract Treg suppression and even promote differentiation into pathogenic Th17 cells.13
MCs may directly or indirectly sustain the availability of inflammatory signals that locally inhibit Treg suppression. The cytokine IL-6, released by innate and adaptive cells on activation, is known to break Treg anergy and suppression14 and to skew Tregs into Th17 cells.13 Among MC-associated membrane molecules, the costimulatory receptor OX40L can prevent de novo differentiation of Tregs and block their function.15,16 Evidence of possible MC-Treg interaction, through a variety of molecular mechanisms, comes from different disease models17,18 and have been envisioned in the pathogenesis of CNS autoimmune disease.4 Here we showed that, in vitro, MCs broke Treg anergy and suppression while promoting Th17 cell differentiation, through mechanisms that require IL-6 and OX40 engagement. We documented MC-Treg-Th17 interaction in vivo in 2 different inflammatory conditions, AHR and EAE, by showing IL-6–producing MCs in association with TGF-β–producing Tregs and IL-17–secreting effector T cells. Colocalization of MCs with Tregs and Th17 cells could be detected in lymph nodes draining the immunization site during EAE initiation. Therefore, MCs appear to play a key role in the development of deviated adaptive responses in inflammatory diseases by inhibiting Treg suppression and modulating Th17 differentiation.
Methods
Mice and reagents
C57BL/6 WT and OTII transgenic mice were purchased from Charles River. C57BL/6 CD45.1-congenic mice were from The Jackson Laboratory. C57BL/6 OX40-deficient mice (Tnfrsf4−/−) were from Nigel Killeen (University of California, San Francisco). Bone marrow and spleens from C57BL/6 IL-6–deficient mice (il6−/−) were kindly provided by Juan Rivera (National Institutes of Health, Bethesda, MD). Mice were maintained under pathogen-free conditions at the animal facility of Fondazione Istituti di Ricovero e Cura a Carattere Scientifico Istituto Nazionale dei Tumori (Milan, Italy). Animal experiments were authorized by the Institute Ethical Committee of Fondazione Istituti di Ricovero e Cura a Carattere Scientifico Istituto Nazionale dei Tumori and performed in accordance to institutional guidelines and national law (DL116/92). Shipped mice were kept for 2 to 3 weeks in our facility before being entered into the experiments. 2,4-Dinitrophenol (DNP)-specific IgE were kindly provided by Juan Rivera (National Institutes of Health). Recombinant IL-6 and IL-3 were purchased from PeproTech. Anti-IL-6Rα-blocking monoclonal antibody (clone 15A7) was a kind gift of Gennaro Ciliberto (IRBM, Pomezia, Italy).
AHR induction and analysis
Neutrophilic airway hyperreactivity was induced in OTII mice by intranasal injection of 50 μg OVA (grade 5; Sigma-Aldrich) dissolved in 20 μL phosphate-buffered saline (PBS) once daily for 3 consecutive days. Twenty-four hours after the last inhalation, bronchoalveolar lavage (BAL) cells were collected and analyzed by flow cytometry.
EAE immunization and analysis
EAE was induced with MOG35-55 (MEVGWYRSPFSRVVHLYRNGK) as previously described.19 Briefly, MOG35-55 peptide was dissolved in PBS and emulsified with an equal volume of incomplete Freund adjuvant supplemented with 4 mg/mL heat-killed Mycobacterium tuberculosis H37Ra (Difco). Mice were injected subcutaneously with the peptide emulsion containing 200 μg of MOG35-55/mouse and, on the same day and 48 hours later, injected intravenously with 200 ng of Bordetella pertussis Toxin (List Laboratories) dissolved in PBS. Mice were assessed daily for clinical signs of EAE according to a 5-point scale.19 Cell suspensions obtained from spinal cords were resuspended in 37% isotonic Percoll, overlaid on 70% isotonic Percoll, and centrifuged at 600g for 25 minutes. Pellets containing enriched MC preparations were collected and extensively washed before staining.
Histopathology and immunohistochemistry
Sections from formalin-fixed, paraffin-embedded lymph nodes and spinal cords were stained with hematoxylin and eosin or Giemsa. Single-marker immunohistochemistry was performed with the streptavidin-biotin-peroxidase complex method using anti-IL-6 (M-19) or anti-IL-17 (H-132) with either 3,3′-diaminobenzidine or aminoethylcarbazole as chromogen. Double immunostainings were performed by a 2-step single-staining approach. The first stainings were performed using streptavidin-conjugated alkaline phosphatase (LSAB+ kit; Dako) and Fast Red or nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate chromogenic substrates (Dako). Second staining was performed by incubating slides with the anti-Foxp3 (eBioscience) or anti-IL-17 and specific secondary horseradish peroxidase–conjugated antibodies with either 3,3′-diaminobenzidine or aminoethylcarbazole as chromogen. All the immunostained sections (but those involving nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate chromogen) were counterstained with hematoxylin. Slides were analyzed under a Leica DM2000 optical microscope, and microphotographs were collected using a Leica DFC320 digital camera.
BMMC–T-cell coculture and cytokine detection
Bone marrow-derived MCs (BMMCs) were differentiated in vitro from bone marrow cells as described.20 After 5 weeks, BMMCs were monitored for FcϵRI and c-kit expression by flow cytometry. Purity was usually more than 97%; 106/mL BMMCs were sensitized in medium without IL-3 for 4 hours with 1 μg/mL of DNP-specific IgE and then treated for 20 minutes at 4°C with anti-CD16/CD32 (eBioscience). CD4+CD25− (Teffs) or CD4+CD25+ (Tregs) were purified from splenocytes with the CD25+ T-cell isolation kit (Miltenyi Biotec) and labeled by incubation with 5 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) for 15 minutes at 37°C. A total of 7.5 × 104/well Teffs and/or Tregs were cultured with 5 × 104 accessory cells (consisting of the whole spleen irradiated with 30 Gy) with or without 7.5 × 104/well BMMCs for 72 hours in complete medium containing RPMI 1640 (Sigma-Aldrich) supplemented with 5% fetal calf serum, 2 mM l-glutamine, 200 U penicillin, and 200 mg/mL streptomycin (Sigma-Aldrich). For stimulation, 1 μg/mL of purified anti-CD3 (clone 145-2C11, eBioscience) was added. Where indicated, 100 ng/mL DNP–human serum albumin (Sigma-Aldrich) was added (Ag).
Flow cytometry
Phycoerythrin (PE)–anti-CD45.2, PE-anti-OX40 (OX86), PE-Cy7-anti-CD4, fluorescein isothiocyanate (FITC)– and PE-Cy7-anti–c-kit, and FITC–anti-FcϵRI-α (MAR-1) were purchased from eBioscience. BMMCs were stained with purified anti-OX40L (RM134L, eBioscience) followed by biotin–anti-rat IgG (Sigma-Aldrich) and PE-streptavidin (eBioscience).
Intracellular staining with PE-anti-Foxp3 (eBioscience), FITC-anti-TGF-β1 (1D11, RnD Systems), and Alexa Fluor 647–anti-IL17A (eBioscience) was performed after 4 hours of stimulation in the presence of monensin solution (eBioscience), 50 ng/mL phorbol myristate acetate, and 500 ng/mL ionomycin (Sigma-Aldrich). For evaluation of IL-6 secretion, cells were stimulated for 4 hours with 5 μg/mL brefeldin A (Sigma-Aldrich), fixed in 2% PFA, stained with PE-anti–IL-6 (MP5-20F3; eBioscience) in 0.5% saponin (Sigma-Aldrich), and then surface-stained. Cytokine amounts in supernatants were analyzed through Cytokine Beads Array (BD Biosciences; inflammation and Th1/Th2 cytokine kits) according to the manufacturer's instructions. Flow cytometry data were acquired on a FACSCalibur (BD Biosciences) and analyzed with FlowJo software (Version 8.5.2; TreeStar).
Statistical analysis
Results are expressed as the mean plus or minus SEM. Data were analyzed using a nonpaired Student t test (Prism software; GraphPad).
Results
MCs inhibit Treg suppression and promote Treg and Teff expansion
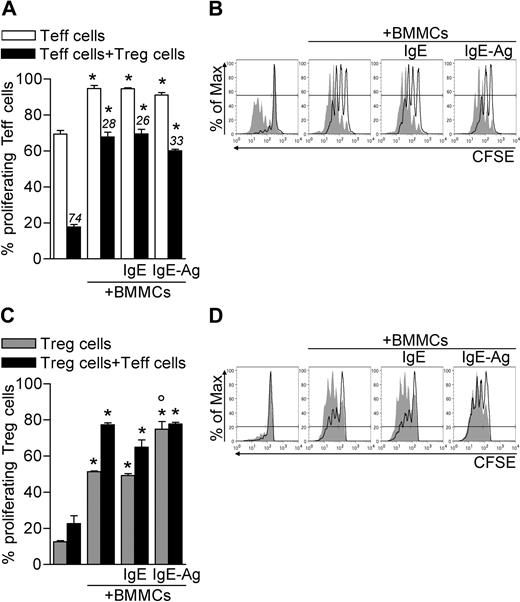
It is well documented that T-cell proliferation can be enhanced or inhibited by MCs and Tregs, respectively.12,21 To investigate the net effect of MCs and Tregs on the proliferation of nonregulatory T cells, CD45.2 CD4+CD25− effector T cells (Teffs) and CD45.1 CD4+CD25+ congenic Tregs were both labeled with CFSE and polyclonally activated in the absence or presence of BMMCs, either unstimulated or previously stimulated with IgE or IgE and Ag. After 3 days, CD4+ T cells were stained with CD45.2 antibody to discriminate CFSE dilution in the 2 T-cell populations. In the absence of Tregs, BMMCs increased Teff proliferation (from 69.33 ± 2.00 to 94.70 ± 1.67, P < .005), independently from their activation status. Strikingly, BMMCs significantly reduced the ability of Tregs to suppress the proliferation of Teffs (from 17.76 ± 1.35 to 65.75 ± 2.68, P < .001; Figure 1A-B). This effect was not simply the result of BMMC-mediated Teff expansion because BMMCs significantly reduced the rate of Treg suppression in each condition (from 74.39 ± 1.41 to 26.67 ± 3.71, P < .001; Figure 1A). BMMCs inhibited Treg suppression in a dose-dependent fashion, as proven by scaling down the BMMC/T-cell ratio (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article).
BMMCs expand both Teffs and Tregs and inhibit Treg suppression. CD45.2+ Teffs and CD45.1+ Tregs were CFSE-labeled and polyclonally activated in the presence of unstimulated, IgE-sensitized, or IgE-Ag–challenged BMMCs. After 72 hours, CFSE dilution in gated Teffs or Tregs was evaluated by flow cytometry as a function of proliferation. (A) Percentage of proliferating (CFSElow) Teffs. Numbers above bars indicate percentage inhibition of Tregs in each condition. (B) Representative histogram plots showing CFSE dilution in Teffs in the absence (filled areas) or presence (solid lines) of Tregs. (C) Percentages of proliferating (CFSElow) Tregs. (D) Representative histogram plots showing CFSE dilution in Tregs in the absence (solid lines) or presence (filled areas) of Teffs. *P < .05, compared with the corresponding condition in the absence of BMMCs. °P < .05, compared with the corresponding condition in the absence of Ag stimulation to BMMCs. All data are from 1 representative of 3 independent experiments.
BMMCs expand both Teffs and Tregs and inhibit Treg suppression. CD45.2+ Teffs and CD45.1+ Tregs were CFSE-labeled and polyclonally activated in the presence of unstimulated, IgE-sensitized, or IgE-Ag–challenged BMMCs. After 72 hours, CFSE dilution in gated Teffs or Tregs was evaluated by flow cytometry as a function of proliferation. (A) Percentage of proliferating (CFSElow) Teffs. Numbers above bars indicate percentage inhibition of Tregs in each condition. (B) Representative histogram plots showing CFSE dilution in Teffs in the absence (filled areas) or presence (solid lines) of Tregs. (C) Percentages of proliferating (CFSElow) Tregs. (D) Representative histogram plots showing CFSE dilution in Tregs in the absence (solid lines) or presence (filled areas) of Teffs. *P < .05, compared with the corresponding condition in the absence of BMMCs. °P < .05, compared with the corresponding condition in the absence of Ag stimulation to BMMCs. All data are from 1 representative of 3 independent experiments.
Treg suppression and anergy are either similarly or differentially regulated by antigen-presenting cells depending on the experimental system.22-25 In the absence of BMMCs, we found Tregs poorly proliferating while suppressing Teffs, as shown by others.22,25 Notably, in the presence of BMMCs, Tregs extensively proliferated even without Teffs (from 12.50 ± 0.64 to 50.25 ± 0.74, P < .001). Such proliferation was further enhanced when sensitized and Ag-activated BMMCs were added to the culture (to 74.80 ± 4.24, P < .005; Figure 1C-D). Teffs synergized with BMMCs in enhancing Treg proliferation. In addition, BMMC-mediated Treg expansion depended on the BMMC/T-cell ratio (supplemental Figure 1B).
Overall these data suggest that BMMCs break Treg anergy and suppressive function, allowing Teff proliferation.
MC-mediated inhibition of Treg suppression requires IL-6 and OX40
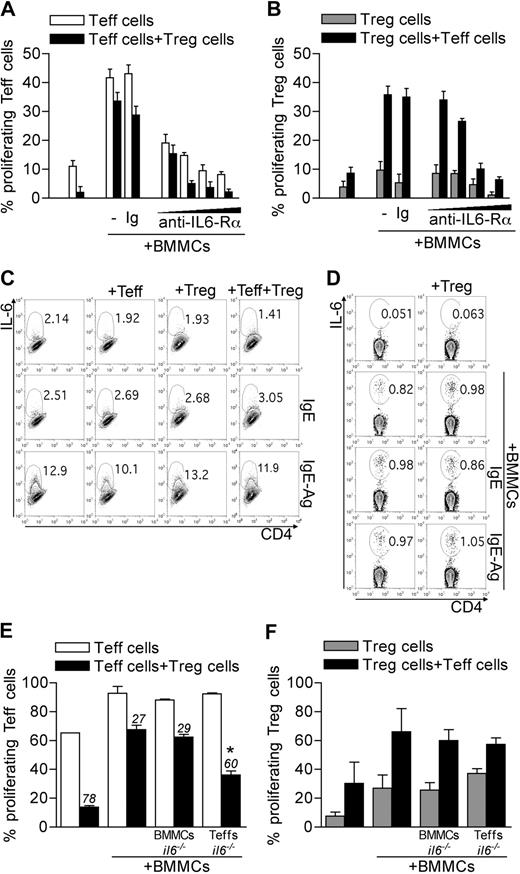
The cytokine IL-6 has been associated with reversal of Treg anergy,24 suppression,14 and phenotype13 ; therefore, it is a suitable candidate for BMMC-mediated inhibition of Treg functions. Indeed, the addition of an antibody blocking IL-6Rα (clone 15A7) reverted, in a dose-dependent manner, BMMC-induced Teff and Treg expansion and rescued Treg suppressive function (Figure 2A-B). Both Teffs and BMMCs could be sources of IL-6. BMMCs produced IL-6 only when IgE-sensitized and Ag-stimulated, regardless of the presence of Tregs and/or Teffs (Figure 2C). Conversely, Teffs produced IL-6 when stimulated with BMMCs, independently from their activation and from the concomitant presence of Tregs (Figure 2D).
Roles of T-cell-derived IL-6 in BMMC effects. (A-B) CD45.2 Teffs and CD45.1 congenic Tregs, both CFSE-labeled, were cultured with BMMCs. Where indicated, IL-6Rα–blocking monoclonal antibody (clone 15A7) was added scaling the final concentration at 50, 20, 10, or 5 μg/mL. As control, no antibody or an isotype-matched antibody (Ig) was added. After 72 hours, CFSE dilution as a function of proliferation was evaluated in gated Teffs (A) or Tregs (B). Percentages of CFSElow cells are indicated in each subset. Data are from 1 representative of 2 independent experiments. (C-D) BMMCs were left unstimulated, IgE-sensitized, or IgE-Ag–activated and cultured alone or with equal numbers of Tregs, Teffs, or both, in the presence of anti-CD3 to stimulate T cells, for 20 hours. IL-6 intracellular staining was performed on gated BMMCs (CD4− cells) (C) or Teffs (D) in the coculture. Representative plots are here shown of 2 independent experiments. (E-F) WT CD45.2 Teffs and CD45.1 Tregs were CFSE labeled and stimulated in the presence of WT or il6−/− BMMCs. Alternatively, il6−/− CD45.2 Teffs were used. After 72 hours, CFSE dilution was evaluated by flow cytometry in gated Teffs (E) or Tregs (F). Numbers above bars indicate percentage inhibition of Tregs in each condition. *P < .05, compared with the corresponding condition with WT BMMCs and WT Teffs. Data are from 1 representative of 2 independent experiments.
Roles of T-cell-derived IL-6 in BMMC effects. (A-B) CD45.2 Teffs and CD45.1 congenic Tregs, both CFSE-labeled, were cultured with BMMCs. Where indicated, IL-6Rα–blocking monoclonal antibody (clone 15A7) was added scaling the final concentration at 50, 20, 10, or 5 μg/mL. As control, no antibody or an isotype-matched antibody (Ig) was added. After 72 hours, CFSE dilution as a function of proliferation was evaluated in gated Teffs (A) or Tregs (B). Percentages of CFSElow cells are indicated in each subset. Data are from 1 representative of 2 independent experiments. (C-D) BMMCs were left unstimulated, IgE-sensitized, or IgE-Ag–activated and cultured alone or with equal numbers of Tregs, Teffs, or both, in the presence of anti-CD3 to stimulate T cells, for 20 hours. IL-6 intracellular staining was performed on gated BMMCs (CD4− cells) (C) or Teffs (D) in the coculture. Representative plots are here shown of 2 independent experiments. (E-F) WT CD45.2 Teffs and CD45.1 Tregs were CFSE labeled and stimulated in the presence of WT or il6−/− BMMCs. Alternatively, il6−/− CD45.2 Teffs were used. After 72 hours, CFSE dilution was evaluated by flow cytometry in gated Teffs (E) or Tregs (F). Numbers above bars indicate percentage inhibition of Tregs in each condition. *P < .05, compared with the corresponding condition with WT BMMCs and WT Teffs. Data are from 1 representative of 2 independent experiments.
To discriminate the role of IL-6 derived from BMMCs or Teffs, il6−/− mice were used as source of BMMCs or of Teffs. IL-6–deficient BMMCs were still able to revert Treg anergy and suppression similarly to their WT counterpart. On the contrary, IL-6 deficiency in Teffs restored Treg suppression in the presence of BMMCs, without significantly affecting Treg expansion (Figure 2E-F). We concluded that Teffs were the functionally relevant source of this cytokine. Nonetheless, IL-6 effect on Treg functions was detectable only in the presence of BMMCs.
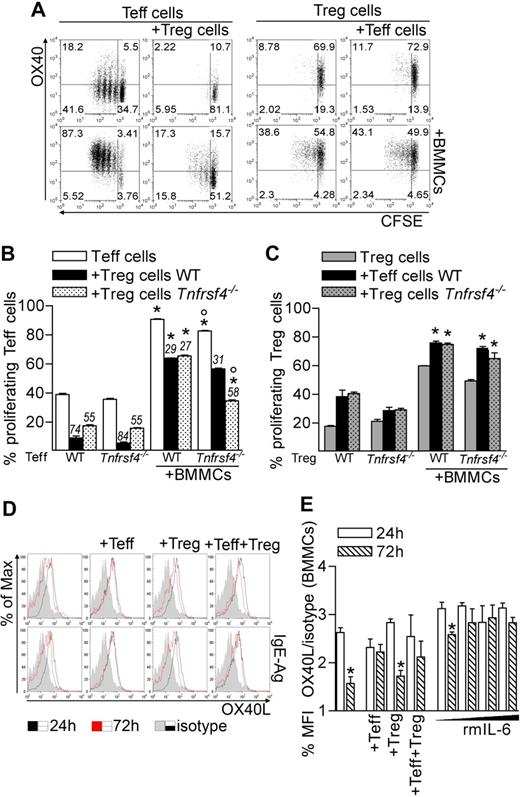
Molecular mechanisms underlying BMMC-Teff cooperation in the inhibition of Treg function may include both soluble and membrane-associated signals. Indeed, besides IL-6, several costimulatory molecules associated with the cell surface have been functionally linked to the reversal of Treg suppression.22,23 Furthermore, IL-6 might regulate the expression of the membrane receptors possibly involved in BMMC–T-cell crosstalk. Among costimulatory molecules that can bridge such interaction, the OX40L/OX40 axis has been recognized capable of bidirectional signaling. Indeed, the expression of OX40L on BMMCs is constitutive and tunable by soluble factors, such as TNF-α.21 OX40L interacts with OX40 (encoded by Tnfrsf4 gene) on the surface of activated Teffs, boosting their proliferation and survival.26 On the Treg side, OX40 is constitutively expressed and conveys functional inhibition on triggering.15,16 We have recently uncovered that Tregs suppress BMMC histamine degranulation, but not cytokine secretion, through OX40L signaling.18 Based on these premises, we tested whether OX40 expression on Tregs and Teffs was modulated in the coculture with BMMCs. In the presence of BMMCs, in line with the proliferation rate, Teffs expressed high or low levels of OX40 in the absence or in the presence of Tregs, respectively (Figure 3A). Contrary to Teffs, but in line with their own proliferation rate, Tregs expressed high levels of OX40 that became even brighter in the presence of BMMCs and/or Teffs (Figure 3A). The distinct effect of OX40 triggering on Teffs and Tregs was investigated by coculturing BMMCs with each T-cell subset from WT or Tnfrsf4−/− donors. In the absence of BMMCs, Tnfrsf4−/− Teff proliferation and Tnfrsf4−/− Treg suppression were slightly reduced compared with their WT counterparts, according to previous results.16,27 In the presence of BMMCs, Teff expansion by BMMCs was minimally affected by OX40 deficiency. Strikingly, only if both Tregs and Teffs were OX40-deficient, Treg suppression was partially restored despite BMMCs addition (Figure 3B). This suggested that OX40 triggering might reduce Teff susceptibility to Treg suppression on the one hand, while directly hindering Treg function on the other. It also might imply that both events were concurrently needed to establish the level of T-cell activation. Thus, OX40L on MCs was shown to be a key signal that maximized the immune response by acting on both Tregs and Teffs, as we have shown in antitumor immunity.28 We observed no relevant effect of OX40 in BMMC-mediated Treg proliferation (Figure 3C).
OX40/OX40L axis plays a role in BMMC functions. (A) OX40 expression and CFSE dilution in gated CD45.2 Teffs and CD45.1 Tregs after 72 hours of coculture with BMMCs. (B-C) Proliferation of Teffs (B) and Tregs (C) obtained from WT or Tnfrsf4−/− mice, alternatively labeled with CFSE, and seeded in different combinations, in the presence or not of BMMCs. Numbers above bars indicate percentage inhibition of Tregs. *P < .05, compared with the corresponding condition in the absence of BMMCs. °P < .05, compared with the corresponding condition in the presence of WT T cells. (D-E) OX40L expression in BMMCs after 24 or 72 hours of culture alone or in the presence of Teffs, Tregs, or both. When indicated, BMMCs received scaled amounts of recombinant IL-6 (rmIL-6, 1, 5, 20, and 50 ng/mL). (E) Mean fluorescence intensity of OX40L staining (relative to isotype staining) in BMMCs. *P < .05, compared with the same condition at 24 hours. Data are from 1 representative of 2 independent experiments.
OX40/OX40L axis plays a role in BMMC functions. (A) OX40 expression and CFSE dilution in gated CD45.2 Teffs and CD45.1 Tregs after 72 hours of coculture with BMMCs. (B-C) Proliferation of Teffs (B) and Tregs (C) obtained from WT or Tnfrsf4−/− mice, alternatively labeled with CFSE, and seeded in different combinations, in the presence or not of BMMCs. Numbers above bars indicate percentage inhibition of Tregs. *P < .05, compared with the corresponding condition in the absence of BMMCs. °P < .05, compared with the corresponding condition in the presence of WT T cells. (D-E) OX40L expression in BMMCs after 24 or 72 hours of culture alone or in the presence of Teffs, Tregs, or both. When indicated, BMMCs received scaled amounts of recombinant IL-6 (rmIL-6, 1, 5, 20, and 50 ng/mL). (E) Mean fluorescence intensity of OX40L staining (relative to isotype staining) in BMMCs. *P < .05, compared with the same condition at 24 hours. Data are from 1 representative of 2 independent experiments.
On BMMCs, OX40L expression was detectable regardless of IgE sensitization or IgE-Ag activation while down-regulated after 72 hours in vitro unless BMMCs were cocultured with Teffs but not Tregs (Figure 3D-E). Interestingly, addition of recombinant IL-6 could substitute Teffs in maintaining high levels of OX40L in a dose-dependent fashion, suggesting that Teff-derived IL-6 might amplify BMMC–T-cell cross-talk through a prolonged OX40L/OX40 interaction (Figure 3E). Thus, the OX40/OX40L axis is sustained by IL-6 and contributes to BMMC-driven blockade of Treg function.
MCs organize the cytokine milieu favorable to Th17 cell differentiation
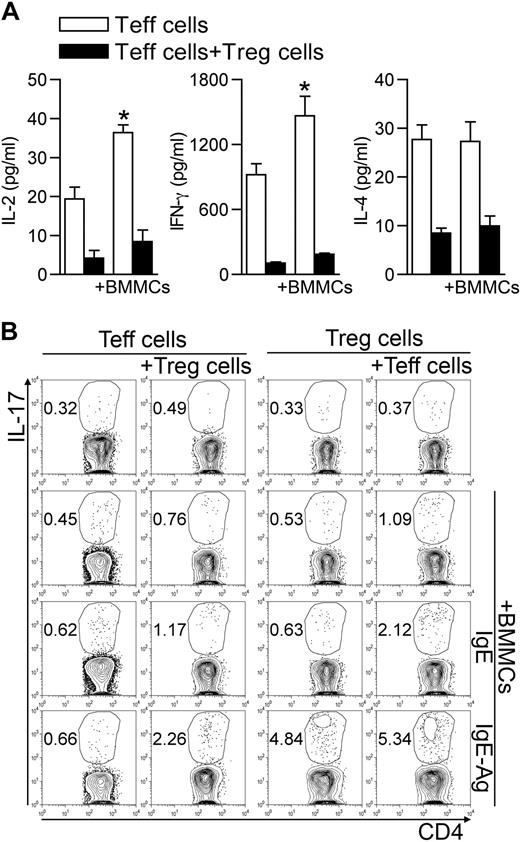
It has been previously reported that, under some circumstances, Tregs can suppress cytokine production without affecting T-cell proliferation.22,25 To characterize the BMMC-mediated Treg inhibition, we analyzed the array of cytokines detectable in supernatants of BMMC–T-cell cocultures. The release of Teff-associated cytokines, such as IL-2, interferon-γ, and IL-4, although increased in the presence of BMMCs, was inhibited by Tregs (Figure 4A). However, Tregs did not inhibit the release of proinflammatory cytokines, such as IL-6, TNF-α, and monocyte chemoattractant protein-1 (not shown). Thus, in the presence of BMMCs, Tregs were still able to inhibit Th1 (IL-2 and interferon-γ) and Th2 (IL-4) cytokine production, although they were unable to suppress Teff proliferation and the release of proinflammatory mediators, as well as to preserve their own anergic condition.
BMMCs create an optimal cytokine milieu for differentiation of Th17 cells. (A) Teffs were activated alone or in the presence of Tregs, and IgE-Ag–activated BMMCs were added or not to the T-cell culture. After 72 hours, supernatants were collected and analyzed for cytokine production. Among all cytokines analyzed, those showing significant variation between different groups are shown. *P < .05, compared with the corresponding condition in the absence of BMMCs. (B) CD45.2 Teffs and CD45.1 congenic Tregs were cultured alone or with unstimulated, IgE-sensitized, or IgE-Ag–activated BMMCs in equal amounts. After 72 hours, intracellular IL-17 content was evaluated by flow cytometry in each T-cell subset, gated according to the respective CD45 variant. Data are from 1 representative of 2 (A) or 3 (B) independent experiments.
BMMCs create an optimal cytokine milieu for differentiation of Th17 cells. (A) Teffs were activated alone or in the presence of Tregs, and IgE-Ag–activated BMMCs were added or not to the T-cell culture. After 72 hours, supernatants were collected and analyzed for cytokine production. Among all cytokines analyzed, those showing significant variation between different groups are shown. *P < .05, compared with the corresponding condition in the absence of BMMCs. (B) CD45.2 Teffs and CD45.1 congenic Tregs were cultured alone or with unstimulated, IgE-sensitized, or IgE-Ag–activated BMMCs in equal amounts. After 72 hours, intracellular IL-17 content was evaluated by flow cytometry in each T-cell subset, gated according to the respective CD45 variant. Data are from 1 representative of 2 (A) or 3 (B) independent experiments.
Hampered Th1/Th2 differentiation and availability of IL-6 and TNF-α and of Treg-derived TGF-β may favor Th17 differentiation.13 To investigate this possibility, we evaluated the IL-17 intracellular content in CD45.2+ Teffs and CD45.1+ Tregs after 3 days of coculture. Addition of IgE-Ag–activated, but not resting, BMMCs induced IL-17 production in Teffs only if Tregs were part of the trio. Conversely, the percentage of IL-17–producing Tregs was increased by IgE-Ag–stimulated BMMCs (from 0.39 ± 0.14 to 4.57 ± 0.59, P < .001) independently from the presence of Teffs (Figure 4B). Surprisingly, BMMCs also produced IL-17, and their contribution became evident in testing IL-17 content by enzyme-linked immunosorbent assay in the 3-cell culture supernatants (supplemental Figure 2).
In summary, these data suggest that activated BMMCs turn Tregs from anergic and suppressive into proliferating and IL-17–producing cells, an effect that spreads the pathogenic potential to nearby T cells, which gain competence for proliferation and Th17 differentiation.
MCs colocalize in vivo with Tregs and Th17 cells in different inflammatory conditions
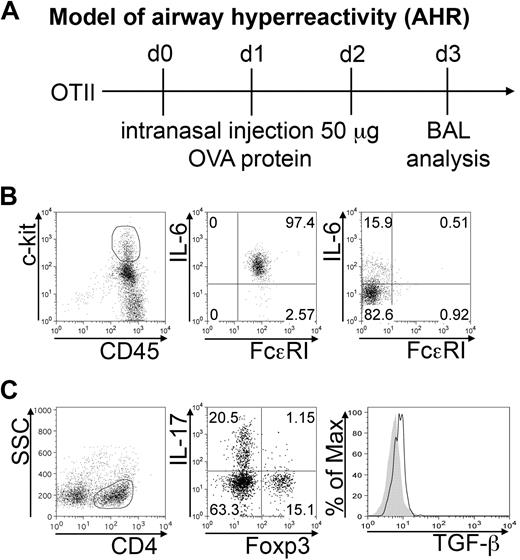
To visualize the interactions engaged by MCs with Tregs and Th17 cells in vivo, we analyzed 2 well-characterized models of inflammatory disease in which MCs and Th17 cells have a pathogenic role.
In transgenic OTII mice, intranasal ovalbumin challenge induces the development of Th17 cells that are responsible for neutrophil infiltration in the airways. BAL was harvested after 3 daily intranasal OVA injections (Figure 5A). More than 90% of MCs, identified as c-kithigh FcϵRI+ cells, produced IL-6 (Figure 5B middle panel). However, it should be noted that MCs were not the only source of IL-6 among the collected cells, as also lymphocytes produced IL-6 in vivo (Figure 5B right panel). The CD4+ component of BAL cells included Th17 cells and Foxp3+ Tregs, in roughly similar proportion. A minority (∼ 1%) of double IL-17 Foxp3+ cells was also detected. Intracellular staining revealed active TGF-β production by Foxp3+ Tregs in vivo (Figure 5C). Therefore, IL-6–producing MCs and TGF-β–producing Tregs populate the same inflammatory milieu in association with skewed Th17 effector cells.
BMMCs, Tregs, and Th17 cells populate inflamed airways. AHR was induced in OTII mice by intranasal OVA injection (A). BAL cells were collected 24 hours after the last inhalation. (B) Representative plots showing surface FcϵRI expression and IL-6 intracellular content in gated CD45+ c-kithigh (middle panel), representing MCs among harvested BAL cells, and in gated CD3+ lymphocytes (right panel). Among gated CD4+ cells, IL-17 versus Foxp3 expression is depicted (C). Histogram shows intracellular TGF-β expression in gated CD4+Foxp3+ Tregs (solid line) compared with isotype control (filled area). The shown data are representative from 3 experiments, each including at least 5 mice.
BMMCs, Tregs, and Th17 cells populate inflamed airways. AHR was induced in OTII mice by intranasal OVA injection (A). BAL cells were collected 24 hours after the last inhalation. (B) Representative plots showing surface FcϵRI expression and IL-6 intracellular content in gated CD45+ c-kithigh (middle panel), representing MCs among harvested BAL cells, and in gated CD3+ lymphocytes (right panel). Among gated CD4+ cells, IL-17 versus Foxp3 expression is depicted (C). Histogram shows intracellular TGF-β expression in gated CD4+Foxp3+ Tregs (solid line) compared with isotype control (filled area). The shown data are representative from 3 experiments, each including at least 5 mice.
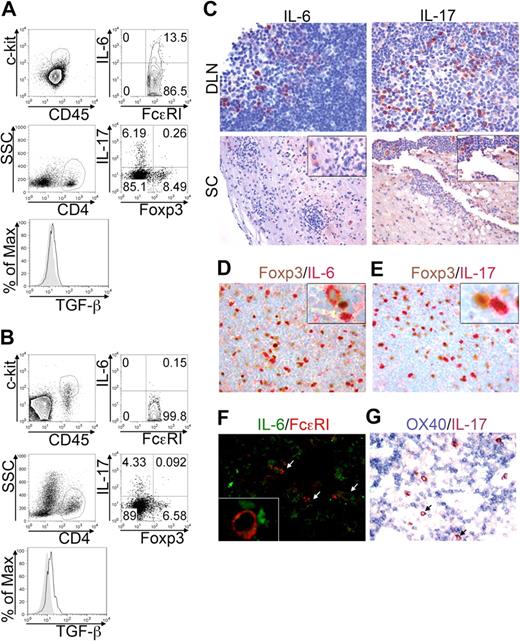
EAE is a model of autoimmunity involving MCs,7 Th17,29 and Tregs30 participation, at least in the effector phase. The frequency and the behavior of these cells were analyzed in draining lymph nodes (DLNs) and spinal cord (SC), at the acute phase of MOG35-55-induced EAE. This setting allowed distinguishing between the priming site and the target organ of the disease. The frequency of c-kit+ FcϵRI+ MCs was sizeable in DLNs (Figure 6A top panels) and SC (Figure 6B top panels) during disease onset. A relevant proportion (8.53% ± 3.49%) of DLN-populating MCs, but very few (0.11% ± 0.12%) of CNS-residing MCs, actively produced IL-6 in the acute phase, indicating that they might affect Th17 cell priming and differentiation primarily at the immunization site (Figure 6A-B top panels). However, MCs were not the only source of IL-6 in any condition analyzed (not shown), indicating that also in vivo other cells, probably T cells, might be an important source for this cytokine. Among CD4+ T cells, Th17 cells accumulated preferentially in DLNs rather than in SC at the acute phase (Figure 6A-B bottom panels) while recruited to SC at more advanced stages (not shown). Foxp3+ Tregs, detected in both DLNs and SC, were actively producing TGF-β (Figure 6A-B bottom panels). Notably, a minority (< 0.5%) of IL-17 Foxp3 double-positive T cells were detected, supporting the idea that Tregs may differentiate into Th17 cells in an inflammatory microenvironment.13,31
BMMCs, Tregs, and Th17 cells colocalize in EAE priming and effector sites. EAE was induced in C57BL/6 mice by MOG35-55 immunization. Animals were killed after 10 days during the acute phase. Lymph nodes draining the MOG35-55 injection site (DLN) and spinal cords (SC) were analyzed by flow cytometry. (A) Representative dot plots showing IL-6–producing cells among FcϵRI+c-kit+ MCs (top panels) and IL-17 versus Foxp3 expression in gated CD4+ T cells (bottom panels) in DLN of immunized mice. Histogram shows TGF-β expression in gated CD4+Foxp3+ Tregs (solid line) overlaid to isotype staining (filled area). (B) Same analysis as in panel A, performed on SC samples. (C) Formalin-fixed, paraffin-embedded sections obtained from DLN and SC were evaluated by immunohistochemistry for IL-6 (left panels) and IL-17 (right panels) expression (original magnification ×200). (D-E) In DLN, expression of Foxp3 (brown) versus IL-6 (red, D) or versus IL-17 (red, E) was analyzed. Insets show (at higher magnification) the proximity of Tregs with Th17 cells or IL-6+ cells (original magnification ×200). (F) Double fluorescence confocal microscopy for IL-6 (green) and FcϵRI (red) reveals a few IL6+ FcϵRI+ MCs (white arrows) scattered among IL-6+ FcϵRI− lymphocytes. The inset shows (at higher magnification) the interaction between MCs and IL-6–producing T cells (original magnification ×200). (G) Double immunohistochemistry for OX40 (blue) and IL-17 (red) shows IL-17+ cells (cytoplasmatic reactivity) intermingling with small clusters of OX40+ lymphocytes (membrane reactivity). IL-17–expressing cells also showed some degree of reactivity to anti-OX40 on the cell surface (arrows) Original magnification ×200. Data are from 2 independent immunization experiments, each including at least 7 mice.
BMMCs, Tregs, and Th17 cells colocalize in EAE priming and effector sites. EAE was induced in C57BL/6 mice by MOG35-55 immunization. Animals were killed after 10 days during the acute phase. Lymph nodes draining the MOG35-55 injection site (DLN) and spinal cords (SC) were analyzed by flow cytometry. (A) Representative dot plots showing IL-6–producing cells among FcϵRI+c-kit+ MCs (top panels) and IL-17 versus Foxp3 expression in gated CD4+ T cells (bottom panels) in DLN of immunized mice. Histogram shows TGF-β expression in gated CD4+Foxp3+ Tregs (solid line) overlaid to isotype staining (filled area). (B) Same analysis as in panel A, performed on SC samples. (C) Formalin-fixed, paraffin-embedded sections obtained from DLN and SC were evaluated by immunohistochemistry for IL-6 (left panels) and IL-17 (right panels) expression (original magnification ×200). (D-E) In DLN, expression of Foxp3 (brown) versus IL-6 (red, D) or versus IL-17 (red, E) was analyzed. Insets show (at higher magnification) the proximity of Tregs with Th17 cells or IL-6+ cells (original magnification ×200). (F) Double fluorescence confocal microscopy for IL-6 (green) and FcϵRI (red) reveals a few IL6+ FcϵRI+ MCs (white arrows) scattered among IL-6+ FcϵRI− lymphocytes. The inset shows (at higher magnification) the interaction between MCs and IL-6–producing T cells (original magnification ×200). (G) Double immunohistochemistry for OX40 (blue) and IL-17 (red) shows IL-17+ cells (cytoplasmatic reactivity) intermingling with small clusters of OX40+ lymphocytes (membrane reactivity). IL-17–expressing cells also showed some degree of reactivity to anti-OX40 on the cell surface (arrows) Original magnification ×200. Data are from 2 independent immunization experiments, each including at least 7 mice.
The tissue distribution, the reciprocal relationship, and the molecular products of Th17 cells, Tregs, and MCs were then analyzed by immunohistochemistry in lymphoid organs and CNS of MOG35-55-immunized mice. The cytokine IL-6 was found as intracellular product in DLNs and as both intracellular and extracellular molecule in CNS (Figure 6C left). In DLNs, IL-6+ cells were mostly found in the T-cell–rich paracortical area and consisted of small- to medium-sized cells. Laser scanning confocal microscopy for FcϵRI and IL-6 (Figure 6F) and immunohistochemical analysis of Giemsa-stained sections (supplemental Figure 3) allowed the identification of some IL-6–producing MCs scattered among IL-6+ T cells in lymph nodes. Overall, most of the IL-6–producing cells were FcϵRI− and IL-6 signal in FcϵRI+ cells was dimmer than that observed in FcϵRI− cells, supporting a prominent T-cell derivation of IL-6 (Figure 6F, supplemental Figure 3). IL-17–producing cells were mainly found in DLNs during acute phase and in perivascular lymphoid aggregates in SC. Extracellular IL-17 was detected, especially in acutely inflamed SC (Figure 6C right panels). Some IL-17–secreting cells were found in close proximity to Foxp3+ Tregs in DLNs, indicating that the 2 cell types might establish cognate or direct interactions, as suggested,13 allowing Tregs to contribute to Th17 skewing (Figure 6E). Close proximity was occasionally detected in DLNs between Tregs and IL-6–secreting MCs and T cells, suggesting again that Tregs might directly take part in innate and adaptive inflammatory response (Figure 6D). Double immunohistochemistry for OX40 and IL-17 showed several IL-17–reactive cells among small clusters of OX40-expressing lymphocytes (Figure 6G). In many cases, IL-17+ cells were adjacent to one or more OX40+ lymphocytes and some IL-17–expressing cells showed surface reactivity to anti-OX40 (Figure 6G arrows). In conclusion, the MC-Tregs-Th17 interplay, here documented in vivo, may be involved in the initiation, in addition to the effector phase, of inflammatory diseases.
Discussion
It has been proposed that a proinflammatory microenvironment may profoundly alter Treg program in vivo, which may be more dynamic than so far perceived, but the link between Tregs and inflammation, if functionally related, remains, mechanistically, poorly understood. Innate immune cells may represent a significant source of pro- and anti-inflammatory mediators both in situ and in locoregional lymphoid organs. Poorly studied outside the allergy field, MCs can orchestrate inflammation through a plethora of soluble and cell surface molecules acting on nearby cells. Here we showed, for the first time in inflamed tissues, the direct interaction between MCs and Tregs in vivo in 2 disease models, such as AHR and EAE. Such MC-Treg interaction was also detected in lymph nodes draining the EAE immunization site, suggesting a role for MCs in T-cell priming. In vitro, MCs reverted the anergic and suppressive phenotype of Tregs and promoted a Th17-skewed immune response. Soluble and contact-mediated mechanisms, such as IL-6 and OX40/OX40L, respectively, were shown to take part in this process.
Although confirming that BMMCs boosted Teff proliferation and cytokine production in vitro,21 here we have added the evidence that, in the presence of BMMCs, Tregs markedly decreased their suppressive activity on Teffs. This might occur because of an MC effect on Treg-intrinsic function and/or an MC effect on Teff susceptibility to suppression. However, BMMCs directly induced Treg proliferation and IL-17 production, even in the absence of Teffs. Because Treg anergy and suppressive ability may be functionally linked,23 this suggested that BMMCs might directly affect Treg functional program. Nonetheless, Treg proliferation was further enhanced in the concurrent presence of BMMCs and Teffs, suggesting that the BMMC effect on Tregs may be, at least partially, the result of Teff boosting.
Germane was the BMMCs-induced reversal of Treg suppression restricted to responder T-cell proliferation because Teff production of Th1/Th2 cytokines remained inhibited by Tregs. Thus, a sort of “split” functional paralysis of Tregs can be envisaged in this 3-cell system that, preserving Treg inhibition of cytokine production, allows T-cell expansion that, once blocked in the Th1/Th2 program, could deflect to alternative differentiation pathways.
A sizeable pool of Tregs can be detected in lymphoid organs and in tissues during inflammatory diseases, such as CNS in EAE and hyperreactive airways in AHR. However, the in vivo role of Tregs in these contexts remains largely unexplained. They can alternatively, but not exclusively, suppress immune responses, without the strength necessary to overcome pathology, or remain neutral because blocked in their functions, or become detrimental being shifted from a regulatory to a pathogenic phenotype. The first possibility is sustained by disease exacerbation on Treg depletion.30 In favor of the second hypothesis, myelin-specific Tregs from inflamed CNS are able to suppress ex vivo naive but not CNS-derived encephalitogenic Teffs, producing TNF-α and IL-6.32 Third, the role of Treg-derived TGF-β in Th17 skewing13 suggests that Tregs can take part in inflammatory diseases. It could be proposed that Tregs are not irreversibly differentiated, but rather plastic and tunable depending on the disease phase. Indeed, we found that IL-17–gaining Tregs still retained high/intermediate levels of Foxp3 expression in vitro (not shown), suggesting that Tregs may transiently undergo a double-positive stage before becoming purely inflammatory cells.31 Accordingly, ex vivo evaluation of Treg function cannot reproduce their in vivo behavior that is under the influence of locally released factors. However, the lack of knowledge on Treg significance in inflammatory pathology justifies the in vitro assay considering the multiple players operating in vivo.
We have dissected here the complex relationships of a 3-cell culture comprising MCs, Tregs, and Teffs. Blockade of IL-6Rα signal reverted BMMC effects in a dose-dependent manner, indicating IL-6 as key factor unleashing Tregs from anergy and inhibiting their suppressive function. The alternative use of il6−/− mice as donors of BMMCs or Teffs indicated that IL-6-null BMMCs, likewise the WT counterpart, still blocked Treg suppression. Instead, Tregs inhibited the proliferation of IL-6–deficient Teffs despite the presence of IL-6 competent BMMCs. Of note, IL-6Rα blockade reverted BMMC effects irrespective of their activation status (not shown), thus correlating with the pattern of IL-6 production in T cells rather than in BMMCs.
T-cell production of IL-6 was early in the coculture, but the initial trigger for this activation remains unknown. A possibility is that a strong costimulation conveyed by BMMCs promotes IL-6 secretion by Teffs that in turn initiate BMMC-mediated amplification of the T-cell response. This likely occurs through the OX40/OX40L pathway that takes part in later events rather than in early triggering of the T-cell response. Indeed, the expression of OX40L on BMMCs during 72-hour culture required the presence of Teffs or the addition of recombinant IL-6, indicating that early Teff-derived IL-6 sustains prolonged OX40 engagement. Accordingly, the expression of OX40 on Teffs began 16 hours after activation, thus excluding its role in earlier phases of BMMC–T-cell interaction. As a candidate for such initial Teff activation, direct TCR stimulation by anti-CD3 cross-linked on BMMC-associated Fcγ-receptors was doubtful because Fc-blocked BMMCs were used. Also excluded were the CD80/CD86 molecules that are not expressed by BMMCs.21 In light of previous data showing that BMMCs-derived TNF-α boosted T-cell activation,21 we tested TNF-α-null BMMCs that however phenocopied the WT counterparts in terms of Teff proliferation and Treg expansion/suppressive function (not shown). Thus, a still unknown “magic bullet” triggered IL-6 production in T cells that, sensed by innate components such as MCs, instigated their complicity to amplify a response only drafted by adaptive cells (for a summarizing model, see supplemental Figure 4A).
The activation status of BMMCs had different impact on certain T-cell functions. Indeed, increased Teff and Treg proliferation and inhibition of Treg suppression occurred without difference when unstimulated, IgE-sensitized, or IgE-Ag–activated BMMCs were used. Conversely, only IgE-sensitized Ag-stimulated BMMCs specifically induced Th17 arousal. IL-6 production by Teffs and OX40L/OX40 expression levels remained unaltered regardless of the presence of unstimulated or stimulated BMMCs, suggesting that they might be involved in a nonpolarized T-cell activation rather than in active Th17 skewing. We are tempted to speculate that steady-state BMMCs have the intrinsic property to foster T-cell activation, whereas their specific stimulation should be required to induce their secretion of IL-6 but also of TNF-α and IL-1, all necessary for a complete Th17 response (supplemental Figure 4B).
We have recently shown that Tregs directly inhibit MC degranulation in vitro and in vivo through OX40/OX40L reverse signaling in the MC.18 Of note, Tregs were shown to suppress the release of histamine-preformed granules, thus inhibiting immediate anaphylactic response, without affecting TNF-α granule secretion or IL-6 production. On the same line, some of us have recently found that histamine might protect from EAE because mice unable to synthesize histamine because of a genetic deficit in the histidine decarboxylase develop exacerbated disease on MOG35-55 immunization.33 A recent report shows Tregs inhibiting FcϵRI expression in MC,34 a process that may result in the impairment of certain MC functions and, as a consequence, in altered T-cell activation. However, we could not find any difference in FcϵRI expression on MC, in coculture with or without Tregs (not shown), or in Treg-depleted or replete animals in vivo.18 Thus, a sort of split suppression seems to occur from Tregs to MCs; accordingly, Tregs selectively suppress the release of protective mediators while preserving MC secretion of proinflammatory cytokines. MC-Treg interaction has a role in the tolerance to skin allograft, depending on Treg-derived IL-9 that recruits and sustains MC infiltration into the graft.17 In this setting, MCs maintain tolerance until forced to degranulate. Again, different contexts may be characterized by split tolerance and variable interactions between regulatory and inflammatory cells.
IL-6 and OX40 have been independently associated with the same inflammatory diseases. il6−/− mice develop milder EAE, even though IL-21 can substitute IL-6 in Th17 induction.35 Tnfrsf4−/− mice also show attenuated EAE,36 and administration of an OX40/OX40L-blocking antibody has been shown to ameliorate EAE symptoms.37 However, they have never been studied together or in the context of MC-Treg interactions. Moreover, very little is known about the contribution of OX40-expressing Tregs and Teffs in disease or about the mechanisms driving distinct molecular and functional responses in the 2 T-cell subsets on the stimulation of a shared molecule. Reconstitution of MC-genetically deficient mice with WT or il6−/− BMMCs will allow ascribing a precise role to MC-derived IL-6 in disease. We plan to induce EAE in the widely used MC-deficient strain KitW-sh/W-sh, even though we cannot exclude that the defects in granulocytic populations described in these mice38 may mask the actual role of MCs in EAE. In vivo, we found both MCs and T cells producing IL-6 in close contact with Tregs in EAE priming site. This suggests that multiple IL-6 sources are possible and that MCs may promote EAE in the lymphoid compartment during the initiation phase, rather than in the inflamed tissue during effector phase; accordingly, BMMCs injected into MC-deficient mice have been shown to rescue EAE susceptibility, albeit unable to infiltrate CNS.9
The interplay between innate and adaptive immune cells contributes to the complexity of autoimmune disorders. T cell–initiated activation may evoke the aid of potent innate players, as MCs, to subvert suppressive barriers, and even to transform regulatory cells into cells with pathogenic activity. Thus, blocking this harmful cascade would represent a suitable goal for treatment of inflammatory disease. This complex picture requires further investigation but warns the scientific community to avoid straightforward therapeutic approaches that block, instead of just selectively limiting, MC functions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Juan Rivera, Brigitta Stockinger, and Massimo Costanza for helpful discussion, Juan Rivera for providing bone marrow and spleen from il6−/− mice, and Giuseppe Penna and Luciano Adorini for help with intracellular staining.
This work was supported by grants from the Italian Ministry of Health, Associazione Italiana Ricerca sul Cancro, Ministero dell'Istruzione, Università e Ricerca (PRIN 2005), Agenzia Spaziale Italiana (Progetto OSMA), LR.11 del Friuli Venezia Giulia, and the National Multiple Sclerosis Society. S.P. is supported by a fellowship from Fondazione Italiana Ricerca sul Cancro.
Authorship
Contribution: S.P. and G.G. contributed to discussion of experimental design and data analysis and wrote the manuscript; S.P. performed in vitro and in vivo functional regulatory T-cell analysis and AHR model; G.G. set up the in vitro mast cell—T-cell coculture and intracellular staining; C.T. performed histopathologic analyses, immunohistochemistry, and immunofluorescence experiments and imaging; S.M. set up in vivo EAE immunization; A.G. and B.F. helped with experiments; R.P. provided scientific assistance for the EAE model and discussed the results; and M.P.C. and C.E.P. directed the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mario P. Colombo, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Istituto Nazionale dei Tumori, via Venezian 1, 20133 Milan, Italy; e-mail: mario.colombo@istitutotumori.mi.it; or Carlo E. Pucillo, University of Udine, P. le M. Kolbe 4, 33100 Udine, Italy; e-mail: carlo.pucillo@uniud.it.
References
Author notes
*S.P. and G.G. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal