Protein kinase C-δ (PKC-δ) is expressed in platelets and activated downstream of protease-activated receptors (PARs) and glycoprotein VI (GPVI) receptors. We have previously shown that PKC-δ positively regulates PAR-mediated dense granule secretion, whereas it negatively regulates GPVI-mediated dense granule secretion. We further investigated the mechanism of such differential regulation of dense granule release by PKC-δ in platelets. SH2 domain–containing inositol phosphatase-1 (SHIP-1) is phosphorylated on Y1020, a marker for its activation, upon stimulation of human platelets with PAR agonists SFLLRN and AYPGKF or GPVI agonist convulxin. GPVI-mediated SHIP-1 phosphorylation occurred rapidly at 15 seconds, whereas PAR-mediated phosphorylation was delayed, occurring at 1 minute. Lyn and SHIP-1, but not SHIP-2 or Shc, preferentially associated with PKC-δ on stimulation of platelets with a GPVI agonist, but not with a PAR agonist. In PKC-δ–null murine platelets, convulxin-induced SHIP-1 phosphorylation was inhibited. Furthermore, in Lyn null murine platelets, GPVI-mediated phosphorylations on Y-1020 of SHIP-1 and Y311 of PKC-δ were inhibited. In murine platelets lacking Lyn or SHIP-1, GPVI-mediated dense granule secretions are potentiated, whereas PAR-mediated dense granule secretions are inhibited. Therefore, we conclude that Lyn-mediated phosphorylations of PKC-δ and SHIP-1 and their associations negatively regulate GPVI-mediated dense granule secretion in platelets.
Introduction
Platelet-collagen interactions are thought to have the greatest significance at medium or high shear rates found in arteries and diseased vessels.1 Glycoprotein VI (GPVI) is the major signaling receptor for collagen on the platelet surface.1 The critical role played by platelets in hemostasis, thrombosis and vascular remodeling, and healing is related to their function as exocytotic cells that secrete important effector molecules at the site of vascular injury. Platelets normally contain at least 3 types of large intracellular granules, such as α, dense, and lysosomal granules. There are 3 to 8 dense granules per platelet, and the contents from these dense granules are important for recruiting more platelets to the site of injury.2,3
Protein kinase C (PKC) has been implicated in platelet secretion.4 Protein kinase Cs are members of the extended AGC (protein kinases A, G, and C) family of differentially expressed serine/threonine kinases implicated in a diverse array of cellular functions. After activation, these kinases migrate to different subcellular locations, including the plasma membrane and cytoskeletal elements where they regulate different physiologic functions.5 PKC isoforms are subdivided into 3 groups based on their lipid and cofactor requirements: the diacylglycerol and calcium-sensitive conventional isoforms (α, βI, βII and γ), the diacylglycerol-sensitive and calcium-insensitive novel isoforms (δ, η, θ, and ϵ), and the phosphatidylinositide trisphosphate-sensitive atypical isoforms (ζ, ι, μ, and λ).6
PKC-δ plays a key role in growth regulation and tissue remodeling in other cells7 and differentially regulates dense granule secretion in platelets. It positively regulates protease-activated receptor (PAR)–mediated dense granule release and negatively regulates GPVI-mediated dense granule release.8,–10 Intrinsic function of PKCs is regulated by 3 mechanisms: (1) binding of the cofactor that allosterically activates the enzyme, (2) phosphorylation on the activation loop residue that primes the enzyme for catalysis, and (3) interaction with proteins that position it close to its regulators and substrates.11
In immune cells, a 145-kDa protein associates with Shc on cellular activation with multiple cytokines and plays an important role in the negative regulation of degranulation mediated by FcγRIIB receptor by associating with PKC-δ.12,–14 This 145-kDa protein is SH2 domain–containing inositol phosphatase (SHIP), a 5′inositol phosphatase, that dephosphorylates inositol 1,3,4,5-tetrakisphosphate and phosphatidyl inositol 3,4,5-trisphosphate (PIP3) and is expressed in a variety of hematopoietic cells.15,16 There are 2 isoforms of SHIP that are expressed in platelets, SHIP-1 and SHIP-2, which share 37% homology between them. Among the 2 isoforms expressed in platelets, SHIP-1 is a major regulator of PIP3 levels.17 The tyrosine phosphorylation of SHIP and its translocation to the membrane on thrombin stimulation in platelets occurs in an integrin-dependent manner.18 Therefore, SHIP-1 is considered to be downstream of αIIbβ3 upon PAR stimulation.19 However, the role of SHIP-1 downstream of GPVI stimulation is not clearly understood.
Src family kinases (SFKs) are nonreceptor tyrosine kinases that mediate integrin-dependent outside-in signaling in platelets.20 Among SFKs, Lyn, is reported to have negative regulatory roles in lymphocytes and macrophages.21,22 Studies in RBL-2H3 cells show that Lyn associates with PKC-δ.23 However, the mechanism underlying the regulation of PKC-δ's function by Lyn resulting from association is unknown.
In this study, we investigated the mechanism of differential regulation of dense granule secretion and show that the regulation of PKC-δ and SHIP-1 phosphorylations by Lyn and their associations negatively regulate GPVI-mediated dense granule secretion in platelets.
Methods
Materials
Approval for this study was obtained from the Institutional Review Board of Temple University.
Convulxin (CVX), apyrase grade VII, thrombin, and acetylsalicylic acid were obtained from Sigma-Aldrich. Collagen-related peptide (CRP) was purchased from Dr Richard Farndale (Reader in Receptor Biochemistry, University of Cambridge). Collagen and Luciferin-luciferase reagent were purchased from Chrono-Log. Phospho-SHIP-1 (Y1020) and PKC-δ antibodies were obtained from Cell Signaling Technologies and Santa Cruz Biotechnology, respectively. The antibodies against Src family members were obtained from Cell Signaling Technologies. The protease inhibitor cocktail for lysis buffer was purchased from Roche Diagnostics. All the other reagents were of reagent grade, and deionized water was used throughout.
Animals
PKC-δ −/− (C57BL/6 strain) mice were a generous gift from Dr Keiko Nakayama (Division of Developmental Genetics, Tohoku University Graduate School of Medicine) and wild-type (WT) C57/BL6 littermates were used as controls. The 129/Sv Lyntm1Sor/J−/− mice, 129-Fyntm1Sor/J−/− mice, Inpp5dtm1Dmt/J SHIP-1−/− mice, and the corresponding age- and sex-matched WT controls were purchased from Jackson Laboratory. Src−/− mice were a generous gift from Dr Archana Sanjay (Department of Anatomy and Cell Biology, Temple University School of Medicine). The mice were used for physiologic measurements using the protocol approved by the Institutional Animal Care and Use Committee. The animals used for the experiment were subject to very minimal distress and killed with an overdose of pentobarbital before decapitation at the end of experimentation.
Preparation of washed human platelets
Whole blood was drawn from healthy human volunteers selected from students, staff, or workers at the Temple University who provided their informed consent in accordance with the Declaration of Helsinki. Donated blood was collected in tubes containing one-sixth volume of acid citrate dextrose (2.5 g sodium citrate, 1.5 g citric acid, and 2 g glucose in 100 mL deionized water). Citrated blood was centrifuged (Eppendorf 5810R centrifuge) at 230g for 20 minutes at room temperature to obtain platelet-rich plasma (PRP). The PRP was then incubated with 1 mM acetylsalicylic acid (aspirin) for 30 minutes at 37°C and then allowed to remain at room temperature for 5 minutes. The PRP was then centrifuged for 10 minutes at 980 relative centrifugal force (RCF) at room temperature. Platelet pellet was resuspended in calcium-free Tyrode buffer (138 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 3 mM NaH2PO4, 5 mM glucose, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid adjusted to pH 7.4) containing 0.1 units/mL apyrase. Cells were counted using the Z1 Coulter Particle Counter and adjusted to a concentration of 2 × 108/mL.
Preparation of washed mouse platelets
Blood was collected from anesthetized mice by cardiac puncture into syringes containing 3.8% sodium citrate as anticoagulant. The whole blood was centrifuged (IEC Micromax centrifuge; International Equipment) at 100g for 10 minutes to isolate the PRP. Prostaglandin E1 (1 μM) was added to PRP. The platelets were centrifuged at 400g for 10 minutes, and the pellet was resuspended in Tyrode buffer containing 0.1 units/mL apyrase.
Measurement of platelet-dense granule secretion in human and mouse platelets
Adenosine triphosphate (ATP) secretion from platelet-dense granules was determined in aspirinated human platelets or 10 μM indomethacin-treated murine platelets using the Luciferin-Luciferase assay. The platelets were stimulated in a lumi-aggregometer at 37°C with stirring at 900 rotations per minute (rpm) × 100, and the corresponding luminescence was measured. The data are represented in the form of actual secretion tracings, and the ATP released was quantitated in nanomoles and the values shown next to the secretion tracings.
Immunoprecipitation and Western blotting
Platelets were stimulated with agonists for the appropriate time, and the reaction was stopped by the addition of equal volumes of the 2× cell lysis buffer (50 mM Tris-HCl, 0.5% NP-40, 0.25% sodium deoxycholate, 0.15 M NaCl, 1 mM ethylenediaminetetraacetic acid, pH 8.0, 1 mM phenylmethylsulfonyl fluoride, 1 μg/mL protease inhibitor cocktail, 1 mM activated sodium vanadate, 1 mM sodium fluoride, 1 mM β-glycerophosphate). The lysates were incubated for 30 minutes in ice for completion of lysis. The samples were then spun at 12 000g at 4°C for 10 minutes to bring down the cytoskeleton. The cell lysates were isolated, and 1.5 μg of total PKC-δ or 4.123 μg of total SHIP immunoprecipitating antibodies were added, respectively, and incubated overnight at 4°C. A negative control sample was also prepared by immunoprecipitating for rabbit IgG. Protein A/G beads were added the next day, and the immune complexes were allowed to rock on a rocker for 2 hours, processed for sodium dodecyl sulfate gel electrophoresis, and immunoblotted as described previously. At the same time, Western samples were prepared. All the samples were used for immunoblotting using the primary antibodies followed by alkaline phosphatase–coupled secondary antibodies per established protocols. The membranes were finally incubated with CDP-Star chemiluminescent substrate (Tropix) for 10 minutes at room temperature, and immunoreactivity was detected using a Fuji Film Luminescent Image Analyzer (LAS-1000 CH).
Statistical analysis
The results were quantitated and expressed as mean plus or minus SD. The data were statistically analyzed using Student t test; P values less than or equal to .05 were considered significant.
Results
PAR- and GPVI-mediated SHIP-1 phosphorylations follow different activation kinetics
SHIP-1 plays an important role in the negative regulation of degranulation in mast cells by associating with PKC-δ.14 Because PKC-δ differentially regulates dense granule secretion in platelets downstream of PARs and GPVI,8,9 we examined whether there are differences in the phosphorylation of SHIP-1 downstream of these receptors. SHIP-1 has 2 tyrosine residues on its C-terminus, Y1020 and Y1166 in humans. The C-terminus is important for its catalytic function.24 SHIP-1 is expressed in platelets, and its tyrosine phosphorylation on thrombin stimulation is aggregation-dependent and integrin-mediated.18 Previous findings from Pasquet et al25 showed that SHIP is tyrosine phosphorylated on GPVI stimulation. However, because of the lack of availability of isoform-specific phospho antibody, the isoform of SHIP that is activated downstream of GPVI receptors was not identified. Therefore, we evaluated GPVI-mediated SHIP-1 phosphorylation at tyrosine residue 1020 (Y1020) as a marker of its activation. We stimulated human platelets with PAR agonist peptides, AYPGKF, SFLLRN, and a GPVI agonist, CVX. Our results showed that PAR-mediated SHIP-1 phosphorylation was delayed and started at 60 seconds and peaked at 120 seconds (Figure 1A-B), whereas GPVI-mediated SHIP-1 phosphorylation was rapid, started as early as 15 seconds and peaked at 60 seconds (Figure 1C). Thus, we concluded that PAR- and GPVI-mediated SHIP-1 phosphorylation occurs at different time points.
Time course of PARs and GPVI-mediated SHIP-1 phosphorylation. Washed and aspirin-treated human platelets were stimulated with 500 μM AYPGKF (A), 10 μM SFLLRN (B), or 100 ng/mL CVX (C) for various time periods at 37°C under stirring conditions, and the blots were probed with the antibody for pSHIP-1 Y1020. Each blot is a representative of 3 experiments from 3 different donors.
Time course of PARs and GPVI-mediated SHIP-1 phosphorylation. Washed and aspirin-treated human platelets were stimulated with 500 μM AYPGKF (A), 10 μM SFLLRN (B), or 100 ng/mL CVX (C) for various time periods at 37°C under stirring conditions, and the blots were probed with the antibody for pSHIP-1 Y1020. Each blot is a representative of 3 experiments from 3 different donors.
SHIP-1, but not SHIP-2 or Shc, associates with PKC-δ on stimulation of GPVI receptors
There are 2 isoforms of 5′-inositol phosphatase, SHIP-1 and SHIP-2, expressed in human blood platelets; and SHIP-1, rather than SHIP-2, plays a major role in regulating PIP3 levels in platelets.17 The association of SHIP-1 with PKC-δ negatively regulates mast cell degranulation.14 In addition, Shc was shown to associate with both SHIP-1 and PKC-δ in mast cells.14 Therefore, we investigated the association of SHIP-1, SHIP-2, and Shc with PKC-δ in platelets using coimmunoprecipitation. When PKC-δ was immunoprecipitated and probed for phospho-SHIP-1, there was no constitutive association of SHIP-1 with PKC-δ (Figure 2A). Furthermore, SHIP-1 did not associate with PKC-δ on stimulation of platelets with 500 μM AYPGKF. However, SHIP-1 preferentially associated with PKC-δ on GPVI stimulation with CVX (Figure 2A). We confirmed these results with reverse-immunoprecipitation experiments by immunoprecipitating with total SHIP-1 and probing for PKC-δ. These experiments confirmed that the association of SHIP-1 with PKC-δ occurs only on GPVI activation (Figure 2B). Because SHIP-2 and Shc are also expressed in platelets, we investigated the association of SHIP-2 and Shc with PKC-δ. In coimmunoprecipitation experiments, we did not detect any association of SHIP-2 or Shc with PKC-δ either constitutively, or on PAR or GPVI receptor stimulation in platelets (data not shown). Rabbit IgG served as a negative control for our immunoprecipitation experiments. These data suggest differential association of SHIP-1 with PKC-δ on GPVI activation.
Association of phospho-SHIP-1 with PKC-δ. Washed aspirin-treated human platelets were stimulated with 500 μM AYPGKF or 100 ng/mL CVX for 60 seconds at 37°C under stirring conditions, and then the samples were immunoprecipitated with total PKC-δ and immunoblotted for phospho-SHIP-1 (A) as described in “Immunoprecipitation and Western blotting.” Reverse immunoprecipitation experiments were carried out by immunoprecipitating total SHIP-1 and immunoblotting for total PKC-δ (B). Rabbit IgG served as a negative control for all our immunoprecipitation experiments.
Association of phospho-SHIP-1 with PKC-δ. Washed aspirin-treated human platelets were stimulated with 500 μM AYPGKF or 100 ng/mL CVX for 60 seconds at 37°C under stirring conditions, and then the samples were immunoprecipitated with total PKC-δ and immunoblotted for phospho-SHIP-1 (A) as described in “Immunoprecipitation and Western blotting.” Reverse immunoprecipitation experiments were carried out by immunoprecipitating total SHIP-1 and immunoblotting for total PKC-δ (B). Rabbit IgG served as a negative control for all our immunoprecipitation experiments.
SHIP-1 phosphorylation is inhibited in PKC-δ–null murine platelets on GPVI stimulation
Because PKC-δ associates with SHIP-1 in human platelets on GPVI stimulation, we investigated whether this association has any functional implications on SHIP-1 phosphorylation. Hence we evaluated the phosphorylation of SHIP-1 by CVX stimulation in PKC-δ−/− murine platelets. As shown in Figure 3A, AYPGKF-induced SHIP-1 phosphorylation was not affected in the PKC-δ−/− murine platelets. However, CVX-induced SHIP-1 phosphorylation was inhibited in PKC-δ−/− murine platelets compared with the WT littermates (Figure 3B). These data indicate that the association of PKC-δ with SHIP-1 regulates SHIP-1 tyrosine phosphorylation only on GPVI stimulation and not on PAR stimulation. However, because PKC-δ is a serine threonine kinase, it may be indirectly regulating tyrosine phosphorylation of SHIP-1 possibly through an adapter function.
PKC-δ regulates GPVI-, but not PAR-mediated, phosphorylation of SHIP-1. Washed and indomethacin-treated murine platelets from PKC-δ−/− and age-matched WT littermates were stimulated with (A) PAR4 agonists or (B) CVX for 2 minutes. The samples were then lysed with sample buffer containing dithiothreitol (DTT) and probed for pSHIP-1 Y1020 antibody. Total Erk and β-actin were used as lane-loading controls.
PKC-δ regulates GPVI-, but not PAR-mediated, phosphorylation of SHIP-1. Washed and indomethacin-treated murine platelets from PKC-δ−/− and age-matched WT littermates were stimulated with (A) PAR4 agonists or (B) CVX for 2 minutes. The samples were then lysed with sample buffer containing dithiothreitol (DTT) and probed for pSHIP-1 Y1020 antibody. Total Erk and β-actin were used as lane-loading controls.
Lyn regulates GPVI-mediated SHIP-1 phosphorylation
The signaling function of SHIP-1 is subject to the regulation by SFK members.22,26,–28 Pan SFK inhibitor PP2 totally abolished GPVI-mediated SHIP-1 phosphorylation in platelets (data not shown). To clearly delineate the specific member of SFKs regulating GPVI-mediated SHIP-1 phosphorylation, we used Fyn−/−, Src−/−, and Lyn−/− murine platelets. Washed indomethacin-treated murine platelets were stimulated with GPVI agonists CVX or collagen, and aggregation was measured. Aggregation was inhibited in Fyn−/− platelets on stimulation with CVX (Figure 4A) consistent with previous findings.29 There was no effect on GPVI-mediated SHIP-1 phosphorylation in Fyn−/− murine platelets compared with WT littermates (Figure 4B). Src−/− mice showed no defect in aggregation (Figure 4C) or no inhibition of SHIP-1 phosphorylation on stimulation with GPVI agonists (Figure 4D). On the other hand, Lyn−/− murine platelets showed a potentiation of CVX-induced aggregation (Figure 4E) and inhibition of collagen-induced SHIP-1 phosphorylation (Figure 4F). Consistent with the data using collagen as the GPVI agonist, CVX-induced SHIP-1 phosphorylation was also inhibited in Lyn−/− murine platelets but not in Fyn−/− or Src−/− murine platelets compared with their WT littermates (data not shown). Furthermore, there was no difference in SHIP-1 phosphorylation in Lyn−/− murine platelets compared with their WT littermates upon PAR stimulation (data not shown). These data suggest an important role for Lyn in regulating GPVI-mediated SHIP-1 phosphorylation in murine platelets.
Regulation of GPVI-mediated SHIP-1 phosphorylation by Fyn, Src, and Lyn in murine platelets. Washed and indomethacin-treated WT, Fyn−/− (A-B), Src−/− (C-D), and Lyn−/− (E-F) murine platelets were stimulated with 60 ng/mL CVX or 20 μg/mL collagen under stirring conditions for 60 seconds at 37°C. The reaction was stopped using 3× sample loading buffer (with DTT). The lysates were subjected to Western blotting analysis and probed for pSHIP-1 (Y1020). Total Erk and β-actin served as lane-loading controls.
Regulation of GPVI-mediated SHIP-1 phosphorylation by Fyn, Src, and Lyn in murine platelets. Washed and indomethacin-treated WT, Fyn−/− (A-B), Src−/− (C-D), and Lyn−/− (E-F) murine platelets were stimulated with 60 ng/mL CVX or 20 μg/mL collagen under stirring conditions for 60 seconds at 37°C. The reaction was stopped using 3× sample loading buffer (with DTT). The lysates were subjected to Western blotting analysis and probed for pSHIP-1 (Y1020). Total Erk and β-actin served as lane-loading controls.
Lyn associates with PKC-δ and regulates PKC-δ Y311 phosphorylation
Because it was shown that Lyn associates with PKC-δ in RBL-2H3 cells,23 we evaluated such association in platelets. Platelets were activated with 500 μM AYPGKF or 100 ng/mL CVX followed by immunoprecipitation with total PKC-δ and immunoblotted with total Lyn. As shown in Figure 5A, Lyn associates with PKC-δ on stimulation with only CVX. However, there is no constitutive association of Lyn and PKC-δ in human platelets. In addition, we failed to see such association between Lyn and SHIP-1 (data not shown). Because Lyn associated with PKC-δ on GPVI stimulation, we investigated the implication of such association. Phosphorylation on Y311 of PKC-δ is known to occur downstream of GPVI stimulation of platelets.30 In Lyn−/− mice, Y311 phosphorylation of PKC-δ was inhibited on stimulation with CVX or collagen, compared with WT littermates (Figure 5B). These results indicate that Lyn regulates Y311 phosphorylation of PKC-δ on GPVI stimulation.
Lyn associates with PKC-δ and regulates PKC-δ Y311 phosphorylation on GPVI stimulation. Washed aspirin-treated human and indomethacin-treated murine platelets were used. Human platelets were stimulated with 500 μM AYPGKF or 100 ng/mL CVX and immunoprecipitated with total PKC-δ and probed for total Lyn (A). Washed indomethacin-treated murine platelets from Lyn−/− mice and corresponding WT littermates were stimulated with 60 ng/mL CVX or 20 μg/mL collagen for 1 minute. The samples were then lysed with sample buffer containing DTT and probed for PKC-δ Y311 (B). Total PKC-δ served as a lane-loading control in this experiment.
Lyn associates with PKC-δ and regulates PKC-δ Y311 phosphorylation on GPVI stimulation. Washed aspirin-treated human and indomethacin-treated murine platelets were used. Human platelets were stimulated with 500 μM AYPGKF or 100 ng/mL CVX and immunoprecipitated with total PKC-δ and probed for total Lyn (A). Washed indomethacin-treated murine platelets from Lyn−/− mice and corresponding WT littermates were stimulated with 60 ng/mL CVX or 20 μg/mL collagen for 1 minute. The samples were then lysed with sample buffer containing DTT and probed for PKC-δ Y311 (B). Total PKC-δ served as a lane-loading control in this experiment.
Lyn-mediated tyrosine phosphorylation regulates the association of SHIP-1 with PKC-δ
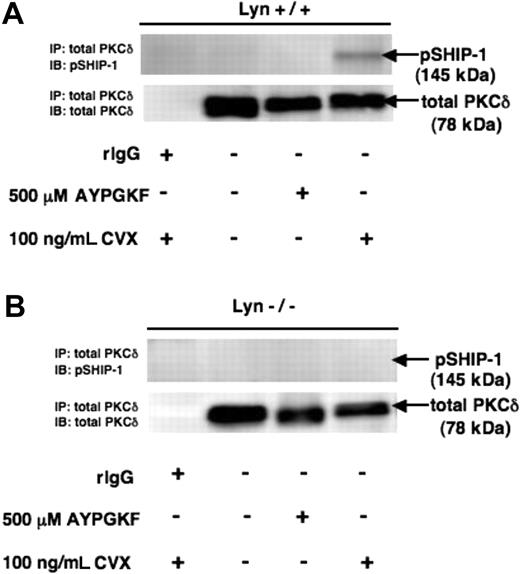
Lyn and SHIP-1 have been shown to associate on antigen stimulation in immune cells22 and are known regulators of hematopoiesis.31 SHIP-1 also associates with PKC-δ through a trimolecular complex with Shc in immune cells.14 Our data show that Lyn phosphorylates SHIP-1 on GPVI stimulation of platelets (Figure 4F). To investigate the role of Lyn in the regulation of the association between PKC-δ and SHIP-1, we carried out coimmunoprecipitation experiments in Lyn−/− murine platelets and corresponding WT littermates (Lyn+/+). We immunoprecipitated the murine platelets with PKC-δ and immunoblotted for phospho-SHIP-1. Our results revealed that SHIP-1 associates with PKC-δ in WT murine platelets (Figure 6) but not in Lyn−/− littermates on CVX-stimulation. There was no association of SHIP-1 with PKC-δ in Lyn−/− or Lyn+/+ littermates on stimulation with AYPGKF (Figure 6). These data indicate that Lyn regulates the association of SHIP-1 with PKC-δ in platelets, possibly through phosphorylation of PKC-δ and/or SHIP-1, on GPVI receptor stimulation.
Lyn regulates the association between PKC-δ and SHIP-1. Washed, indomethacin-treated WT littermates (A) and Lyn−/− mice (B) were stimulated with 500 μM AYPGKF or 100 ng/mL CVX, immunoprecipitated with total PKC-δ, and probed for pSHIP-1 and total PKC-δ, respectively. Rabbit IgG was used as a negative control for the coimmunoprecipitation experiment.
Lyn regulates the association between PKC-δ and SHIP-1. Washed, indomethacin-treated WT littermates (A) and Lyn−/− mice (B) were stimulated with 500 μM AYPGKF or 100 ng/mL CVX, immunoprecipitated with total PKC-δ, and probed for pSHIP-1 and total PKC-δ, respectively. Rabbit IgG was used as a negative control for the coimmunoprecipitation experiment.
Lyn and SHIP-1 negatively regulate GPVI-mediated dense granule secretion in murine platelets
Studies have established hematopoietic-specific SHIP to be a negative regulator of mast cell degranulation.32,33 SHIP-1 is a major regulator of PIP3 levels in platelets.17 Our studies show that SHIP-1 is phosphorylated by Lyn (Figure 4F), and this phosphorylation is regulated by PKC-δ (Figure 3). In addition, we have shown that SHIP-1 and PKC-δ, and Lyn and PKC-δ associate with each other on GPVI stimulation in platelets. If these associations are important for the regulation of dense granule release by GPVI pathways, then absence of any of these signaling molecules should have a functional implication on secretion. Because we and others have shown that CVX-induced dense granule secretion is potentiated by pharmacologic inhibitors of PKC-δ,8,9 we hypothesized that, in the absence of Lyn or SHIP-1, the dense granule secretions would also be potentiated on GPVI stimulation. On stimulation of indomethacin-treated washed murine platelets with PAR-4 agonist peptide AYPGKF (100 μM) or thrombin (0.05 U/mL) and GPVI agonists, CVX (60 ng/mL), or collagen (20 μg/mL) at submaximal doses for 3 minutes, platelet aggregations and dense granule secretions were slightly inhibited on PAR stimulation with AYPGKF (Figure 7A) or thrombin (Figure 7B) and potentiated on CVX (Figure 7C) or collagen (Figure 7D) stimulation in Lyn−/− mice. Similarly, in SHIP-1−/− murine platelets, AYPGKF-induced dense granule secretion was inhibited (Figure 7E), whereas CVX- or collagen-induced dense granule secretion (Figure 7F-G) was potentiated. Similar results were obtained with CRP, a GPVI agonist (data not shown). These data suggest that the intermolecular interactions between Lyn, SHIP-1, and PKC-δ negatively regulate collagen-induced dense granule secretion in platelets.
Dense granule secretion measurements in WT, Lyn−/−, and SHIP-1−/− murine platelets. Washed indomethacin-treated WT and Lyn−/− or SHIP-1−/− murine platelets were stimulated with 100 μM AYPGKF (A,E), 0.05 U/mL thrombin (B), 60 ng/mL CVX (C,F) or 20 μg/mL collagen (D,G) for 3 minutes at 37°C as indicated under stirring conditions. The activation of platelets was performed in a lumi-aggregometer at 37°C with stirring at 900 rotations per minute (rpm) × 100, and the secretion was measured and expressed in as nanomoles of ATP released/108 platelets. The data are represented as normal secretion tracings, and the actual ATP released was represented in nanomoles and analyzed by Student t test. P ≤ .05 was considered significant.
Dense granule secretion measurements in WT, Lyn−/−, and SHIP-1−/− murine platelets. Washed indomethacin-treated WT and Lyn−/− or SHIP-1−/− murine platelets were stimulated with 100 μM AYPGKF (A,E), 0.05 U/mL thrombin (B), 60 ng/mL CVX (C,F) or 20 μg/mL collagen (D,G) for 3 minutes at 37°C as indicated under stirring conditions. The activation of platelets was performed in a lumi-aggregometer at 37°C with stirring at 900 rotations per minute (rpm) × 100, and the secretion was measured and expressed in as nanomoles of ATP released/108 platelets. The data are represented as normal secretion tracings, and the actual ATP released was represented in nanomoles and analyzed by Student t test. P ≤ .05 was considered significant.
Discussion
PKC isoforms mediate several platelet functional responses, including platelet secretion, and thereby amplify platelet responses. PKC-δ isoform differentially regulates secretion in many cells.9,14,34 This isoform positively regulates PAR-mediated dense granule secretion, whereas it negatively regulates GPVI-mediated dense granule secretion. In the current study, we investigated the mechanism of such differential regulation of platelet-dense granule secretion by PKC-δ.
Our results also show that the rapid phosphorylation of SHIP-1 on GPVI stimulation of platelets results in its association with PKC-δ. Whereas GPVI-mediated SHIP-1 phosphorylation is rapid, started as early as 15 seconds and peaked at 60 seconds, PAR-mediated SHIP-1 phosphorylation is delayed, started at 60 seconds and peaked at 120 seconds. This delay is consistent with the previous findings that PAR-mediated tyrosine phosphorylation of SHIP-1 is aggregation-dependent and occurs on integrin engagement.18 Our previous studies have shown that PKC-δ is also rapidly phosphorylated (< 30 seconds) on Thr 505, a marker of its activation, downstream of both GPVI and PARs.9 Thus, the activation of PKC-δ and SHIP-1 downstream of PAR stimulation do not occur in the same time frame. However, platelet-dense granule secretion is a rapid process that usually occurs within 30 seconds, coinciding with PKC-δ activation and SHIP-1 phosphorylation downstream of GPVI receptors. Hence, we investigated whether the rapid phosphorylation of SHIP-1 downstream of GPVI receptors may regulate platelet-dense granule secretion.
GPVI signaling and immune receptor signaling mimic each other and involve immunoreceptor tyrosine-based activation motifs and phospholipase C-γ (PLC-γ).35 In mast cells, which signal through immune receptors, PKC-δ and SHIP-1 associate through an adapter protein, Shc. However, we did not see any association of either PKC-δ or SHIP-1 with Shc in platelets on GPVI stimulation. GPVI receptors signal through PLC-γ2, whereas PAR signaling involves activation of PLC-β2. Therefore, the differences in the signaling downstream of PARs and GPVI receptors may result in the differential association of PKC-δ and SHIP-1 leading to differential regulation of platelet-dense granule secretion.
SHIP-1 is a major regulator of PIP3 levels in platelets,17 and the membrane localization of SHIP-1 is very crucial for its catalytic activity.27 The association of tyrosine-phosphorylated SHIP-1 with PKC-δ may enhance its membrane localization whereby the catalytic activity of SHIP-1 is increased causing a reduction in PIP3 levels. PIP3 levels are also positively regulated by the activation of PI3 kinases.36 The inhibition of PI3 kinases, with wortmannin and LY294002, and reduction of PIP3 levels have been known to reduce platelet functional responses.37 Thus, the association of PKC-δ with SHIP-1 might decrease PIP3 levels and thereby negatively regulate platelet-dense granule secretion.
SHIP-1 has 3 important structural domains: N-terminal SH2 domain, central catalytic domain, and C-terminal proline-rich domain.38 The SH2 domain binds tyrosine-phosphorylated proteins, and PKC-δ has been shown to be tyrosine phosphorylated in platelets.39 Hence, the interaction between PKC-δ and SHIP-1 might involve phosphotyrosine residues on PKC-δ and SH2 domain of SHIP-1. Although PKC-δ has 9 tyrosine residues, Hall et al30 have shown that Y52, Y155, Y525, and Y332 are not phosphorylated on GPVI stimulation. As our data show that Y311 phosphorylation on PKC-δ is regulated by Lyn, this or other phosphotyrosine residues on PKC-δ might mediate the association with SHIP-1. It is also very probable that Lyn may not be the sole regulator of Y311 phosphorylation on PKC-δ as the phosphorylation is not completely abolished in Lyn null murine platelets.
SHIP-1 has a proline-rich domain that may interact with Src family kinases via their SH3 domains. This interaction might lead to the Lyn-mediated SHIP-1 phosphorylation on Y1020. Our studies show that Lyn specifically phosphorylates SHIP-1 on Y1020 in platelets on GPVI stimulation. We cannot rule out an interaction between phosphorylated Y1020 on SHIP-1 with PKC-δ that is essential for their association. Because there is no association of PKC-δ and SHIP-1 in Lyn null murine platelets, we can only conclude that tyrosine phosphorylations by Lyn are important for this association. In addition, because we failed to detect an association between Lyn and SHIP-1, we cannot rule out the role for another tyrosine kinase downstream of Lyn that phosphorylates SHIP-1.
The functional implications of the association between a kinase and a phosphatase are not clear. PKC-δ has been shown to associate and regulate the catalytic activity of a serine threonine phosphatase PP2Ac and modulate the dopamine production in the neuronal cells.40 From our study, PKC-δ regulates GPVI-mediated SHIP-1 phosphorylation and not PAR-mediated SHIP-1 phosphorylation (Figure 3). As the phosphorylation of SHIP-1 in PKC-δ null murine platelets is not completely ablated, we predict a role for other kinases in phosphorylating SHIP-1 on GPVI stimulation. The association of PKC-δ with SHIP-1 might cause serine/threonine phosphorylations on SHIP-1 that enhance the catalytic activity of SHIP-1. Thus, PKC-δ associated SHIP-1 might reduce the PIP3 levels more readily and negatively regulate platelet functional responses. However, whether the serine/threonine phosphorylations on SHIP regulate its catalytic function is not known.
SHIP-1 is tyrosine phosphorylated on stimulation of PARs or GPVI receptors. This tyrosine-phosphorylated SHIP-1 preferentially associated with PKC-δ on GPVI stimulation. As reported previously, both PAR- and GPVI-mediated SHIP-1 phosphorylations are regulated by SFKs because SFK inhibitors PP1 and PP2 inhibited SHIP-1 phosphorylation. However, studies in immune cells have shown that Lyn regulates the tyrosine phosphorylation of SHIP.22,41 Studies by these groups also showed a reduced SHIP phosphatase activity in the Lyn−/− bone marrow-derived mast cells, indicating that Lyn regulates the phosphatase catalytic activity of SHIP by retaining SHIP at the membrane. Thus, the tyrosine phosphorylation of SHIP-1 by Lyn is required for its catalytic function.22 Consistent with these findings, GPVI-mediated SHIP-1 phosphorylation was inhibited in Lyn−/− murine platelets, whereas it was unaffected in Fyn−/− and Src−/− murine platelets. These findings suggest a critical role for Lyn in regulating the function of SHIP-1. Furthermore, Lyn preferentially associated with PKC-δ on GPVI receptor stimulation, but not PAR stimulation, consistent with the findings that have been reported in RBL-2H3 cell lines.23
Although it is possible that PKC-δ might associate with some other signaling molecules and phosphorylate them to regulate dense granule release, this is doubtful as PKC-δ is also activated downstream of PARs and positively regulates this functional response. Therefore, it is the formation of a signaling complex downstream of GPVI stimulation that involves PKC-δ, which differentially regulates platelet-dense granule secretion.
Downstream of GPVI receptors, there is a signaling complex of Lyn, PKC-δ, and SHIP-1. Lyn not only regulates SHIP-1 phosphorylation but also associates with PKC-δ and regulates the Y311 phosphorylation of PKC-δ. These phosphorylations result in potentiation of dense granule secretions in Lyn−/− or SHIP-1−/− murine platelets on GPVI stimulation and inhibition on PAR stimulation. Previous studies have also shown that PAR-mediated aggregations and dense granule secretions were inhibited in Lyn−/− murine platelets.42 Severin et al43 reported that GPVI-mediated platelet-dense granule secretion was unaffected in SHIP-1−/− mice. The reason for these differences could be attributed to stimulation of SHIP-1−/− murine platelets with low doses of collagen and failure to treat the platelets with indomethacin to eliminate feedback mediators. For all the dense granule measurements in their studies, the platelets were stimulated with only one GPVI agonist, collagen. We consistently showed a potentiation of dense granule secretion in SHIP-1−/− murine platelets using CVX, CRP, and collagen as GPVI agonists with indomethacin treatment to strengthen our findings.
In conclusion, we have shown that (1) Lyn-mediated tyrosine phosphorylation of SHIP-1 and PKC-δ regulates their association; and (2) intermolecular interactions of Lyn, SHIP-1, and PKC-δ are required for the negative regulation of GPVI-mediated dense granule secretion in platelets.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Keiko Nakayama, Tohoku Graduate School of Medicine, Sendai Miyagi, Japan, for providing PKC-δ null mice and Ms Monica Dupon for her technical assistance.
This work was supported by the National Institutes of Health (research grants HL60683, HL80444, HL81322, and HL 93231) and predoctoral fellowships from the American Heart Association, Great Rivers affiliate (R.C., S.M.).
National Institutes of Health
Authorship
Contribution: R.C. designed and performed experiments, analyzed data, and wrote the paper; S.K. performed experiments and analyzed data; S.M. analyzed data; A.S. provided essential tools; J.L.D. interpreted results and analyzed data; and S.P.K. provided overall direction, designed experiments, and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satya P. Kunapuli, Department of Physiology, Temple University, Rm 217 MRB, 3420 N Broad St, Philadelphia, PA 19140; e-mail: spk@temple.edu.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal