Abstract
Stroke is a leading cause of death and disability. The only therapy available is recombinant tissue plasminogen activator, but side effects limit its use. Platelets play a crucial role during stroke, and the inflammatory reaction promotes neurodegeneration. von Willebrand factor (VWF), an adhesion molecule for platelets, is elevated in patients with acute stroke. The activity of VWF is modulated by ADAMTS13 (a disintegrin-like and metalloprotease with thrombospondin type I repeats-13) that cleaves VWF to smaller less-active forms. We recently documented that ADAMTS13 negatively regulates both thrombosis and inflammation. We report that deficiency or reduction of VWF reduces infarct volume up to 2-fold after focal cerebral ischemia in mice, thus showing the importance of VWF in stroke injury. In contrast, ADAMTS13 deficiency results in larger infarctions, but only in mice that have VWF. Importantly, infusion of a high dose of recombinant human ADAMTS13 into a wild-type mouse immediately before reperfusion reduces infarct volume and improves functional outcome without producing cerebral hemorrhage. Furthermore, recombinant ADAMTS13 did not enhance bleeding in a hemorrhagic stroke model. Our findings show the importance of VWF in regulating infarction and suggest that recombinant ADAMTS13 could be considered as a new therapeutic agent for prevention and/or treatment of stroke.
Introduction
Ischemic stroke is a leading cause of death and disability around the world. Each year in the United States approximately 795 000 persons have a new or recurrent stroke.1 Thrombolytic therapy with tissue plasminogen activator (tPA), which leads to fibrin degradation and promotes clot lysis, is beneficial for ischemic stroke.2,3 However, tPA use is restricted to the first few hours after stroke. In addition, tPA may increase the incidence and severity of cerebral hemorrhage and edema formation.2,3 Thus, there remains a clear need to identify new therapeutic agents for minimizing the effects of stroke. In addition to their effect on coagulation, such agents could also target platelet adhesion and the inflammatory process that follows ischemic stroke.
von Willebrand factor (VWF) is a large multimeric glycoprotein that is important in platelet adhesion and thrombus formation.4 At least in mice, VWF appears more important in arterial thrombosis than fibrin,5 the substrate of tPA/plasmin. More recently, VWF was also shown to contribute to leukocyte adhesion and inflammatory cell recruitment.6,7 VWF is stored in an ultra-large form (UL-VWF; > 20 million kDa) in platelet α-granules and Weibel-Palade bodies of endothelial cells from which it is released during injury or inflammation.8,9 If not immediately consumed for platelet adhesion, the UL-VWF is cleaved by ADAMTS13 (a disintegrin-like and metalloprotease with thrombospondin type I repeats-13) to smaller less-adhesive multimers that circulate in plasma. ADAMTS13 is a metalloprotease of approximately 190 kDa10 present in plasma at a concentration of 0.5 to1 μg/mL in humans.11 Ischemia is a potent inducer of Weibel-Palade body secretion,12 thus making the infarct area highly thrombogenic even after thrombolysis.
VWF deficiency is associated with the most common bleeding disorder in humans, von Willebrand disease.13 In contrast, deficiency of ADAMTS13 is seen in patients with thrombotic thrombocytopenic purpura, which is often characterized by neurologic symptoms because of cerebral ischemia caused by microthrombi in the cerebral microvasculature.14-16 In a mouse model of ADAMTS13 deficiency,17 we have shown that ADAMTS13 down-regulates both thrombosis18 and inflammation6 and that recombinant human ADAMTS13 (r-hu ADAMTS13) infusion in wild-type (WT) mice reduced both platelet adhesion and aggregation.18 However, the role of VWF and ADAMTS13 in stroke has not been experimentally addressed. In this study, using the VWF−/−, Adamts13−/−, and Adamts13−/−/VWF−/− mice, we have demonstrated a critical role of VWF in stroke produced by middle cerebral artery occlusion (MCAO). We also show that ADAMTS13 negatively regulates infarct size after stroke and that infusion of recombinant ADAMTS13 improves stroke outcome in mice.
Methods
Mice
The Adamts13−/−,17 VWF−/−,19 and Adamts13−/−/VWF−/−20 mice described in this study were on C57BL/6J background. The control WT mice on C57BL/6J background were purchased from The Jackson Laboratory. The mice used were 8- to 10-week-old males. Animals were bred at the Immune Disease Institute, and experimental procedures were approved by its Animal Care and Use Committee.
Preparation of ADAMTS13 protein
r-hu ADAMTS13 was expressed by stably transfected HEK 293 (human embryonic kidney 293) or CHO (Chinese hamster ovary) cell lines in serum-free medium. After a volume reduction by ultradiafiltration, r-hu ADAMTS13 was purified by applying a conventional multistep chromatography. r-hu ADAMTS13 purified to homogeneity was characterized by SDS–polyacrylamide gel electrophoresis under reducing and nonreducing conditions and Western blotting with a rabbit polyclonal anti-ADAMTS13 antibody.18 The activity was assessed by the FRETS-VWF73 assay as described.21 r-hu ADAMTS13 protein was dissolved in 150 mmol NaCl/20 mmol Histidin/2% Sucrose/0.05% Crillet 4HP (Tween 80), pH 7.4 (Baxter Bioscience). Control (vehicle) used in experiments was buffer in which r-hu ADAMTS13 was dissolved.
MCAO stroke model
Transient focal cerebral ischemia was induced by 2-hour occlusion of the right middle cerebral artery with a 7.0-siliconized filament in male mice.22,23 We checked by black ink infusion that the architecture of blood vessels in the middle cerebral artery region did not show any obvious differences among the mouse genotypes used in this study. Mice were anesthetized with 1% to 1.5% isoflurane in 30% oxygen. Body temperature was maintained at 37°C (± 1.0°C) using a heating pad. Laser Doppler flowmetry was used in all mice to confirm induction of ischemia and reperfusion (Table 1). At 10 minutes before reperfusion (110 minutes after MCAO), r-hu ADAMTS13 (3460 U/kg; Baxter Bioscience) or vehicle was injected intravenously. At 22 hours after MCAO, mice were killed. Eight 1-mm coronal sections were stained with 2% triphenyl-2,3,5-tetrazolium-chloride (TTC). Sections were photographed with a digital Nikon D70 camera and infarct areas (white) were measured blindly using Image J software (National Institutes of Health; http://isbweb;nih.gov/U/).
Mean recordings of regional cerebral blood flow
| Mouse/treatment . | Figure . | n . | MCAO, % before ischemia . | Reperfusion, % before ischemia . |
|---|---|---|---|---|
| WT | 1 | 13 | 12.1 | 79.9 |
| VWF+/− | 1 | 13 | 12.5 | 86.6 |
| VWF−/− | 1 | 10 | 15.6 | 77.9 |
| WT | 2 | 13 | 13.9 | 84.7 |
| ADAMTS13−/− | 2 | 15 | 13.4 | 87.9 |
| VWF−/−ADAMTS13−/− | 2 | 6 | 12.7 | 78.0 |
| WT | 3 | 9 | 11.8 | 82.0 |
| r-hu ADAMTS13 (HEK) | 3 | 9 | 13 | 75.7 |
| WT | 3 | 8 | 12.4 | 78.8 |
| r-hu ADAMTS13 (CHO) | 3 | 9 | 10.7 | 89.2 |
| Mouse/treatment . | Figure . | n . | MCAO, % before ischemia . | Reperfusion, % before ischemia . |
|---|---|---|---|---|
| WT | 1 | 13 | 12.1 | 79.9 |
| VWF+/− | 1 | 13 | 12.5 | 86.6 |
| VWF−/− | 1 | 10 | 15.6 | 77.9 |
| WT | 2 | 13 | 13.9 | 84.7 |
| ADAMTS13−/− | 2 | 15 | 13.4 | 87.9 |
| VWF−/−ADAMTS13−/− | 2 | 6 | 12.7 | 78.0 |
| WT | 3 | 9 | 11.8 | 82.0 |
| r-hu ADAMTS13 (HEK) | 3 | 9 | 13 | 75.7 |
| WT | 3 | 8 | 12.4 | 78.8 |
| r-hu ADAMTS13 (CHO) | 3 | 9 | 10.7 | 89.2 |
Laser-Doppler flow was continuously recorded during induction of focal cerebral ischemia and reperfusion. No major differences were observed between the groups of animals.
Quantification of hemorrhage in brain after stroke
WT mice were subjected to 90 minutes of MCAO. Ten minutes after MCAO mice were injected intravenously with a platelet-depleting anti-GPIbα antibody (rat IgG anti-GPIbα mAbs; emfret Analytics) or control antibody (rat IgG nonimmune rat antibodies; emfret Analytics; 2 μg/g body weight). r-hu ADAMTS13 (derived from Chinese CHO cell, 3460 U/kg) or vehicle were injected 10 minutes before filament removal. Brains were extracted at 22 hours after filament removal and separated into 2 hemispheres. Each hemisphere was weighed and blended in 500 μL Drabkin reagent. The homogenate was spun at 16 000g for 10 minutes, and the supernatant was taken for analysis. The OD was measured at 530 nm (reference wavelength set at 630 nm) on a plate reader. A standard curve was generated by blending normal brain samples with different volumes of fresh mouse blood.
Tape removal test
Mice were subjected to 1 hour of MCAO. They were injected with r-hu ADAMTS13 (derived from CHO cell, 3460 U/kg; Baxter Bioscience) or vehicle 10 minutes before reperfusion (50 minutes after MCAO) and were tested 24 hours after surgery. The tape removal test allows the assessment of sensory and motor impairments in forepaw function and was adapted from previous studies in rats.24 Mice were held, and 6-mm diameter round tapes were placed onto the plantar surface of the 2 forepaws so that they covered the hairless part of the forepaws. The animal was then placed in a box (40 cm × 30 cm), and the time the animal took to remove the pieces of tape from the ipsilateral and contralateral paws was recorded. The animals were given a maximum of 180 seconds to sense the tapes and then remove them and were scored as 180 seconds if they did not succeed.
Measurements of IL-6 levels in plasma
Blood samples were obtained 22 hours after 2-hour MCAO by retroorbital bleeding into tubes containing 30 U/mL Enoxaparin (Aventis Pharmaceutical Products) in phosphate-buffered saline (PBS). Plasma was separated by centrifugation. Interleukin-6 (IL-6) protein concentration was measured by enzyme-linked immunoabsorbent assay (R&D Systems) according to the manufacturer's guidelines.
Quantification of neutrophils
Twenty-two hours after MCAO (2 hours), mice were killed by overdose of isoflurane, perfused with ice-cold PBS (pH 7.4), and brains were harvested. Brain cryosections (20 μm) were stained with H&E, and the extra vascular neutrophils were counted blindly in the peri-infarct areas with a light microscope (Olympus BX40) at 40×/0.75 magnification. For each animal, 3 fields in 3 sections (2 mm apart) from the ischemic hemisphere were analyzed. Values represent the number of neutrophils per square millimeter. Three animals were evaluated per group.
Bleeding time
Mice (8-9 weeks old) were anesthetized with 2.5% Avertin (15 μL/g mouse body weight; IP), and a 3-mm segment of tail was amputated. The tail was immersed in PBS at 37°C, and the time required for the stream of blood to stop for more than 30 seconds was defined as the bleeding time.25
Statistical analysis
Results are reported as the mean plus or minus SEM. Statistical comparisons were performed using ANOVA followed by Fisher PLSD test or Bonferroni multiple comparison test. P values less than .05 were considered significant. For IL-6 measurement in plasma, the statistical significance was assayed using the Kruskal-Wallis nonparametric test followed by the Dunn multiple comparison test. P values less than .05 were considered significant.
Results
VWF plays an essential role in cerebral ischemia
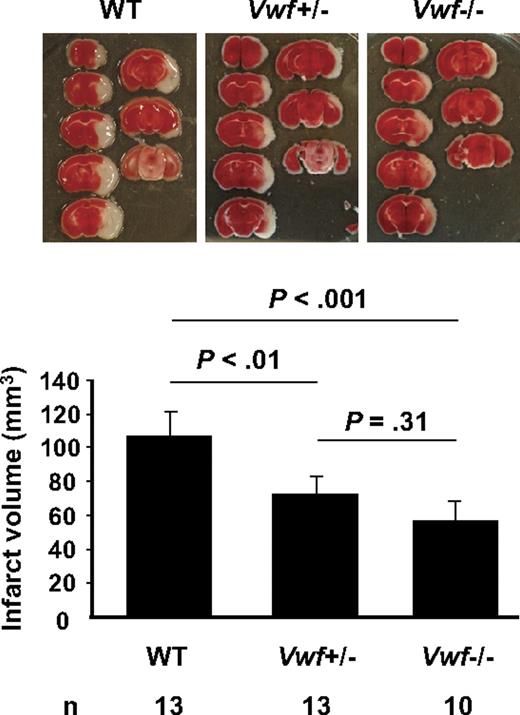
To address the importance of VWF levels in stroke outcome, we subjected WT, VWF+/−, and VWF−/− mice to 2 hours of focal cerebral ischemia using the MCAO stroke model and examined mouse brains 22 hours later using TTC staining to quantify infarct size (Figure 1). We observed that deficiency in VWF caused a 2-fold reduction in infarct volume compared with WT (P < .001). In the VWF+/− mice the infarct volume was reduced by nearly 40% (P < .01; Figure 1), showing that decreasing VWF to 50%19 is sufficient to drastically reduce stroke effect.
Level of VWF regulates infarct volume after ischemic stroke in mice. Representative TTC stain of coronal brain sections of 1 mouse for each strain 22 hours after MCAO (top) and brain infarct volumes (bottom) in WT, VWF+/−, and VWF−/− mice. Deficiency or heterozygosity of VWF resulted in a significant decrease in infarct volume compared with WT.
Level of VWF regulates infarct volume after ischemic stroke in mice. Representative TTC stain of coronal brain sections of 1 mouse for each strain 22 hours after MCAO (top) and brain infarct volumes (bottom) in WT, VWF+/−, and VWF−/− mice. Deficiency or heterozygosity of VWF resulted in a significant decrease in infarct volume compared with WT.
ADAMTS13 negatively regulates infarction after cerebral ischemia
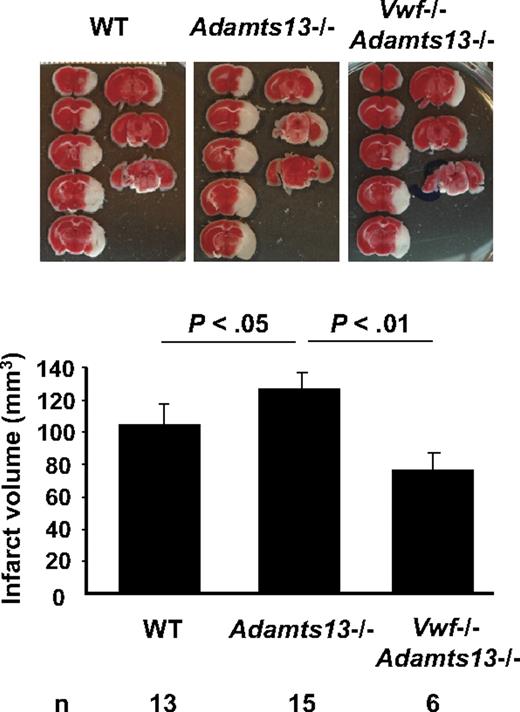
We next evaluated the role of ADAMTS13 in ischemic stroke. We hypothesized that it has a protective role. Indeed, Adamts13−/− mice showed significantly increased infarct volume after MCAO compared with WT mice (124.12 ± 6.59 mm3 vs 103.65 ± 6.69 mm3; P < .05; Figure 2). The function of ADAMTS13 in stroke depended on its action on VWF, because mice deficient in both ADAMTS13 and VWF had infarct volume similar to mice deficient in VWF alone (P = .28; Figures 1–2).
ADAMTS13 regulates infarct volume after ischemic stroke in mice. Representative TTC stain of coronal brain sections of 1 mouse for each strain 22 hours after focal cerebral ischemia in WT, Adamts13−/−, and Adamts13−/−/VWF−/− (top) and corresponding brain infarct volumes quantification (bottom). Increase in infarct volume in Adamts13−/− mice, compared with WT, depended on the presence of VWF.
ADAMTS13 regulates infarct volume after ischemic stroke in mice. Representative TTC stain of coronal brain sections of 1 mouse for each strain 22 hours after focal cerebral ischemia in WT, Adamts13−/−, and Adamts13−/−/VWF−/− (top) and corresponding brain infarct volumes quantification (bottom). Increase in infarct volume in Adamts13−/− mice, compared with WT, depended on the presence of VWF.
Because ADAMTS13 can regulate both thrombosis and inflammation,6,18 we wanted to compare the inflammatory response of WT and Adamts13−/− mice to stroke. At 22 hours after the MCAO, we did not observe differences in neutrophil recruitment to the peri-infarct region as determined by counting the neutrophils in H&E-stained brain sections (WT, 36 ± 4/mm2; Adamts13−/−, 40 ± 9/mm2; NS). Within the infarct, neutrophil counts were lower although similar in these 2 groups. IL-6 was reported to be increased in plasma of mice 1 day after MCAO, showing activation of the peripheral immune system.26 We determined plasma levels of IL-6 at 22 hours after 2-hour MCAO. Compared with sham-operated WT mice, we confirmed a significant elevation of IL-6 in the plasma of WT mice that underwent MCAO (Table 2). However, there was no difference in plasma levels of IL-6 between WT and Adamts13−/− mice after MCAO surgery. Therefore, it is unlikely that the larger infarcts observed in the Adamts13−/− are a result of a significantly enhanced inflammatory response in these mice.
Plasma levels of IL-6 in wild-type and ADAMTS13−/− mice 22 hours after cerebral ischemia and in sham-operated wild-type mice
| Mouse/treatment . | n . | IL-6, pg/mL plasma . |
|---|---|---|
| Wild type/sham | 10 | 42.2 ± 11.3 |
| Wild type/MCAO | 15 | 252.8 ± 82.2 |
| Adamts13−/−/MCAO | 10 | 242.9 ± 67.7 |
| Mouse/treatment . | n . | IL-6, pg/mL plasma . |
|---|---|---|
| Wild type/sham | 10 | 42.2 ± 11.3 |
| Wild type/MCAO | 15 | 252.8 ± 82.2 |
| Adamts13−/−/MCAO | 10 | 242.9 ± 67.7 |
Significant difference was found for wild type/sham versus wild type/MCAO (P < .01). Wild type/MCAO and Adamts13−/−/MCAO were not significantly different.
r-hu ADAMTS13 improves stroke outcome after cerebral ischemia
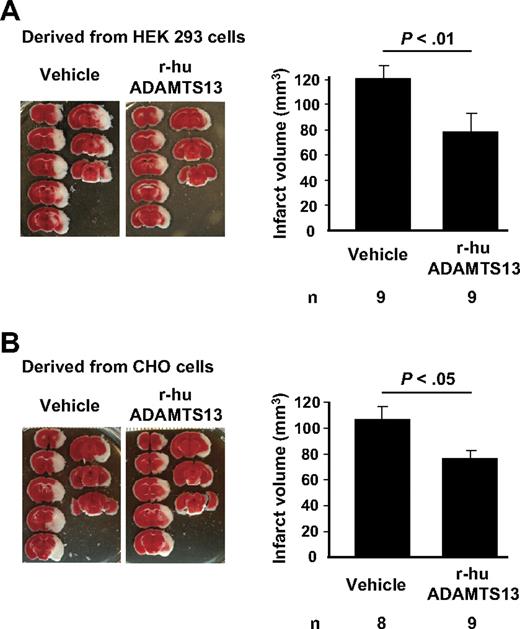
Because the lack of endogenous ADAMTS13 aggravates consequences of ischemic stroke, we next evaluated the therapeutic potential of infusion of additional r-hu ADAMTS13 into WT mice. To try to emulate clinical situations, we infused the protein 110 minutes after ischemic occlusion, that is, just before removing the blocking filament resulting in reperfusion. During the period of stasis, thrombi form in the artery because this MCAO stroke model is highly dependent on platelets and their adhesion receptors, including the receptors for VWF, β3 integrin27 and GPIbα.28 In addition, we could visualize thrombi in the microcirculation in the infarcted area (not shown). We prepared r-hu ADAMTS13 in 2 different cell lines (HEK293 and CHO cells), in case the glycosylation of the resulting protein might be different. Indeed there were differences in the glycosylation pattern, with CHO cell product being more extensively sialylated, probably resulting in a different half-life of the 2 preparations in mouse circulation (HEK293 ADAMTS13 < 1 hour and CHO cell ADAMTS13 several hours; E. Muchitsch, Baxter Innovations GmbH, unpublished observations, March 2007). We have previously shown that r-hu ADAMTS13, prepared in HEK293, was effective in reducing platelet plug size in the ferric chloride arterial injury model in mice.18 The r-hu ADAMTS13 cleaved both mouse and human VWF with similar efficiency (B. Plaimaver, Baxter Innovations GmbH, unpublished observations, August 2008). We found that, despite their different half-lives at the high concentration infused, both of the r-hu ADAMTS13 preparations were similarly effective, reducing infarct volume by approximately 30% (Figure 3A-B).
r-hu ADAMTS13 reduces infarct volume after focal cerebral ischemia in WT mice. Representative TTC staining of coronal brain sections of 1 mouse for each treatment and infarct volumes 22 hours after focal cerebral ischemia in mice treated with (A) vehicle or r-hu ADAMTS13 (derived from HEK293 cells) and (B) vehicle or r-hu ADAMTS13 (derived from CHO cells) are shown.
r-hu ADAMTS13 reduces infarct volume after focal cerebral ischemia in WT mice. Representative TTC staining of coronal brain sections of 1 mouse for each treatment and infarct volumes 22 hours after focal cerebral ischemia in mice treated with (A) vehicle or r-hu ADAMTS13 (derived from HEK293 cells) and (B) vehicle or r-hu ADAMTS13 (derived from CHO cells) are shown.
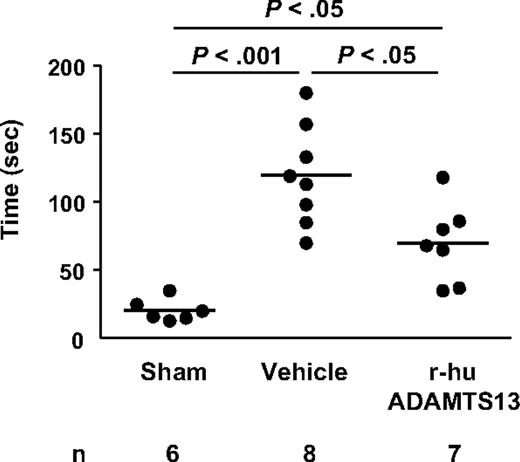
To test whether the reduction in infarct volume actually improves functional outcome, we performed the tape removal test, a technique that assesses sensory and motor impairments in forepaw function.24 Twenty-four hours after surgery, mice that underwent 1-hour MCAO showed an increase in the time needed to remove adhesive tape from the contralateral and ipsilateral paws compared with sham-operated mice (Figure 4), consistent with previous reports.24 Interestingly, treatment with r-hu ADAMTS13 (derived from CHO cells) significantly shortened the time to remove the adhesive tapes from the paws compared with vehicle-treated mice (P < .05), indicating a profound improvement in sensorimotor performance of the mice treated with r-hu ADAMTS13. Taken together, these results show a protective effect of r-hu ADAMTS13 when infused after cerebral ischemia.
r-hu ADAMTS13 improves performances in the tape removal test after ischemic stroke. Time needed to remove tapes from both the contralateral and ipsilateral paws was recorded on sham-operated mice and mice that underwent MCAO (1 hour) injected intravenously with r-hu ADAMTS13 or vehicle 10 minutes before reperfusion. Global differences between groups were found for each parameter (P < .05).
r-hu ADAMTS13 improves performances in the tape removal test after ischemic stroke. Time needed to remove tapes from both the contralateral and ipsilateral paws was recorded on sham-operated mice and mice that underwent MCAO (1 hour) injected intravenously with r-hu ADAMTS13 or vehicle 10 minutes before reperfusion. Global differences between groups were found for each parameter (P < .05).
Effects of r-hu ADAMTS13 infusion on cerebral hemorrhage and hemostatic function of the mice
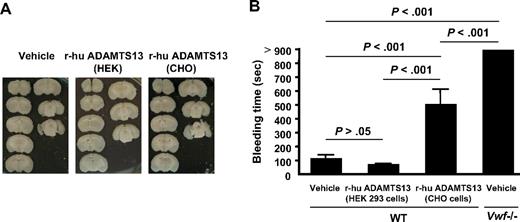
We did not detect cerebral hemorrhage in any WT mice treated with either r-hu ADAMTS13 preparation (Figure 5A). Interestingly, we did not detect cerebral hemorrhage in VWF−/− or VWF+/− mice either. However, we have previously reported that antibody-mediated platelet depletion in this MCAO model causes significant bleeding in the affected hemisphere.22 Thus, the platelets' role in prevention of hemorrhage at stroke sites is preserved in VWF deficiency.
Effect of the r-hu ADAMTS13 preparations on cerebral hemorrhage and tail bleeding time. (A) Representative unstained coronal brain sections of 1 mouse for each treatment show a lack of hemorrhage in r-hu ADAMTS13-treated mice (derived from HEK 293 and CHO cells). (B) Bleeding time measurements show highly increased bleeding in VWF−/− mice compared with WT mice. All the VWF−/− mice were cauterized at 900 seconds to stop bleeding. r-hu ADAMTS13-treated mice (5 hours) had a bleeding time comparable with WT mice (prepared in HEK cells) or prolonged bleeding time (prepared in CHO cells) but significantly shorter than the VWF−/− mice. n = 8 each group.
Effect of the r-hu ADAMTS13 preparations on cerebral hemorrhage and tail bleeding time. (A) Representative unstained coronal brain sections of 1 mouse for each treatment show a lack of hemorrhage in r-hu ADAMTS13-treated mice (derived from HEK 293 and CHO cells). (B) Bleeding time measurements show highly increased bleeding in VWF−/− mice compared with WT mice. All the VWF−/− mice were cauterized at 900 seconds to stop bleeding. r-hu ADAMTS13-treated mice (5 hours) had a bleeding time comparable with WT mice (prepared in HEK cells) or prolonged bleeding time (prepared in CHO cells) but significantly shorter than the VWF−/− mice. n = 8 each group.
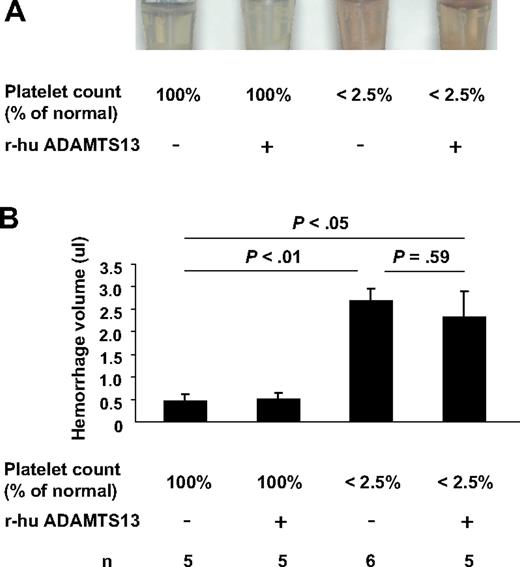
To address the possibility that infusion of r-hu ADAMTS13 may sensitize mice for cerebral hemorrhage, we have used this thrombocytopenia-induced intracerebral hemorrhage model22 in WT mice and examined the effects of r-hu ADAMTS13 infusion on hemoglobin level in the brain in this model. In platelet-depleted or not platelet-depleted mice after 90-minute MCAO, we injected r-hu ADAMTS13 (from CHO cells) or vehicle 10 minutes before reperfusion. Hemorrhage was determined at 22 hours. There was no difference in hemorrhage volume between r-hu ADAMTS13- and vehicle-treated mice (Figure 6). However, the platelet-depleted mice had greater than 6-fold increased hemorrhage compared with the mice of normal platelet count. Therefore, treatment with r-hu ADAMTS13 did not cause hemorrhage or sensitize mice to brain hemorrhage after ischemic stroke.
Effect of r-hu ADAMTS13 infusion on intracerebral hemorrhage after stroke in thrombocytopenic mice and mice with normal platelet count. (A) Supernatants of right (injured) hemisphere homogenates. (B) Hemorrhage volume as determined by hemoglobin brain tissue analysis. Hemoglobin level (corresponding to a volume of blood in μL) was determined in the right infarcted hemisphere. The levels of hemoglobin from the right hemispheres with normal platelet count were low (the same as in the noninjured left hemispheres whether platelet depleted or not depleted; not shown). In contrast, hemorrhage was significant in the right hemispheres of platelet-depleted mice. Infusion of r-hu ADAMTS13 did not affect the hemorrhage volume.
Effect of r-hu ADAMTS13 infusion on intracerebral hemorrhage after stroke in thrombocytopenic mice and mice with normal platelet count. (A) Supernatants of right (injured) hemisphere homogenates. (B) Hemorrhage volume as determined by hemoglobin brain tissue analysis. Hemoglobin level (corresponding to a volume of blood in μL) was determined in the right infarcted hemisphere. The levels of hemoglobin from the right hemispheres with normal platelet count were low (the same as in the noninjured left hemispheres whether platelet depleted or not depleted; not shown). In contrast, hemorrhage was significant in the right hemispheres of platelet-depleted mice. Infusion of r-hu ADAMTS13 did not affect the hemorrhage volume.
To examine to what extent r-hu ADAMTS13 affects hemostasis in the periphery, we also measured tail bleeding time in WT mice 5 hours after infusion with r-hu ADAMTS13 and compared that with mice treated with vehicle and to VWF−/− mice. The VWF−/− mice had a highly prolonged bleeding time (Figure 5B), with all of the animals requiring cauterization, confirming on a pure background the severe bleeding phenotype of these mice.19 The HEK293 preparation with short half-life of r-hu ADAMTS13 did not affect bleeding, whereas the CHO cell preparation with long half-life prolonged bleeding time but to a lesser extent than VWF deficiency (Figure 5B). Thus, reducing the VWF multimer size by ADAMTS13 had a less drastic effect on bleeding time than VWF deficiency because the shorter VWF species retained hemostatic activity.
Discussion
This study has identified VWF as an important regulator of cerebral ischemia. By cleaving VWF, ADAMTS13 provided a significant protective effect after experimental stroke in mice.
High plasma levels of VWF are weakly associated with coronary heart disease.29 They also are found in patients with acute ischemic stroke30 and were suggested to predispose to ischemic stroke.31 This prompted us to investigate whether VWF plays an active role in ischemic stroke. Our results show that deficiency of VWF dramatically reduced infarct volume at 22 hours after cerebral ischemia. Surprisingly, VWF heterozygosity also significantly reduced infarct size, which we confirmed in a second double-blinded study (not shown). This VWF haploinsufficiency, not detected in previous studies of these mice, shows the importance of VWF level in thrombosis, specifically in the brain. For example, we could not detect a defect in ferric chloride–induced thrombosis in arterioles of the mesentery in the heterozygotes (not shown). While our manuscript was being finished, an interesting study by Kleinschnitz et al32 reported a significant 40% reduction in infarct volume in VWF−/− mice 24 hours after a 60-minute MCAO. The motor and global neurologic functions of the VWF−/− mice were better than WT mice undergoing the same surgery. Magnetic resonance imaging evaluation over a 7-day period of these mice also showed no excessive bleeding in the VWF-deficient brains, corroborating our own observations.
The plasma levels of VWF in humans vary over a 6-fold range,33 and, on the basis of our observation that VWF levels modulate infarction, it could be hypothesized that the outcome of stroke would be worse in persons with high VWF. ADAMTS13 regulates VWF activity, not by decreasing VWF antigen levels, but by cleaving the UL-VWF into smaller, less-adhesive multimers. Here, we observed that ADAMTS13 deficiency increased infarct size after cerebral ischemia, indicating the importance of VWF size on stroke outcome. We also observed that r-hu ADAMTS13 prepared in the 2 different cell lines significantly reduced infarct volume when infused 110 minutes after cerebral ischemia, indicating that r-hu ADAMTS13 infusion after an ischemic event could diminish its deleterious consequences. Importantly, infusion of r-hu ADAMTS13 improved the sensorimotor performance of the WT mice (Figure 4) in a test shown to be useful in evaluating outcome of ischemia produced by MCAO in the mouse.24
The exact mechanism of the protective role of ADAMTS13 in stroke is not known and should be investigated further. The r-hu ADAMTS13 probably helps dismantle existing thrombi formed during ischemia and prevents new thrombi from assembling by cleaving the VWF multimers present in the thrombus and the UL-VWF released locally from Weibel-Palade bodies. Furthermore, neither of the r-hu ADAMTS13 preparations produced cerebral hemorrhage in any of the treated brains. In contrast, tPA induces gross cerebral hemorrhage at 24 hours in the MCAO model,34 as does blockade of the platelet integrin receptor αIIbβ3.28 Interestingly, the r-hu ADAMTS13 preparation with short half-life was equally effective in reducing infarct volume (Figure 3) without affecting bleeding time (Figure 5). Taken together, our results indicate that treatment of ischemic stroke with r-hu ADAMTS13 appears safer than tPA or αIIbβ3 blockade in mice. Because we used the ischemia/reperfusion model in this study, it should be noted that reperfusion may not follow ischemic stroke in humans. Further studies should clarify whether ADAMTS13 could induce thrombolysis or whether it can be used together with tPA.
In conclusion, we have uncovered a crucial role for the VWF-ADAMTS13 axis in regulating ischemic stroke. Both VWF level and its thrombotic activity, as reflected by multimer size, heavily impact stroke outcome. ADAMTS13 provides a significant protective effect by reducing final infarct volume. Whether measurement of VWF and ADAMTS13 levels may provide good indicators for the likelihood of transient ischemic attacks and stroke in humans remains to be seen. Importantly, infusion of r-hu ADAMTS13 into WT mice reduced infarct size and improved functional outcome without inducing cerebral hemorrhage. We propose that r-hu ADAMTS13 could offer a new option for treatment of ischemic stroke.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Lesley Cowan for help with preparation of the manuscript.
This work was supported by a Sponsored Research Agreement from Baxter Bioscience, Vienna, Austria (A.K.C. and D.D.W.) and by the National Heart, Lung, and Blood Institute of the National Institutes of Health (grant R37 HL041002; D.D.W.).
National Institutes of Health
Authorship
Contribution: B.-Q.Z. designed the study, performed most of the experiments, analyzed the results, and wrote the manuscript; A.K.C. helped with study design, measured bleeding time, and cowrote manuscript; M.C. provided helpful suggestions, performed the tape removal test, and wrote sections of the manuscript; I.S.P. blindly quantified most of the data; J.J.Y. measured the IL-6 levels in plasma, blindly counted the neutrophils, and quantified brain hemorrhage; F.S. and M.D. provided the well-characterized, purified r-human ADAMTS13 preparations and helpful discussions; and D.D.W. helped with the study design, analysis of results, and cowrote the manuscript.
Conflict-of-interest disclosure: F.S. and M.D. are employees of Baxter Bioscience. The remaining authors declare no competing financial interests.
The current address for A.K.C. is Department of Internal Medicine, Division of Hematology/Oncology, University of Iowa College of Medicine, Iowa City, IA.
Correspondence: Denisa D. Wagner, Immune Disease Institute, 3 Blackfan Cir, Boston, MA 02115; e-mail: wagner@idi.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal