Off-patent drugs with previously unrecognized anticancer activity could be rapidly repurposed for this new indication. To identify such compounds, we conducted 2 independent cell-based chemical screens and identified the antimicrobial ciclopirox olamine (CPX) in both screens. CPX decreased cell growth and viability of malignant leukemia, myeloma, and solid tumor cell lines as well as primary AML patient samples at low-micromolar concentrations that appear pharmacologically achievable. Furthermore, oral CPX decreased tumor weight and volume in 3 mouse models of leukemia by up to 65% compared with control without evidence of weight loss or gross organ toxicity. In addition, oral CPX prevented the engraftment of primary AML cells in nonobese diabetic/severe combined immunodeficiency mouse models, thereby establishing its ability to target leukemia stem cells. Mechanistically, CPX bound intracellular iron, and this intracellular iron chelation was functionally important for its cytotoxicity. By electron paramagnetic resonance, CPX inhibited the iron-dependent enzyme ribonucleotide reductase at concentrations associated with cell death. Thus, in summary, CPX has previously unrecognized anticancer activity at concentrations that are pharmacologically achievable. Therefore, CPX could be rapidly repurposed for the treatment of malignancies, including leukemia and myeloma.
Introduction
Off-patent drugs with previously unrecognized anticancer activity could be rapidly repurposed for this new indication given their prior safety and toxicity testing in humans and animals. Remarkably, prior studies have identified such compounds with unanticipated anticancer activities. For example, the antifungal ketoconazole was discovered to inhibit the production of androgens from the testes and adrenals in rat models.1 Given this finding, ketoconazole was rapidly advanced into clinical trials for patients with prostate cancer where it displayed clinical efficacy in early studies.2,–4 Likewise, through a somewhat serendipitous route, thalidomide was discovered to have antimyeloma activity.5 Subsequent studies confirmed the activity of thalidomide in myeloma6,–8 and myelodysplasia.9,10 This drug, as well as its second-generation derivative lenalidamide, changed the standard of care for patients with these diseases.11,12
Here we used a systematic approach to identify compounds with unanticipated anticancer activity by testing libraries of off-patent drugs in 2 parallel chemical screens. Both screens independently identified the antimicrobial ciclopirox olamine (CPX). CPX is currently approved for the topical treatment of cutaneous fungal infections13 but has not been previously evaluated as a systemic agent for the treatment of malignancy. However, as part of the development of this agent as a topical therapy, CPX was administered systemically to animals and humans to evaluate its safety and toxicity. These studies demonstrated that CPX was well tolerated with an LD50 value in mice, rats, and rabbits that ranged from 1700 to 3290 mg/kg after oral administration.14 CPX serum concentrations of 10 μM were achievable after repeated administration of CPX to rats and dogs and were not toxic.14 In healthy human volunteers, 10 mg/kg of oral CPX produced no toxicity. In our current study, we demonstrated that CPX displayed preclinical activity against hematologic malignancies at concentrations that appear pharmacologically achievable.15 Mechanistically, CPX-induced cell death was dependent on chelation of intracellular iron and the inhibition of the iron-dependent enzyme ribonucleotide reductase.
Methods
Cell culture
Cell lines.
Leukemia (HL-60, K562, Jurkat, U937, MDAY-D2), solid tumor cell lines (PPC-1, HeLa, HT-29), and GMO5757 human lung fibroblasts16 were cultured in RPMI 1640 medium. HepG2 hepatoma cells and MRC5 human lung fibroblasts were grown in Dulbecco modified Eagle medium. OCI-M2, OCI-AML2, and NB4 leukemia cell lines, and all tested myeloma cell lines were maintained in Iscove modified Dulbecco medium (IMDM). LF1 human lung fibroblasts were maintained in HAM medium. Media were supplemented with 10% fetal bovine serum (FBS), 100 μg/mL penicillin, and 100 units/mL streptomycin (all from HyClone Laboratories). TEX cells were maintained in IMDM, 15% FBS, 2 mM l-glutamine, 1% penicillin-streptomycin, 20 ng/mL stem cell factor (SCF), and 2 ng/mL interleukin-3 (IL-3). M9-ENL1 cells were maintained in minimal essential medium, 20% FBS, 5% human adult plasma, 2 mM l-glutamine, 1% penicillin-streptomycin, 100 ng/mL SCF, 10 ng/mL IL-3, 5 ng/mL IL-7, and 5 ng/mL Fms-like tyrosine kinase-3 ligand. Cells were incubated at 37°C in a humidified air atmosphere supplemented with 5% CO2.
Primary cells.
Primary human acute myeloid leukemia (AML) samples were isolated from peripheral blood samples from consenting patients with AML, who had at least 80% malignant cells among the mononuclear cells in their peripheral blood and cultured at 37°C in IMDM, 10% BIT-9500, 5 mg/mL low- density lipoproteins, 55 μM 2-mercaptoethanol, 2 mM l-glutamine, 1% penicillin-streptomycin, 100 ng/mL SCF, 100 ng/mL Fms-like tyrosine kinase-3 ligand, 20 ng/mL granulocyte colony-stimulating factor, 20 ng/mL IL-6, 50 ng/mL thrombopoietin, 20 ng/mL IL-3, and 20 ng/mL granulocyte-macrophage colony-stimulating factor. Cytogenetic risk groups were classified as previously described.17 Primary normal hematopoietic cells were obtained from healthy volunteers donating their peripheral blood stem cells or bone marrow for allotransplantation. Mononuclear cells were isolated from the samples by Ficoll density gradient centrifugation. The collection and use of human tissue for this study were approved by the local ethics review board (University Health Network, Toronto, ON), and informed consent was obtained in accordance with the Declaration of Helsinki.
Reagents
CPX analogs (1-hydroxy-4,6-diphenyl-20(1H)-pyridinone) (DPOH), (1-(1-methylethoxy)-4,6-diphenyl-2(1H)-pyridinone) (MDP), and (1-amino-4,6-diphenyl-2(1H)-pyridinone) (ADP) were purchased from Katritzky Group, Florida Center for Heterocyclic Compounds, Department of Chemistry, University of Florida. Alamar Blue was purchased from AbD-Serotec. Cytokines were obtained from Amgen or from PeproTech. Methylcellulose, StemSep Human Progenitor Enrichment Cocktail, and BIT-9500 were from Stem Cell Technologies. Low-density lipoproteins were from EMD Chemicals.
Cloning and transfections
The full-length survivin promoter (−1059 upstream of the initiating ATG; National Institute of Environmental Health Sciences-SNPs, Environmental Genome Project, National Institute of Environmental Health Sciences ES15478, Department of Genome Sciences) was isolated from HeLa genomic DNA using the forward primer 3′-CCGCTCGAGTGAAAAAGACAGTGGAGGCACCAGGC-5′ and the reverse primer 3′-CCCAAGCTTTGCCGCCGCCGCCACCTCT-5′. The promoter was subcloned into the GL4.20 firefly luciferase reporter vector (Promega). Clones were sequence-verified for orientation and integrity using a CEQ 8000 Genetic Analysis System (Beckman).
HeLa cells were transfected with survivin promoter construct alone or vector alone using Lipofectamine (Invitrogen) according to the manufacturer's instructions and stable clones selected with puromycin (4 μg/mL; Sigma-Aldrich).
Identification of inhibitors of survivin transactivation
HeLa cells stably overexpressing the survivin promoter driving luciferase (15 000 cells/well) were plated in 96-well plates. After adhering to the plates, cells were treated with aliquots of chemicals from the LOPAC (Sigma-Aldrich), Prestwick (Prestwick Chemical), Spectrum (Microsource), and Biomol libraries of off-patent drugs, chemicals, and natural products. The final concentration of compounds was 5 μM (0.05% dimethyl sulfoxide). Cells were incubated with the molecules for 24 hours. After incubation, survivin promoter activity was assessed by the luciferase assay described in “Luciferase assay.” Results were normalized and corrected for systematic errors using the B score.18 Compounds with a B score value lower than 3 times the SD were empirically considered hits in the assay.
Luciferase assay
Luciferase activity was measured according to the manufacturer's instructions and as previously described (Promega).19
Viability assays
Methylcellulose colony formation
Primary normal mononuclear hematopoietic cells obtained from healthy volunteers donating their peripheral blood stem cells or bone marrow for allotransplantation (1 × 105/mL) were plated in duplicate 35-mm dishes in MethoCult GF H4434 medium (Stem Cell Technologies) with increasing concentrations of CPX. Cells were incubated at 37°C, 5% CO2 with 95% humidity for 14 days. After incubation, the number of granulocyte macrophage-colony forming units and erythroid-burst forming units was counted on an inverted microscope. To confirm the lineage of colonies where necessary, cells were picked from individual colonies, stained with May-Grünwald-Giemsa, and their morphology examined as previously described.22 A plating efficiency of 0.1% was routinely achieved.
Immunoblotting
Total cell lysates were prepared from cells as described previously.23 Briefly, cells were washed with phosphate-buffered saline (PBS), pH 7.4, and suspended in lysis buffer (10 mM Tris, pH 7.4, 150 mM, NaCl, 0.1% Triton X-100, 0.5% sodium deoxycholate, and 5 mM ethylenediaminetetraacetic acid) containing protease inhibitors (Complete tablets; Roche Diagnostics). Protein concentrations were measured by the Bradford assay.24 Equal amounts of protein were subjected to sodium dodecyl sulfate–polyacrylamide gels followed by transfer to polyvinylidene difluoride membranes.
Membranes were probed with polyclonal rabbit anti–human survivin (1 μg/mL; Novus), or with mouse anti–human GAPDH (Trevigen). Secondary antibodies (GE Healthcare) were horseradish peroxidase–conjugated goat anti–mouse IgG (1:10 000 [vol/vol]) and anti–rabbit (1:5000 [vol/vol]). Detection was performed by the enhanced chemical luminescence method (Pierce Chemical).
Assessment of CPX anticancer activity in mouse models of leukemia
MDAY-D2 (MDAY) murine leukemia cells (5 × 105) were injected intraperitoneally into nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice (Ontario Cancer Institute, Toronto, ON), and OCI-AML2 and K562 cells (2 × 106) were injected subcutaneously into both flanks of sublethally irradiated (3.5 Gy) NOD/SCID mice. Mice were then treated daily by oral gavages with CPX (25 mg/kg) in PBS or vehicle control. Tumor volume (tumor length × width2 × 0.5236)25 was measured weekly using calipers. Eight (MDAY-D2), 16 (OCI-AML2), or 30 (K562) days after injection of cells, mice were killed, and the volume and weight of the tumors were measured.
To assess CPX in mouse models of primary AML, primary human AML cells were isolated from fresh peripheral blood samples from patients with AML. For each sample, a frozen aliquot was thawed and slowly diluted in PBS/5% FBS/0.2 mg/mL DNase, counted, and resuspended in serum-free media. The cells (1.7-2.0 × 106) were injected into the right femur of nude/NOD/SCID mice, 20 weeks old, which were irradiated approximately 24 hours previously with 208 cGy from a cesium-137 source. Four weeks after injection, mice were treated daily by oral gavage with CPX (20 mg/kg) in PBS or vehicle control. After 4 weeks of treatment, the mice were killed and the femurs removed. Bones were flushed, and leukemia cell engraftment was quantified using human-specific allophycocyanin-Cy7-CD45 (Beckman Coulter), phycoerythrin-Cy7-CD33, and phycoerythrin-CD19 antibodies (BD Biosciences) using an LSR-II analyzer (BD) and analyzed with FlowJo, Version 8.8 (TreeStar).
All animal studies were carried out according to the regulations of the Canadian Council on Animal Care and with the approval of the Animal Care Committee at the Ontario Cancer Institute.
Intracellular iron measurements
NB-4, MDAY-D2, OCI-M2, OVCAR-3, and PPC-1 cells (2 × 106/mL) were harvested and resuspended in RPMI-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (bicarbonate-free, serum-free, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.3) supplemented with 1 mg/mL bovine serum albumin (Sigma-Aldrich) and 250 nM calcein-AM (BD Biosciences) and incubated at 37°C for 5 minutes. After calcein loading, cells were washed and resuspended (5 × 105/mL) in prewarmed Hanks balanced salt buffer (150 mM NaCl, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.3). Changes in intracellular calcein fluorescence were measured by flow cytometry. Geometric mean fluorescence was calculated by FlowJo analysis software (TreeStar).26,27
Electron paramagnetic spectroscopy studies
OCI-M2 and MDAY-D2 cells (0.5 × 106/mL) were treated with control or CPX (5 and 10 μM) for 24 hours. Cell pellets (3 × 108) were removed and spun at 1500g for 5 minutes. The pellet was pipetted into a quartz tube (outside diameter, 4 mm) and rapidly frozen at 196°C. Electron paramagnetic resonance (EPR) spectra at 77K were obtained using a Varian E-9 spectrometer (Varian E-9 spectrometer located at the National Biomedical EPR Center). With a microwave frequency of 9.122 GHz, microwave power 100 mW, time constant 0.25 seconds, modulation amplitude 10 G, and scan time 2 minutes, 9 scans were averaged.
Statistical analysis
Results are mean plus or minus SD. Treatment effects were compared using Student t test. Calcusyn software (Biosoft) was used to analyze drug combination data.
Results
Screens for off-patent drugs with novel anticancer activity identify CPX
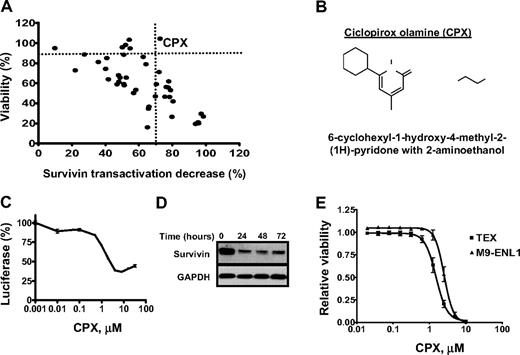
Off-patent drugs with previously unrecognized anticancer activity could be rapidly repurposed for this new indication. To identify such compounds, 2 parallel screens were conducted. In the first screen, we sought to identify compounds that inhibited transactivation of the survivin promoter, as we hypothesized that compounds capable of directly or indirectly inhibiting survivin expression may exert anticancer activity. Here, HeLa cells stably overexpressing the human survivin promoter (−1059 upstream of the initiating ATG) driving firefly luciferase were treated with aliquots of 4800 off-patent drugs and natural products. Twenty-four hours after incubation, survivin promoter activity was measured by the luciferase assay as a marker for compounds with potential anticancer activity. From this screen, we identified 45 compounds (0.94%) that decreased survivin transactivation at least 3 SDs away from the mean B score of the entire population of tested compounds. These 45 compounds were retested in secondary assays that included an MTS assay to exclude nonspecific cytotoxic compounds that would suppress luciferase activity by killing the HeLa cells. In these secondary assays, only 1 of 45 compounds, (6-cyclohexyl-1-hydroxy-4-methyl-2-(1H)-pyridone ethanolammonium salt; CPX), repressed survivin transactivation greater than 60% while maintaining greater than 90% cell viability at 24 hours after treatment (Figure 1A-B). The effects of CPX on survivin transactivation were confirmed in dose-response studies (Figure 1C). Of note, luciferase expression increased slightly at the highest tested concentration of CPX (40 μM) and was probably related to the decreased solubility of CPX at this high concentration. CPX also decreased survivin mRNA and protein expression in wild-type HeLa cells as assessed by quantitative RT-PCR and immunoblotting, respectively (Figure 1D; and data not shown). Of note, and as discussed in “CPX is cytotoxic to maligment cell lines,” 24 hours of treatment with CPX did not alter the viability of HeLa cells, but incubation times of 72 hours were cytotoxic.
Screens for off-patent drugs with unrecognized anticancer activity identify the antifungal CPX. (A) Compounds (n = 45) identified as hits in a primary screen for inhibitors of the survivin promoter were tested for their effects on survivin transactivation and cytotoxicity in HeLa cells stably overexpressing the survivin promoter driving luciferase. HeLa cells were treated with the hit compounds (5 μM) for 24 hours. After incubation, luciferase expression was measured as described in “Luciferase assay,” and cell viability was measured by the MTS assay. Data represent the percentage of viable cells (y-axis) and the percentage luciferase decrease (x-axis) compared with buffer control. (B) Chemical structure of CPX. (C) HeLa cells stably overexpressing the survivin promoter driving luciferase were treated with increasing concentrations of CPX. Twenty-four hours after incubation, luciferase activity was measured. Data represent the mean percentage of luciferase expression ± SD after CPX treatment compared with buffer control from one of 3 representative experiments. (D) HeLa cells were treated with CPX (5 μM). At increasing times after incubation, cells were harvested and total proteins were isolated. Expression of survivin and GAPDH was measured by immunoblotting. (E) TEX and M9-ENL1 cells were treated with increasing concentrations of CPX. Seventy-two hours after incubation, cell viability was measured by the Alamar Blue assay. Data represent the mean percentage of viable cells ± SD from 1 of 3 representative experiments.
Screens for off-patent drugs with unrecognized anticancer activity identify the antifungal CPX. (A) Compounds (n = 45) identified as hits in a primary screen for inhibitors of the survivin promoter were tested for their effects on survivin transactivation and cytotoxicity in HeLa cells stably overexpressing the survivin promoter driving luciferase. HeLa cells were treated with the hit compounds (5 μM) for 24 hours. After incubation, luciferase expression was measured as described in “Luciferase assay,” and cell viability was measured by the MTS assay. Data represent the percentage of viable cells (y-axis) and the percentage luciferase decrease (x-axis) compared with buffer control. (B) Chemical structure of CPX. (C) HeLa cells stably overexpressing the survivin promoter driving luciferase were treated with increasing concentrations of CPX. Twenty-four hours after incubation, luciferase activity was measured. Data represent the mean percentage of luciferase expression ± SD after CPX treatment compared with buffer control from one of 3 representative experiments. (D) HeLa cells were treated with CPX (5 μM). At increasing times after incubation, cells were harvested and total proteins were isolated. Expression of survivin and GAPDH was measured by immunoblotting. (E) TEX and M9-ENL1 cells were treated with increasing concentrations of CPX. Seventy-two hours after incubation, cell viability was measured by the Alamar Blue assay. Data represent the mean percentage of viable cells ± SD from 1 of 3 representative experiments.
CPX was also identified as a potential anticancer agent in a separate screen of these off-patent drugs to identify compounds that are cytotoxic to leukemia stem cells. Specifically, we sought to identify compounds that reduced the viability of TEX and M9-ENL1 cells. TEX and M9-ENL1 cells were derived from lineage-depleted human cord blood cells (Lin− CB) transduced with TLS-ERG28 or MLL-ENL29 oncogenes, respectively, and display properties similar to leukemia stem cells, such as a hierarchal differentiation and marrow repopulation.28,29 In this screen, TEX and M9-ENL1 cells were treated with aliquots of the compound libraries at a final concentration of 1 or 5 μM. Seventy-two hours after incubation, cell viability was measured by the Alamar Blue assay as previously described.21 This screen identified 76 compounds that reduced the viability of TEX and M9-ENL1 cells by at least 75%. These 76 compounds included known cytotoxics, as well as the novel compound, CPX. CPX was therefore evaluated in secondary assays where it reduced the viability of TEX and M9-ENL1 cells with LD50 values of 1.5 and 2.5 μM, respectively (Figure 1E).
CPX is cytotoxic to malignant cell lines
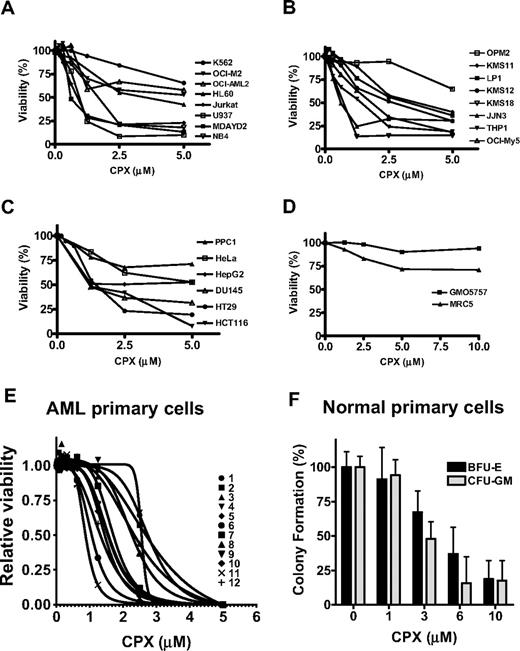
Given the identification of CPX in 2 separate screens for potential anticancer agents, we tested the effects of CPX on cell viability in a panel of leukemia, myeloma, and solid tumor cell lines. Cells were treated with increasing concentrations of CPX. Seventy-two hours after incubation, cell viability was assessed by the MTS assay. At pharmacologically achievable concentrations, CPX reduced cell viability with an LD50 value of 2.5 μM or lower in 5 of 8 leukemia, 4 of 8 myeloma, and 1 of 4 solid tumor cell lines (Figure 2A-C). Cell death was confirmed by propidium iodide staining. In contrast to its effects in these malignant cell lines, CPX did not reduce the viability of nonmalignant lung fibroblast cell lines at a concentration up to 10 μM (Figure 2D). CPX-induced cell death was time dependent. We observed no loss of cell viability at 24 hours, but cell death was detectable at 72 hours (data not shown).
CPX induces cell death in malignant cell lines. Leukemia (A), myeloma (B), solid tumor (C), and nonmalignant lung fibroblast (D) cell lines were treated with increasing concentrations of CPX. Seventy-two hours after incubation, cell viability was measured by MTS assay. Data represent the mean percentage of viable cells ± SD from 1 of at least 3 representative experiments. (E) Primary AML cell samples (n = 12) were treated with increasing concentrations of CPX. Seventy-two hours after incubation, cell viability was measured by the Alamar Blue assay. Data represent the mean percentage of viable cells ± SD where each sample was tested in triplicate. (F) Normal mononuclear cells derived from bone marrow (n = 3) or peripheral blood stem cells (n = 2) were plated in a methylcellulose colony-forming assay with increasing concentrations of CPX. Myeloid (granulocyte macrophage-colony forming units) and erythroid (erythroid-burst forming units) colonies were counted 14 days after plating and normalized to cultures treated with buffer alone. Data represent the mean ± SD of 5 independent experiments performed in duplicate.
CPX induces cell death in malignant cell lines. Leukemia (A), myeloma (B), solid tumor (C), and nonmalignant lung fibroblast (D) cell lines were treated with increasing concentrations of CPX. Seventy-two hours after incubation, cell viability was measured by MTS assay. Data represent the mean percentage of viable cells ± SD from 1 of at least 3 representative experiments. (E) Primary AML cell samples (n = 12) were treated with increasing concentrations of CPX. Seventy-two hours after incubation, cell viability was measured by the Alamar Blue assay. Data represent the mean percentage of viable cells ± SD where each sample was tested in triplicate. (F) Normal mononuclear cells derived from bone marrow (n = 3) or peripheral blood stem cells (n = 2) were plated in a methylcellulose colony-forming assay with increasing concentrations of CPX. Myeloid (granulocyte macrophage-colony forming units) and erythroid (erythroid-burst forming units) colonies were counted 14 days after plating and normalized to cultures treated with buffer alone. Data represent the mean ± SD of 5 independent experiments performed in duplicate.
Consistent with our identification of CPX in a screen for inhibitors of survivin transactivation, CPX decreased survivin expression in 8 of 9 tested cell lines (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The ability of CPX to decrease survivin expression appeared to match its ability to induce cell death as 2.5 μM of CPX did not reduce survivin expression in CPX-resistant OCI-M2 cells. Decreasing survivin expression was probably not important for CPX-induced cell death because silencing of survivin by siRNA did not recapitulate the effects of CPX on cell viability as measured by the MTS assay (data not shown). Rather, reductions in survivin expression by CPX are a biomarker of its activity.
CPX induces cell death in primary malignant cells preferentially over normal hematopoietic cells
Because CPX induced cell death at pharmacologically achievable concentrations in leukemia cell lines, we evaluated its effects on primary AML samples and normal hematopoietic cells. Primary leukemic blasts were isolated from the peripheral blood of patients with AML (intermediate [n = 10] and poor [n = 2] risk cytogenetics) were treated with increasing concentrations of CPX for 72 hours. Cell growth was measured by Alamar Blue assay. CPX reduced the growth of 8 of 12 primary AML samples with an LD50 less than or equal to 2.5 μM (Figure 2E).
We also evaluated the effects of CPX on the clonogenic growth of normal hematopoietic cells. At concentrations of 6 μM or more, CPX inhibited the clonogenic growth of normal hematopoietic cells (Figure 2F) by more than 50%. Thus, there is a narrow difference in cytotoxicity between normal and malignant hematopoietic cells in vitro.
CPX delays tumor growth in mouse models of leukemia
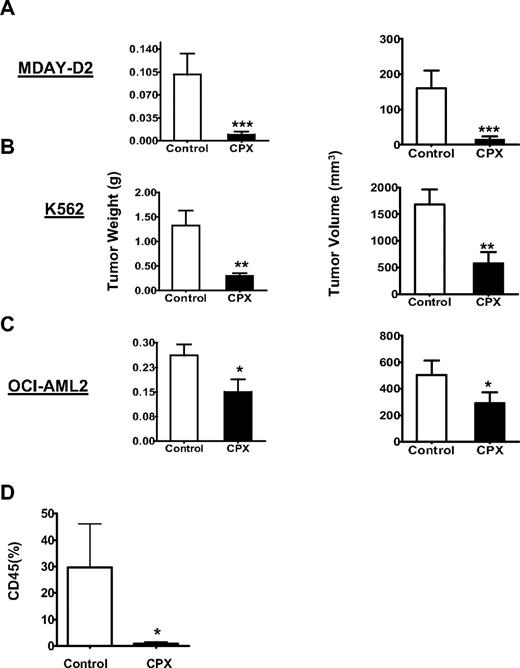
Given the effects of CPX as a potential antileukemic agent, we evaluated CPX in mouse models of leukemia. Leukemia cells were injected intraperitoneally (MDAY-D2) or subcutaneously into the flank (OCI-AML2 and K562) of NOD/SCID mice. Mice were then treated with CPX (25 mg/kg) daily or buffer control by oral gavage. Eight (MDAY-D2), 30 (K562), and 16 (OCI-AML2) days after treatment, mice were killed and the tumors were excised, measured, and weighed. Compared with buffer control, oral CPX decreased tumor weight and volume in all 3 models (Figure 3A-C). No gross organ toxicity or loss of body weight was noted after CPX treatment.
CPX delays tumor growth in mouse models of leukemia. Sublethally irradiated NOD/SCID mice were injected intraperitoneally with (A) MDAY-D2 murine leukemia cells (n = 30; 15 per group), or subcutaneously with (B) K562 cells (n = 30; 15 per group) or (C) OCI-AML2 human leukemia cells (n = 24; 12 per group). After implantation, mice were treated with CPX (25 mg/kg) or vehicle control by oral gavage daily. After 8 (MDAY-D2), 30 (K562), and 16 (OCI-AML2) days, mice were killed and tumors were excised, measured, and weighed. The mean weight and volume ± SD are shown. Means were compared by the Student t test. ***P < .001, **P < .01 (Student t test). One of at least 2 representative experiments is shown. (D) Primary cells from one patient with AML and normal cytogenetics were injected intrafemorally into the right femur of female sublethally irradiated nude/NOD/SCID mice. Four weeks after injection, mice were treated by oral gavage once daily with vehicle (n = 3) or CPX (20 mg/kg; n = 3) for 4 weeks. After treatment, human leukemia cell engraftment in the injected right femur was measured by flow cytometry for human CD45. Data represent the mean ± SD of engrafted human cells from all mice. *P < .05 (Student t test).
CPX delays tumor growth in mouse models of leukemia. Sublethally irradiated NOD/SCID mice were injected intraperitoneally with (A) MDAY-D2 murine leukemia cells (n = 30; 15 per group), or subcutaneously with (B) K562 cells (n = 30; 15 per group) or (C) OCI-AML2 human leukemia cells (n = 24; 12 per group). After implantation, mice were treated with CPX (25 mg/kg) or vehicle control by oral gavage daily. After 8 (MDAY-D2), 30 (K562), and 16 (OCI-AML2) days, mice were killed and tumors were excised, measured, and weighed. The mean weight and volume ± SD are shown. Means were compared by the Student t test. ***P < .001, **P < .01 (Student t test). One of at least 2 representative experiments is shown. (D) Primary cells from one patient with AML and normal cytogenetics were injected intrafemorally into the right femur of female sublethally irradiated nude/NOD/SCID mice. Four weeks after injection, mice were treated by oral gavage once daily with vehicle (n = 3) or CPX (20 mg/kg; n = 3) for 4 weeks. After treatment, human leukemia cell engraftment in the injected right femur was measured by flow cytometry for human CD45. Data represent the mean ± SD of engrafted human cells from all mice. *P < .05 (Student t test).
TEX cells, which are sensitive to CPX, have features similar to AML stem cells. Therefore, we assessed the effects of CPX on the ability of primary AML stem cells to engraft in NOD/SCID mice. We have shown previously that only AML stem cells are able to engraft long-term in NOD/SCID mice.30 Primary AML cells were injected intrafemorally into the right femur31 of sublethally irradiated nude/NOD/SCID female mice. Four weeks after injection, mice were treated by oral gavage with CPX (20 mg/kg) or buffer control 5 of 7 days × 4 weeks. At the end of the experiment, the mice were killed and cells flushed from the femurs. Engraftment of human cells into the marrow was assessed by enumerating the percentage of human CD45 cells using allophycocyanin-Cy7-anti–CD45 and flow cytometry. Engrafted cells were confirmed to be leukemic in origin by the presence of human CD33 and lack of CD19. Compared with mice treated with buffer alone, treatment with CPX significantly decreased the engraftment of primary AML cells without gross organ toxicity or loss of body weight (Figure 3D).
Chelation of intracellular iron is functionally important for CPX-induced cell death
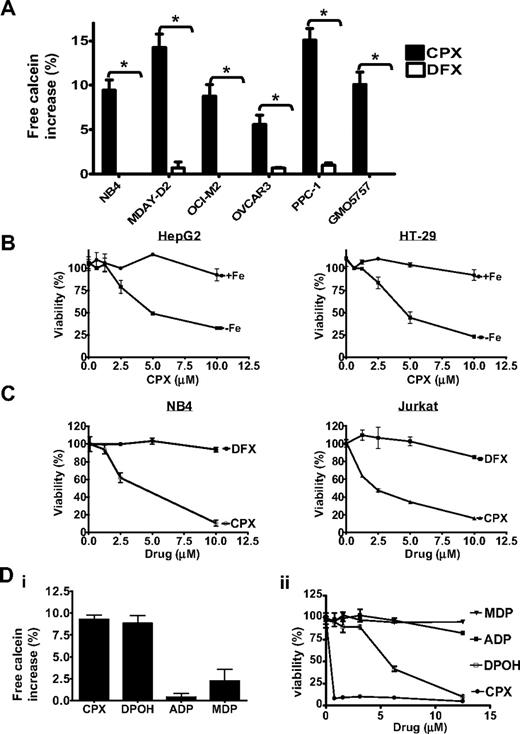
The mechanism of action of CPX as an antifungal agent is not well understood but appears related to its ability to bind intracellular iron. Therefore, we evaluated whether CPX bound intracellular iron in malignant cells as measured by the ability of CPX to displace intracellular calcein bound to iron. Calcein-AM is a nonfluorescent lipophilic ester that penetrates cell membranes where it is rapidly cleaved by cytosolic esterases into an impermeable fluorescent alcohol (calcein) that chelates labile iron. On binding intracellular iron, the fluorescent signal of calcein is quenched.26 To examine the effects of CPX on calcein fluorescence, cells were loaded with calcein-AM followed by treatment with CPX (10 μM) or the more potent extracellular iron chelator deferoxamine32 (100 μM) for 2 minutes. After this brief incubation, calcein fluorescence was measured by flow cytometry (Figure 4A). CPX increased intracellular calcein fluorescence, indicating that it displaced calcein from intracellular iron. In contrast, deferoxamine had no effect on calcein fluorescence as it does not bind intracellular iron. Thus, these results demonstrate that CPX binds intracellular iron in malignant cells and acts through a mechanism distinct from deferoxamine. We also demonstrated that CPX bound intracellular iron in the nonmalignant cell line GMO5757, which was resistant to CPX-induced cell death, indicating that sensitivity and resistance to CPX are not related to its intracellular uptake or its ability to bind intracellular iron.
CPX-induced cell death is iron dependent. (A) Leukemia (NB4, MDAY-D2, and OCI-M2) and solid tumor (OVCAR-3 and PPC-1) cells were loaded with the intracellular iron-chelating fluorescent dye calcein-AM. Cells were then treated with CPX (10 μM) or DFX (100 μM) for 2 minutes. Intracellular iron bound by calcein was measured by flow cytometry. Percentage increase ± SD in the geometric mean intracellular calcein fluorescence is represented *P < .05 (Student t test). One of at least 2 representative experiments performed in triplicate is shown. (B) HT-29(i) and HepG2 (ii) cells were treated with increasing concentrations of CPX with or without iron supplementation with iron in a complex with a transferrin-replacement compound (8 μM). Seventy-two hours after incubation, cell viability was measured by MTS. Data represent the mean ± SD percentage of viable cells from 1 of 3 representative experiments. (C) Jurkat and NB4 leukemia cells were treated with increasing concentrations of CPX or DFX. Seventy-two hours after incubation, cell viability was measured by the MTS assay. Data represent the mean ± SD percentage viable cells from 1 of at least 3 representative experiments performed in triplicate. (D) NB4 cells were loaded with the intracellular iron-chelating fluorescent dye calcein-AM (i). Cells were then treated with 10 μM of CPX or the structurally CPX-related analogs DPOH, ADP, and MDP for 2 minutes. Intracellular iron bound by the compounds was measured by flow cytometry as described in panel A. (ii) NB4 cells were treated with increasing concentrations of CPX or the analogs in panel Di. Seventy-two hours after incubation, cell viability was measured by the MTS assay. Data represent the mean ± SD percentage viable cells from 1 of at least 2 representative experiments performed in triplicate.
CPX-induced cell death is iron dependent. (A) Leukemia (NB4, MDAY-D2, and OCI-M2) and solid tumor (OVCAR-3 and PPC-1) cells were loaded with the intracellular iron-chelating fluorescent dye calcein-AM. Cells were then treated with CPX (10 μM) or DFX (100 μM) for 2 minutes. Intracellular iron bound by calcein was measured by flow cytometry. Percentage increase ± SD in the geometric mean intracellular calcein fluorescence is represented *P < .05 (Student t test). One of at least 2 representative experiments performed in triplicate is shown. (B) HT-29(i) and HepG2 (ii) cells were treated with increasing concentrations of CPX with or without iron supplementation with iron in a complex with a transferrin-replacement compound (8 μM). Seventy-two hours after incubation, cell viability was measured by MTS. Data represent the mean ± SD percentage of viable cells from 1 of 3 representative experiments. (C) Jurkat and NB4 leukemia cells were treated with increasing concentrations of CPX or DFX. Seventy-two hours after incubation, cell viability was measured by the MTS assay. Data represent the mean ± SD percentage viable cells from 1 of at least 3 representative experiments performed in triplicate. (D) NB4 cells were loaded with the intracellular iron-chelating fluorescent dye calcein-AM (i). Cells were then treated with 10 μM of CPX or the structurally CPX-related analogs DPOH, ADP, and MDP for 2 minutes. Intracellular iron bound by the compounds was measured by flow cytometry as described in panel A. (ii) NB4 cells were treated with increasing concentrations of CPX or the analogs in panel Di. Seventy-two hours after incubation, cell viability was measured by the MTS assay. Data represent the mean ± SD percentage viable cells from 1 of at least 2 representative experiments performed in triplicate.
To determine whether chelation of intracellular iron by CPX is functionally important for its cytotoxicity, HepG2 and HT-29 solid tumor and NB4, U937, and K562 leukemia cell lines were treated with increasing concentrations of CPX with and without iron supplementation. For these experiments, cells were supplemented with iron in a complex with a commercial transferrin replacement designed to promote intracellular iron uptake (Sigma-Aldrich). The addition of excess iron abrogated CPX-induced cell death, demonstrating that CPX-mediated cytotoxicity was iron dependent (Figure 4B; and data not shown). However, cytotoxicity of CPX was not the result of chelation of extracellular iron or extraction of intracellular iron, as treatment with up to 10 μM of the more avid iron chelator deferoxamine did not reduce cell viability in Jurkat, MDAY-D2, PPC-1, OVCAR-3, HepG2, and HT29 cell lines (Figure 4C; and data not shown).
As an alternate approach to determine whether intracellular iron chelation is functionally important for the cytotoxicity of CPX, we evaluated analogs of CPX that differed in their ability to bind intracellular iron (supplemental Figure 2). CPX analogs ADP and MDP did not bind intracellular iron and were not cytotoxic. In contrast, the analog DPOH bound intracellular iron and also induced cell death, although it was less potent than CPX (Figure 4D).
CPX inhibits ribonucleotide reductase
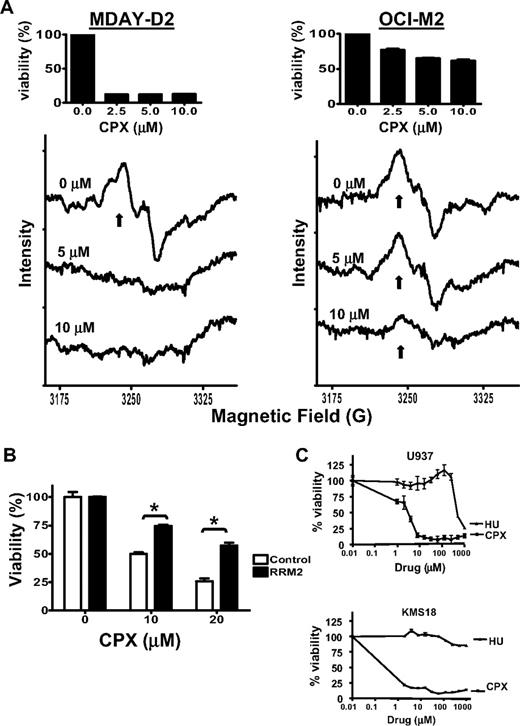
Ribonucleotide reductase is the enzyme responsible for converting nucleoside diphosphates to deoxynucleoside diphosphates, ensuring a balanced supply of deoxyribonucleotides for DNA synthesis.33,–35 This enzyme is iron dependent as its ribonucleotide reductase M2 subunit contains an iron center that is required for the complex's enzymatic activity.36 Given that CPX-induced cell death is iron dependent, we examined the effects of CPX on ribonucleotide reductase activity in CPX-sensitive (MDAY-D2) and -resistant (OCI-M2) cells. MDAY-D2 and OCI-M2 were treated with 5 and 10 μM of CPX for 24 hours. After treatment, the presence of the tyrosyl radical in active ribonucleotide reductase was measured by EPR. At the tested concentrations, CPX inhibited ribonucleotide reductase activity in CPX-sensitive MDAY-D2 but not -resistant OCI-M2 (Figure 5A).
CPX inhibits ribonucleotide reductase. (A) MDAY-D2 and OCI-M2 leukemia cells were treated with CPX (5 and 10 μM) or buffer control for 24 hours. After incubation, cells were harvested, rapidly frozen, and the tyrosyl radical activity (↑) was measured by EPR at a microwave frequency of 9.12 GHz and 77 Kelvin. The averaged of 9 scans is presented. One of 2 representative experiments is shown. (Insets) Mean percentage viability of cells treated with increasing concentrations of CPX. (B) HeLa cervical cancer cells were transfected with cDNA corresponding to RRM2 or vector control and incubated with CPX at increasing concentrations. Seventy-two hours after incubation, cell viability was assessed by the MTS assay. Data represent the mean ± SD percentage viable cells from 1 of 3 representative experiments performed in triplicate; *P < .05 (Student t test). (C) U937 leukemia and KMS18 myeloma cells were treated with increasing concentrations of CPX or hydroxyurea (HU). Seventy-two hours after incubation, cell growth and viability were measured by the MTS assay. Data represent the mean percentage ± SD relative to control cells of 1 of 3 representative experiments performed in triplicate.
CPX inhibits ribonucleotide reductase. (A) MDAY-D2 and OCI-M2 leukemia cells were treated with CPX (5 and 10 μM) or buffer control for 24 hours. After incubation, cells were harvested, rapidly frozen, and the tyrosyl radical activity (↑) was measured by EPR at a microwave frequency of 9.12 GHz and 77 Kelvin. The averaged of 9 scans is presented. One of 2 representative experiments is shown. (Insets) Mean percentage viability of cells treated with increasing concentrations of CPX. (B) HeLa cervical cancer cells were transfected with cDNA corresponding to RRM2 or vector control and incubated with CPX at increasing concentrations. Seventy-two hours after incubation, cell viability was assessed by the MTS assay. Data represent the mean ± SD percentage viable cells from 1 of 3 representative experiments performed in triplicate; *P < .05 (Student t test). (C) U937 leukemia and KMS18 myeloma cells were treated with increasing concentrations of CPX or hydroxyurea (HU). Seventy-two hours after incubation, cell growth and viability were measured by the MTS assay. Data represent the mean percentage ± SD relative to control cells of 1 of 3 representative experiments performed in triplicate.
To determine whether inhibition of ribonucleotide reductase was functionally important for CPX-induced death, we transfected cells with cDNA corresponding to the ribonucleotide reductase M2 subunit of ribonucleotide reductase (RRM2) or vector control. Cells were then treated with increasing concentrations of CPX, and cell viability was measured by the MTS assay. Overexpression of RRM2 abrogated CPX-induced cell death, demonstrating that inhibition of ribonucleotide reductase is functionally important for the cytotoxic effects of CPX (Figure 5B). Of note, CPX was greater than 200-fold more potent than hydroxyurea, a ribonucleotide reductase inhibitor that acts through a mechanism distinct from CPX (Figure 5C). Thus, inhibition of ribonucleotide reductase activity is at least one mechanism by which CPX exerts an anticancer effect.
CPX synergizes with cytarabine
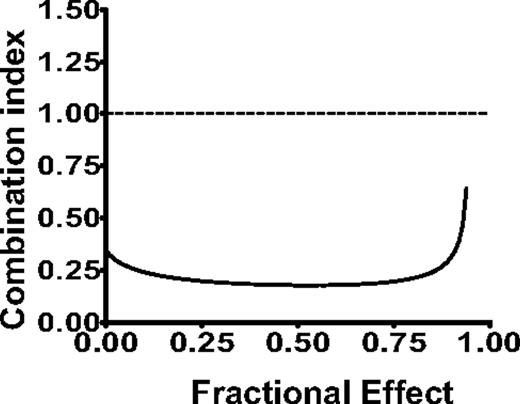
Depletion of intracellular iron37,–39 and inhibition of ribonucleotide reductase40,41 sensitize leukemia cells to cytarabine. Therefore, to further evaluate the therapeutic potential of CPX, we investigated CPX in combination with cytarabine and daunorubicin, chemotherapeutic agents commonly used for the treatment of AML. Here, OCI-AML2 leukemia cells were treated with CPX alone and in combination with cytarabine and daunorubicin. Seventy-two hours later, viability was assessed by the MTS assay. Data were analyzed by the Calcusyn median effect model, in which the combination index (CI) indicates synergism (CI < 0.9), additivity (CI = 0.9-1.1), or antagonism (CI > 1.1). The combination of CPX and cytarabine demonstrated strong synergism (Figure 6) with CI values at the ED50, ED75, and ED90 of 0.18, 0.19, and 0.24, respectively. In contrast, the combination with CPX and daunorubicin was closer to additive with CI values at the ED50, ED75, and ED90 of 0.85, 0.88, and 1.2, respectively. Thus, consistent with its mechanism of action as an intracellular iron chelator and an inhibitor of ribonucleotide reductase, CPX sensitizes cells to cytarabine.
CPX synergizes with cytarabine to induce cell death in leukemia cells. The effects of different concentrations of CPX in combination with cytarabine on the viability of OCI-AML2 cells were measured by MTS assay after 72 hours of incubation. Data were analyzed with Calcusyn software. Combination index (CI) versus fractional effect (FE) plot showing the effect of the combination of CPX and cytarabine. CI < 1 indicates synergism. One of 2 representative isobologram experiments performed in triplicate is shown.
CPX synergizes with cytarabine to induce cell death in leukemia cells. The effects of different concentrations of CPX in combination with cytarabine on the viability of OCI-AML2 cells were measured by MTS assay after 72 hours of incubation. Data were analyzed with Calcusyn software. Combination index (CI) versus fractional effect (FE) plot showing the effect of the combination of CPX and cytarabine. CI < 1 indicates synergism. One of 2 representative isobologram experiments performed in triplicate is shown.
Discussion
In this study, we conducted 2 independent screens for off-patent drugs with previously unrecognized anticancer activity and identified CPX in both screens. In one screen, we sought to identify agents potentially cytotoxic to leukemia stem cells by searching for compounds that inhibited the growth of TEX and M9-ENL1 cell lines. TEX and M9-ENL1 cells display features similar to leukemic stem cells. For example, TEX cells are undifferentiated myeloblasts, are organized as a hierarchy both in vitro and in vivo, and engraft NOD/SCID mice.28 Likewise, M9-ENL1 cells engraft NOD/SCID mice, causing a reproducible, rapid, and fatal B-cell acute lymphoblastic leukemia.29 Consistent with activity of CPX in these cell lines, CPX reduced the viability of primary AML cells and inhibited engraftment of primary AML cells in NOD/SCID mouse models. Thus, CPX appears cytotoxic to leukemia stem cells.
CPX was also identified in a chemical screen for inhibitors of survivin transactivation. Survivin is a regulator of cell cycle and apoptosis42 and is preferentially expressed in malignant cells compared with normal adult cells.43,44 In malignant cells, the aberrant expression of survivin is usually the result of increased transactivation of the survivin promoter rather than mutations in the gene or protein.45 Therefore, we hypothesized that compounds capable of directly or indirectly inhibiting survivin expression would potentially exert anticancer activity. Moreover, investigating compounds that inhibited survivin expression would provide insight into the regulation of this gene in malignancy. Consistent with our identification of CPX as an inhibitor of survivin transactivation, CPX decreased levels of survivin protein and mRNA in the malignant cells. The effects of CPX on survivin expression matched its effects of cell viability as the cells most sensitive to CPX-mediated decreases in survivin expression at 48 hours were most sensitive to CPX-induced cell death at 72 hours. However, our data suggested that decreased survivin expression after CPX treatment was not functionally important for its cytotoxicity. Rather, decreased survivin expression was a biomarker of its intracellular activity and probably reflected its primary action as an intracellular iron chelator with resultant inhibition of iron-containing enzymes, such as ribonucleotide reductase. Nonetheless, in the context of a clinical trial of CPX in patients with malignancy, changes in survivin expression could be followed in primary patient samples as a marker of biologic response to the drug.
CPX is an α-hydroxypyridone that is currently used for the treatment of cutaneous fungal infection.13 Although not previously administered systemically to humans for therapeutic use, its safety after oral and intravenous administration has been extensively tested in animals. Serum concentrations of 3 μg/mL (10 μM) are achievable after repeated administration of CPX to rats and dogs, and these concentrations were not toxic.14 These concentrations are within the range required to produce an antitumor effect based on our current studies. Thus, given the preclinical efficacy demonstrated in this report and the prior toxicology studies performed for its use as an antifungal, CPX could be advanced quickly into clinical trial for patients with refractory hematologic malignancies. However, because of possible differences in iron pools between species, toxicities of CPX not apparent in rodents and dogs may become evident in human clinical trials. Alternatively, CPX may be less effective in patients who have been heavily transfused and have very high levels of iron stores.
Our in vitro studies demonstrated a narrow difference between the cytotoxicity of CPX for primary AML and normal hematopoietic cells, and this finding raises concerns regarding the potential therapeutic window of CPX. However, it is important to note that results of colony formation assays do not always predict clinical toxicity. For example, cytarabine and amsacrine are chemotherapeutic agents routinely used in the treatment of AML but show little or no selectivity for malignant cells over normal cells in colony formation assays.46,47 Moreover, we observed that oral CPX delayed tumor growth in mouse models of leukemia and prevented engraftment of primary AML cells without untoward toxicity. Finally, toxicology studies with CPX conducted in rats and dogs did not report hematologic toxicity. Nonetheless, the small differential sensitivity between primary AML and normal hematopoietic cells raises concerns about the potential hematologic toxicity, and its safety will have to be carefully evaluated in phase 1 clinical trials.
A few lines of evidence from this study support an iron-dependent mechanism of cytotoxicity for CPX. First, supplementing cells with iron inhibited the cytotoxicity of CPX. Second, CPX analogs that did not bind iron were not cytotoxic, whereas analogs that bound iron remained cytotoxic. Third, CPX bound intracellular iron at concentrations that were cytotoxic. Finally, the mechanism of action of CPX as an antifungal relates to its ability to bind iron.48 Nevertheless, this evidence does not preclude an additional iron-independent mechanism of action for CPX.
CPX induced cell death through its ability to chelate intracellular iron. Iron chelators have been previously reported to induce cell death through the inhibition of iron-containing enzymes, such as ribonucleotide reductase,49 and are an emerging therapeutic strategy. Deferoxamine is a hexadentate iron chelator that forms a 1:1 ligand metal complex that chelates extracellular iron.50,51 However, the extracellular iron chelator deferoxamine was significantly less active than CPX in our assays, probably because of the ability of CPX to penetrate through cell membranes and bind intracellular iron. Of note, resistance to CPX was not simply the result of failure of the drug to enter cells or bind iron. Rather, resistance to CPX appeared related to differences in sensitivity to intracellular iron chelation. However, it is not fully understood why cells differ in their sensitivity to intracellular iron chelation. Potentially, differences between cell lines in the abundance of iron pools may explain sensitivity and resistance to CPX.
Ribonucleotide reductase is an iron-dependent enzyme that is critical for DNA replication as it catalyzes the reduction of ribonucleotides into their corresponding deoxyribonucleotides.34 The enzyme is formed by 2 subunits, RRM1 and RRM2.52 The RRM1 subunit contains the active site of the enzyme, whereas the RRM2 subunit contains an Fe(III) center and a tyrosyl radical that is required for the enzymatic activity of the complex.35 Chemical inhibitors of ribonuceotide reductase are efficacious chemotherapeutic agents. For example, gemcitabine inhibits the complex by acting at the active site of the RRM1 subunit,53 and hydroxyurea is a free radical scavenger that inhibits the tyrosyl radical.54 In contrast, iron chelators inhibit ribonucleotide reductase through a different mechanism as they prevent the incorporation of iron into the RRM2 center, thereby inhibiting formation of the tyrosyl radical.51
We demonstrated that CPX inhibited the activity of ribonucleotide reductase. However, CPX probably also affects other iron-containing enzymes. For example, deoxyhypusine hydroxylase is iron-dependent enzyme involved in a unique 2-step reaction with hypusine synthetase, leading to the hypusinylation of the eIF5A.55 eIF5A is a protein involved in cell proliferation and its hypusinylation is a posttranslational modification required for its activation.56 In human umbilical vein endothelial cells, low-micromolar concentrations of CPX inhibited deoxyhypusine hydroxylase and the hypusinylation of eIF5A.57 Thus, it is probable that CPX inhibits iron-dependent targets beyond ribonucleotide reductase in malignant cells.
In conclusion, CPX displays novel activity in malignant cell lines and leukemia stem cells because of its activity as an intracellular iron chelator. Its preclinical efficacy coupled with its prior safety and toxicity record support a clinical trial of this compound in patients with refractory hematologic malignancies.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Panzarella, Princess Margaret Hospital, for statistical advice.
This work was supported by the Leukemia & Lymphoma Society, the Canadian Institute of Health Research (CIHR), the Ontario Institute for Cancer Research and a Summit Award both with funds from the Province of Ontario, Genome Canada through the Ontario Genomics Institute, a Canada Research Chair, and the Canadian Cancer Society and the Terry Fox Foundation. A.D.S. is a Clinical Research Scholar of the Leukemia & Lymphoma Society.
Authorship
Contribution: Y.E. designed research, analyzed data, performed research, and wrote the paper; S.P.M., X.W., and T.E.W. designed research, analyzed data, and performed research; M.G., A.V., and R.H. performed research and analyzed data; A.D., R.A.B., and J.E.D. designed research and analyzed data; J.W. contributed key reagents and supervised research; W.E.A. designed and conducted research, analyzed data, and edited the paper; A.D.S. designed research, analyzed data, and wrote the paper; and all authors reviewed and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Aaron D. Schimmer, Princess Margaret Hospital, Rm 9-516, 610 University Ave, Toronto, ON, Canada M5G 2M9; e-mail: aaron.schimmer@utoronto.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal