Abstract
Although chronic lymphocytic leukemia (CLL) is a disease of elderly patients, subjects older than 65 years are heavily underrepresented in clinical trials. The German CLL study group (GCLLSG) initiated a multicenter phase III trial for CLL patients older than 65 years comparing first-line therapy with fludarabine with chlorambucil. A total of 193 patients with a median age of 70 years were randomized to receive fludarabine (25 mg/m2 for 5 days intravenously, every 28 days, for 6 courses) or chlorambucil (0.4 mg/kg body weight [BW] with an increase to 0.8 mg/kg, every 15 days, for 12 months). Fludarabine resulted in a significantly higher overall and complete remission rate (72% vs 51%, P = .003; 7% vs 0%, P = .011). Time to treatment failure was significantly shorter in the chlorambucil arm (11 vs 18 months; P = .004), but no difference in progression-free survival time was observed (19 months with fludarabine, 18 months with chlorambucil; P = .7). Moreover, fludarabine did not increase the overall survival time (46 months in the fludarabine vs 64 months in the chlorambucil arm; P = .15). Taken together, the results suggest that in elderly CLL patients the first-line therapy with fludarabine alone does not result in a major clinical benefit compared with chlorambucil. This trial is registered with www.isrctn.org under identifier ISRCTN 36294212.
Introduction
For decades, chlorambucil has been the standard first-line treatment for chronic lymphocytic leukemia (CLL). More recently, several phase III trials showed that the purine analogs fludarabine and cladribine produced higher overall response rates and a longer progression-free survival compared with chlorambucil1,2 or CAP polychemotherapy (cyclophosphamide, adriamycin, predniso(lo)ne).3,4 Later, the combination of fludarabine with cyclophosphamide was shown to further improve the response rate and progression-free survival in the first-line treatment of younger CLL patients.5-7 Therefore, purine analogs alone or in combination have become the standard first-line treatment, at least in younger or physically fit CLL patients. However, the median age of patients included in the above studies ranged between 62 and 64 years. It remained unknown whether the larger population of CLL patients beyond 65 years would similarly benefit from fludarabine first-line therapy.
The incidence of CLL is markedly increased in patients older than 65 years, representing approximately three-quarters of all patients with this leukemic disorder.8,9 So far, chlorambucil is still widely used in first-line treatment in elderly patients, because it is easy to administer and less immunosuppressive than fludarabine. Specific clinical trials on elderly patients with CLL have been missing so far. Therefore, the German CLL Study Group (GCLLSG) initiated a prospective multicenter phase III trial, the CLL5 protocol, to compare chlorambucil with fludarabine in the first-line therapy of CLL patients above 65 years.
Methods
Criteria for eligibility
The diagnosis of CLL was confirmed by the criteria established by the National Cancer Institute (NCI)–Sponsored Working Group in 1996.10 The stage of the disease was assessed according to the Binet and Rai classifications11,12 and the requirement of treatment according to the NCI criteria.10 Patients were eligible in Binet stage C and in stage B or A if they had rapid disease progression (lymphocyte doubling time < 3 months) or symptoms from enlarged lymph nodes and organs, or if they had severe B symptoms. All patients between 65 and 80 years without pretreatment were allowed to enter the trial. Patients needed to have a life expectancy of more than 6 months and an Eastern Cooperative Oncology Group performance status of 0 to 2. Severe organ dysfunction not controlled by medication, concomitant or previous other neoplasias, or an autoimmune hemolytic anemia or thrombocytopenia were exclusion criteria.
The protocol has been approved by the institutional review board of the University of Munich. All patients had signed an informed-consent form before study entry in accordance with the Declaration of Helsinki. The trial was registered at www.isrctn.org as no. ISRCTN 36294212.
Randomization and treatment schedule
The randomization was performed at the Institute of Medical Statistics and Epidemiology, Technical University, Munich, Germany. Randomization was balanced according to participating centers. Patients were randomly assigned 1:1 to receive either fludarabine on a 28-day cycle for a maximum of 6 cycles or chlorambucil on a 14-day cycle for a maximum of 24 cycles. Fludarabine was administered daily with 25 mg/m2 intravenously over 30 minutes for 5 days. Chlorambucil, initially dosed with 0.4 mg/kg BW orally on day 1, was increased by 0.1 mg/kg at each treatment course up to 0.8 mg/kg BW if treatment was well tolerated. No routine antibiotic or antiviral prophylaxis or administration of growth factors was provided in either treatment arm.
Treatment was stopped if a life-threatening side effect occurred. In patients without induction of a documented remission after 3 months of chlorambucil treatment and 3 courses of fludarabine treatment, respectively, treatment was stopped. No crossover was scheduled per protocol in case of nonresponse.
Patients who developed either common toxicity criteria (CTC) grade 3 infections, a neutrophil count of less than 109/L, or thrombocytopenia between 20 and 50 × 109/L with concurrent bleeding complications received a dose-reduced regimen at 75% of the initial dose level for the following treatment courses. Patients with neutrophil counts of less than 0.5 × 109/L or thrombocytopenia of less than 20 × 109/L received a 50% dose reduction for the subsequent cycles.
Response assessment
After every course of therapy, patients were evaluated by clinical examination and blood count. After 3 and 6 courses of fludarabine chemotherapy as well as after 3, 6, 9, and 12 months of chlorambucil treatment, the treatment response was assessed by clinical examination, blood count, serum chemistry, and bone marrow biopsy for confirmation of complete response. During follow-up, response was assessed every 3 months by clinical examination and blood count. The clinical response was defined according to the guidelines of the NCI-sponsored working group.10
Serum parameters and cytogenetics
At study entry, serum and heparinized blood samples were obtained in all included patients and shipped to the Institute of Clinical Chemistry, Ludwig-Maximilians-University of Munich as well as to the Department of Internal Medicine III of the University of Ulm.
Serum thymidine kinase (TK) and serum β2 microglobulin were evaluated centrally by a radioimmunoassay and immunometric chemoiluminescence assay, respectively. Serum β2-microglobulin and serum TK were assessed before treatment in 68 of 93 (73%) patients of the fludarabine arm as well as in 74 of 100 (74%) patients before chlorambucil therapy. Cutoff values for elevation of TK and β2-microglobulin were chosen according to previous publications.13,14
Samples for genetic analyses were submitted in 71 (76%) fludarabine-treated patients as well as in 83 (83%) chlorambucil-treated patients. Fluorescence in situ hybridization for detection of chromosomal aberrations and immunoglobulin heavy chain variable regions (IgVH) mutation status were performed as previously described.15,16
Quality of life instrument: European Organization for Research and Treatment of Cancer QLQ C-30
For assessing health-related quality of life (HRQOL), the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 (version 2.0) was used, a 30-item score questionnaire developed by the EORTC.17 The questionnaire included a global health scale, 5 functional scales (physical, role, cognitive, emotional, and social), and 3 symptom scales as well as 6 single items. Raw scores for scales were evaluated by addition of the specific items, which were included in a scale and set into relation to the number of items.17 Raw scores were then transformed to a linear scale ranging from 0 to 100, with a high score in global health and all functional scales correlating with a very good HRQOL. If single items were missing, all completed items were used for calculation, in case that at least half of the items from the scale were answered. If less than half of the items from a scale were answered, a score was not assessed.
As previously published, changes of HRQOL scores between 5 and 10 were interpreted as “little” change to patients, scoring differences between 10 and 20 as “moderate,” and differences of more than 20 as “very large” change.18 The first HRQOL questionnaire at the time point of randomization was handed out by the treating physician. Three additional questionnaires were sent to patients at 6, 12, and 24 months after randomization by mail with a covering letter, explaining the procedure of completion.
Statistical analysis
The primary endpoints of the trial were overall survival and progression-free survival. Secondary endpoints were response rates, quality of response, as well as quality of life and incidence of severe infections. The calculated sample size was 146 evaluable patients, based on 80% power to detect a 20% difference in overall survival after 2 years. Because we anticipated that in elderly CLL patients a high rate of Binet stage C cases would be included, our assumption for the sample size calculation was based on overall survival data of a patient population at poor prognosis (mainly Binet stage C) as described by Binet et al.11 For the calculation of the sample size the program NQUERY Advisor 2.0 (Statistical Solutions) was used.
The study was initiated in July 1999, and recruitment was stopped in September 2004, when at least 146 case report forms were evaluable. Statistical analysis was performed on an intent-to-treat basis including the eligible patients. Time to event was estimated using the Kaplan-Meier method, and treatment comparison was tested with the log-rank test. Overall survival was calculated from randomization to death, progression-free survival from randomization to the time of disease progression according to NCI or death, and time to treatment failure from randomization to time point of disease progression, relapse treatment, or death. Response rates were calculated for all patients with at least 1 cycle and 1 month of therapy, respectively, who were assessable for response. The response recorded was the best achieved at any time point during first-line therapy. Treatment arms were compared by the χ2 test and the Fisher exact test, respectively, if the expected count less than 5 was larger than 20%. Myelotoxicity was also assessed according to the NCI-sponsored Workshop guidelines.10 Multivariate analysis of prognostic factors for progression-free survival and overall survival was performed with a multiple logistic regression model. All statistical tests were 2-sided. Statistical significance was defined at a P value less than 5%. The analysis was performed with SPSS V15.0. The analysis presented here was based on the data collected by August 1, 2007.
Role of the funding source
The pharmaceutical companies providing financial support for the study had no role in study design, data collection, data analysis and interpretation, or writing of the report as well as in the decision to submit the paper for publication. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Baseline characteristics of patients
A total of 206 patients were enrolled in the study protocol. Thirteen patients had to be excluded due to violation of the inclusion criteria (Figure 1); 93 patients were randomized to receive fludarabine, 100 to receive chlorambucil; 8 patients were lost to follow-up. The median follow-up time for the 113 patients alive was 42 months (range, 1-89 months). Although the median observation time was 10 months shorter in the chlorambucil arm (38 vs 48 months), this was not significant (P = .075). Survival data were available in 185 patients, response data in 165 patients, and toxicity data in 181 patients. Comparing the patients' characteristics in the 2 treatment arms, there were no significant differences regarding the main clinical features and risk categories (Table 1).
Trial flow. Of the 206 included patients, 185 patients were available for analysis.
Trial flow. Of the 206 included patients, 185 patients were available for analysis.
Baseline characteristics of the eligible patients
| Characteristics . | Chlorambucil (n = 100) . | Fludarabine (n = 93) . |
|---|---|---|
| Age (y) | 70 (65-78) | 71 (65-78) |
| Age group, % | ||
| 65-69 y | 47 | 45 |
| 70-74 y | 31 | 33 |
| 75-79 y | 22 | 22 |
| Binet stage, % | ||
| A | 16 | 13 |
| B | 44 | 51 |
| C | 40 | 36 |
| Rai stage, % | ||
| 0: low risk | 5 | 5 |
| I or II: intermediate risk | 52 | 55 |
| III or IV: high risk | 43 | 40 |
| Male sex, % | 63 | 66 |
| Median time from initial diagnosis to study entry (mo) | 11 (0-110) | 17 (0-92) |
| ECOG performance status, % | ||
| 0 | 42 | 46 |
| 1 | 49 | 52 |
| 2 | 9 | 2 |
| Concomitant disease, % | ||
| None | 33 | 35 |
| 1 | 35 | 32 |
| 2 or more | 32 | 33 |
| Thymidine kinase (U/L) | 20 (3-108) | 18 (4-71) |
| β2 microglobulin (mg/L) | 4 (2-8) | 4 (2-7) |
| Patients with del(17p), n (%) | 5 (6) | 5 (7) |
| Patients with del(11q), n (%) | 8 (10) | 14 (19) |
| Patients with 12q+, n (%) | 17 (20) | 16 (25) |
| Patients with unmutated IgVH (%) | 27 (59) | 28 (67) |
| Characteristics . | Chlorambucil (n = 100) . | Fludarabine (n = 93) . |
|---|---|---|
| Age (y) | 70 (65-78) | 71 (65-78) |
| Age group, % | ||
| 65-69 y | 47 | 45 |
| 70-74 y | 31 | 33 |
| 75-79 y | 22 | 22 |
| Binet stage, % | ||
| A | 16 | 13 |
| B | 44 | 51 |
| C | 40 | 36 |
| Rai stage, % | ||
| 0: low risk | 5 | 5 |
| I or II: intermediate risk | 52 | 55 |
| III or IV: high risk | 43 | 40 |
| Male sex, % | 63 | 66 |
| Median time from initial diagnosis to study entry (mo) | 11 (0-110) | 17 (0-92) |
| ECOG performance status, % | ||
| 0 | 42 | 46 |
| 1 | 49 | 52 |
| 2 | 9 | 2 |
| Concomitant disease, % | ||
| None | 33 | 35 |
| 1 | 35 | 32 |
| 2 or more | 32 | 33 |
| Thymidine kinase (U/L) | 20 (3-108) | 18 (4-71) |
| β2 microglobulin (mg/L) | 4 (2-8) | 4 (2-7) |
| Patients with del(17p), n (%) | 5 (6) | 5 (7) |
| Patients with del(11q), n (%) | 8 (10) | 14 (19) |
| Patients with 12q+, n (%) | 17 (20) | 16 (25) |
| Patients with unmutated IgVH (%) | 27 (59) | 28 (67) |
Median values are given for continuous variables (5th to 95th percentiles); percentages are given for categorical values.
ECOG indicates Eastern Cooperative Oncology Group.
Dose of the study medication and duration of treatment
The median number of administered courses was 4.9 in the fludarabine arm; the median duration of treatment was 6.5 months in the chlorambucil arm. The median administered chlorambucil dose was 0.5 mg/kg BW. In 20% of the patients, the maximum chlorambucil dose of 0.8 mg/kg was administered; 23% received 12 months of treatment. The main documented cause for treatment withdrawal in the chlorambucil arm was nonresponse in 32 patients (33%), followed by side effects in 25 patients (26%). In the fludarabine arm, 66% of the patients received 6 courses of treatment; 74% received the full dose of 25 mg/m2 on 5 days per cycle. Treatment was stopped earlier in 30 patients mostly because of side effects (17 patients; 19%) or nonresponse (7 patients; 8%).
Response to treatment
Fludarabine induced a significantly higher number of complete remissions according to NCI-WG criteria (7% vs 0%; P = .011) as well as a significantly higher overall response rate (72% vs 51%; P = .003) in the whole cohort (Table 2). Response rates were evaluated in different subgroups. Fludarabine was superior to chlorambucil with regard on overall response rate in early stages Binet A and B, but not in advanced stage Binet C. Moreover, fludarabine showed higher response rates in a high-risk group of patients as defined by elevated serum thymidine kinase levels above 10 U/L or serum β2 microglobulin levels above 3.5 mg/L. In contrast, patients presenting with a del(17p) responded very poorly to chlorambucil (0/5 OR; 0/5 CR) and to fludarabine (1/5 OR; 0/5 CR; Table 2).
NCI criteria defined response rates according to treatment arms in all randomized patients (intent-to-treat population)
| . | No. of patients . | P . | |
|---|---|---|---|
| Chlorambucil (n = 100) . | Fludarabine (n = 93) . | ||
| All patients | |||
| CR | 0 (0) | 6 (7) | .011 |
| Overall response | 51 (51) | 67 (72) | .003 |
| Binet stage A | n = 16 | n = 12 | |
| CR | 0 (0) | 1 (8) | .4 |
| Overall response | 7 (44) | 10 (83) | .04 |
| Binet stage B | n = 43 | n = 46 | |
| CR | 0 (0) | 3 (7) | .2 |
| Overall response | 23 (54) | 36 (78) | .01 |
| Binet stage C | n = 40 | n = 32 | |
| CR | 0 (0) | 2 (6) | .2 |
| Overall response | 21 (53) | 21 (66) | .3 |
| TK > 10 U/L | n = 55 | n = 51 | |
| CR | 0 (0) | 3 (6) | .1 |
| Overall response | 27 (49) | 38 (75) | .007 |
| β2 M > 3.5 mg/L | n = 47 | n = 44 | |
| CR | 0 (0) | 2 (5) | .2 |
| Overall response | 20 (43) | 31 (71) | .007 |
| del(11q) | n = 8 | n = 14 | |
| CR | 0 (0) | 1 (7) | 1.0 |
| Overall response | 3 (38) | 11 (79) | .08 |
| del(17p)− | n = 5 | n = 5 | |
| CR | 0 (0) | 0 (0) | — |
| Overall response | 0 (0) | 1 (20) | 1.0 |
| . | No. of patients . | P . | |
|---|---|---|---|
| Chlorambucil (n = 100) . | Fludarabine (n = 93) . | ||
| All patients | |||
| CR | 0 (0) | 6 (7) | .011 |
| Overall response | 51 (51) | 67 (72) | .003 |
| Binet stage A | n = 16 | n = 12 | |
| CR | 0 (0) | 1 (8) | .4 |
| Overall response | 7 (44) | 10 (83) | .04 |
| Binet stage B | n = 43 | n = 46 | |
| CR | 0 (0) | 3 (7) | .2 |
| Overall response | 23 (54) | 36 (78) | .01 |
| Binet stage C | n = 40 | n = 32 | |
| CR | 0 (0) | 2 (6) | .2 |
| Overall response | 21 (53) | 21 (66) | .3 |
| TK > 10 U/L | n = 55 | n = 51 | |
| CR | 0 (0) | 3 (6) | .1 |
| Overall response | 27 (49) | 38 (75) | .007 |
| β2 M > 3.5 mg/L | n = 47 | n = 44 | |
| CR | 0 (0) | 2 (5) | .2 |
| Overall response | 20 (43) | 31 (71) | .007 |
| del(11q) | n = 8 | n = 14 | |
| CR | 0 (0) | 1 (7) | 1.0 |
| Overall response | 3 (38) | 11 (79) | .08 |
| del(17p)− | n = 5 | n = 5 | |
| CR | 0 (0) | 0 (0) | — |
| Overall response | 0 (0) | 1 (20) | 1.0 |
Complete response (CR) was defined according to the NCI criteria10 as normal physical examination, normal blood count, and less than 30% lymphocytes in a sample obtained by bone marrow biopsy. Nonresponse was defined as stable disease or progression.
TK indicates thymidine kinase; and β2 M, β2 microglobulin.
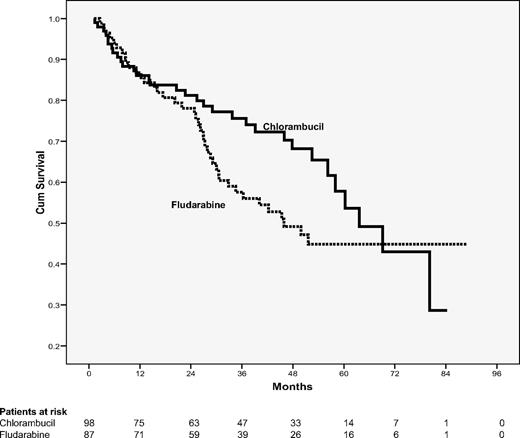
Overall survival
Although there was no significant difference in the overall survival between both treatment arms, median survival time was shorter in the fludarabine arm with 46 months in comparison with 64 months after chlorambucil without reaching statistical significance (P = .15; Figure 2). Main causes of death in the fludarabine arm were progressive CLL (21 patients, 52%), followed by secondary diseases (13 patients, 32%), including secondary malignancies and Richter transformation (8 patients, 20%: 4 transformations, 1 bronchial carcinoma, 1 mantle cell lymphoma, and 1 epithelial carcinoma). In the chlorambucil arm, progressive CLL was also the main cause of death (21 patients; 66%), followed by secondary diseases and malignancies (8 patients, 25%, including 3 with secondary malignancies: 2 Richter transformation and 1 colon carcinoma). Fatal, treatment-related side effects occurred in 4 patients treated with fludarabine and 1 chlorambucil-treated patient (see below).
Overall survival according to randomization. A total of 98 patients assigned to chlorambucil and 87 patients assigned to fludarabine were evaluable for overall survival. Thirty-two patients treated with chlorambucil and 40 patients treated with fludarabine died (P = .15).
Overall survival according to randomization. A total of 98 patients assigned to chlorambucil and 87 patients assigned to fludarabine were evaluable for overall survival. Thirty-two patients treated with chlorambucil and 40 patients treated with fludarabine died (P = .15).
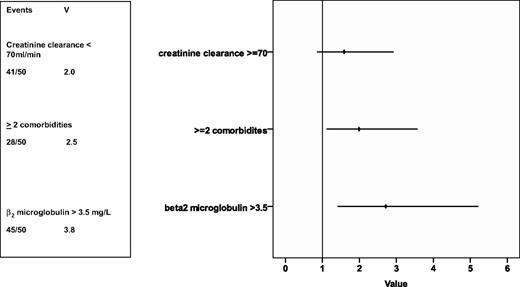
In a univariate analysis, 7 prognostic factors for overall survival were evaluated: sex, Binet stage, age, creatinine clearance, incidence of 2 or more comorbidities, serum thymidine kinase, and serum β2-microglobulin. Serum β2-microglobulin as well as the presence of 2 or more comorbidities were associated with a higher risk of death (see supplemental Figures 1 and 2, available on the Blood website; see the Supplemental Materials link at the top of the online article). A multivariate analysis including the treatment arm in addition to the abovementioned parameters was performed on 132 patients and identified serum β2-microglobulin as well as the incidence of 2 or more comorbidities as a significant prognostic factor predicting a shorter overall survival (hazard ratio [HR]) for serum β2-microglobulin 2.7; 95% confidence interval (CI) 1.4-5.2; HR for 2 or more comorbidities 2.0; 95% CI 1.1-3.6; Figure 3). A detailed fluorescence in-situ hybridization analysis and evaluation of JgVH mutational status as well as of ZAP70 and CD38 will be presented in a separate paper.
HR of prognostic factors on survival. The rhomb represents the HR, the line the 95% confidence interval. Rhombs on the right side of the lane represent an increased risk of death, on the left side a reduced risk of death. V indicates variance.
HR of prognostic factors on survival. The rhomb represents the HR, the line the 95% confidence interval. Rhombs on the right side of the lane represent an increased risk of death, on the left side a reduced risk of death. V indicates variance.
Progression-free survival
The median progression-free survival was similar in both patient groups with 19 months for fludarabine and 18 months for chlorambucil, respectively (P = .7; Figure 4). Both treatment arms were compared in high-risk patients as defined above, but no difference in progression-free survival was found.
Progression-free survival according to randomization. Seventy (80%) of the patients in the fludarabine arm progressed as did 74 (77%) in the chlorambucil arm. Median progression-free survival was 18 months in the chlorambucil arm versus 19 months in the fludarabine arm (P = .7).
Progression-free survival according to randomization. Seventy (80%) of the patients in the fludarabine arm progressed as did 74 (77%) in the chlorambucil arm. Median progression-free survival was 18 months in the chlorambucil arm versus 19 months in the fludarabine arm (P = .7).
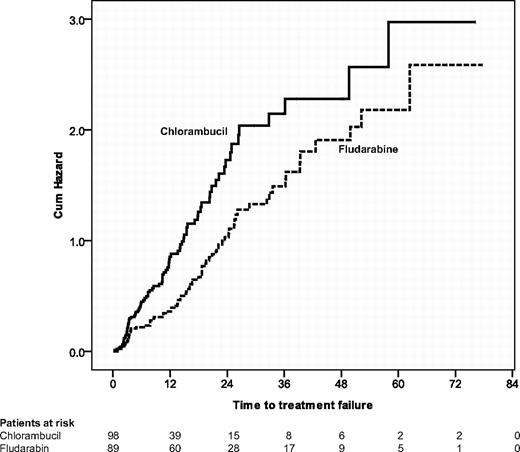
The finding that qualitatively better and higher response rates for fludarabine did not translate into longer progression-free survival was somewhat unexpected. Therefore, we also evaluated the time to treatment failure. Patients treated with chlorambucil had a significantly shorter time to treatment failure than fludarabine-treated patients (11 vs 18 months, P = .004; see Figure 6). This difference in the results for progression-free versus time to treatment failure seems to be explained by a higher proportion of patients in the chlorambucil arm receiving a second-line treatment before becoming progressive: 18 of 61 patients who received a relapse treatment after first-line therapy with chlorambucil had no documented progressive disease (29%) in comparison with 5 of 35 patients after first-line therapy with fludarabine (14%; P = .07).
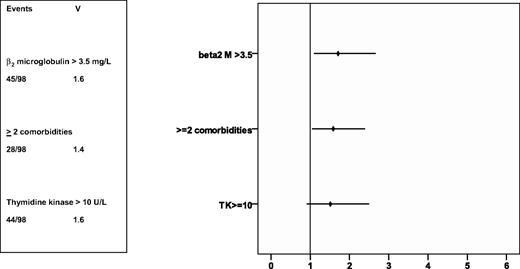
A univariate analysis of the risk parameters (treatment arm, sex, Binet stage, age, creatinine clearance, incidence of 2 or more comorbidities, serum thymidine kinase, and serum β2-microglobulin) evaluating the influence on progression-free survival was performed. No difference in progression-free survival between Binet stages was assessed. Patients with elevated serum TK as well as patients with elevated β2-microglobulin had a significantly shorter progression-free survival time (15 vs 23 months, P = .01; 14 vs 22 months, P = .003). In a multivariate analysis performed in 144 patients, elevated β2--microglobulin and the presence of 2 or more comorbidities were associated with a shorter progression-free survival time, while the proliferation marker serum TK failed to reach statistical significance (Figure 5).
HR of prognostic factors on progression-free survival. The rhomb represents the HR, the line the 95% confidence interval. Rhombs on the right side of the lane represent an increased risk of death, on the left side a reduced risk of death. V indicates variance.
HR of prognostic factors on progression-free survival. The rhomb represents the HR, the line the 95% confidence interval. Rhombs on the right side of the lane represent an increased risk of death, on the left side a reduced risk of death. V indicates variance.
Second-line treatment
A significantly lower proportion of patients initially treated with fludarabine were retreated when disease progressed again (50% vs 77%; P = .006). Except for 1 patient in each arm, more detailed data regarding relapse treatment were available in all patients. The majority of the chlorambucil-treated patients received fludarabine for second-line therapy (26 of 61 patients; 43%), followed by chlorambucil retreatment or continued chlorambucil therapy on the same dose level of the last regular treatment course (12 patients; 20%) and fludarabine-based combinations (10 patients; 16%). Seven patients received bendamustine, 2 CHOP, 1 alemtuzumab, and 2 others conventional chemotherapeutic regimens. In the chlorambucil arm, the overall response to relapse treatment was 60% in 35 assessable patients, whereas 53% of 19 evaluable patients had a response to second-line therapy with fludarabine. Patients in relapse after fludarabine first-line therapy were more frequently treated with combination regimens: 11 patients (32% each) received either fludarabine-based combinations, 8 patients either CHOP or R-CHOP (24%), 6 patients were treated with bendamustine or in combination with mitoxantrone (18%), 3 patients with fludarabine alone or chlorambucil (11% each), 2 received another regimen, and 1 patient alemtuzumab. Response evaluation to relapse treatment in the fludarabine arm was documented in 16 patients; 14 of them showed a response (87%).
Toxicity and secondary malignancy
CTC grade 3 and 4 myelotoxicity was significantly more frequent in the fludarabine arm than in the chlorambucil arm (Table 3). Neutropenias did not occur more frequently in 1 of the 2 treatment arms. Because a weekly neutrophil count was not requested by the protocol, neutrophil counts were not obtained in 19 chlorambucil- and 22 fludarabine-treated patients. No significant difference in the incidence rate of severe infections or infections in general (32% in the chlorambucil arm vs 26% in the fludarabine arm; P = .4) was noted. Three of these severe infections in the fludarabine arm were lethal (3 pneumonias) in comparison with 1 lethal infection (septic shock) during chlorambucil treatment. The difference in the incidence rate of severe autoimmune hemolytic anemia (AIHA) was statistically not significant between both arms (8% vs 2%; P = .08). The rates include 1 lethal AIHA in the fludarabine arm.
Percent of patients with at least one grade 3 or 4 side effect classified by CTC criteria and NCI criteria according to randomization group
| . | Chlorambucil (n = 96) . | Fludarabine (n = 87) . | P . |
|---|---|---|---|
| CTC grade 3 and 4 | |||
| Myelotoxicity in general | 23 | 42 | .005 |
| Leukocytopenia | 3 | 28 | < .001 |
| Neutropenia | 12 | 12 | 1.0 |
| Anemia | 27 | 15 | .05 |
| AIHA | 2 | 8 | .08 |
| Thrombocytopenia | 20 | 15 | .4 |
| Infection | 4 | 8 | .3 |
| NCI grade 3 and 4 | |||
| Neutropenia | 40 | 40 | 1.0 |
| Anemia | 6 | 1 | .08 |
| Thrombocytopenia | 25 | 29 | .5 |
| . | Chlorambucil (n = 96) . | Fludarabine (n = 87) . | P . |
|---|---|---|---|
| CTC grade 3 and 4 | |||
| Myelotoxicity in general | 23 | 42 | .005 |
| Leukocytopenia | 3 | 28 | < .001 |
| Neutropenia | 12 | 12 | 1.0 |
| Anemia | 27 | 15 | .05 |
| AIHA | 2 | 8 | .08 |
| Thrombocytopenia | 20 | 15 | .4 |
| Infection | 4 | 8 | .3 |
| NCI grade 3 and 4 | |||
| Neutropenia | 40 | 40 | 1.0 |
| Anemia | 6 | 1 | .08 |
| Thrombocytopenia | 25 | 29 | .5 |
AIHA indicates autoimmune hemolytic anemia.
In the fludarabine arm, 14 patients (12%) developed a secondary neoplasia (8 patients) or Richter transformation (6 patients) during or after chemotherapy and 7 (7%) in the chlorambucil arm, including 2 Richter transformation, respectively (P = .06). So far, only 1 myelodysplastic syndrome (MDS) occurred after treatment with fludarabine and no secondary acute myeloid leukemia (AML) was observed.
Health-related quality of life
The EORTC C30 questionnaire was completed by 150 of 193 patients (77%) at least once. Baseline and at least 1 follow-up questionnaire were available in 87 patients (45%). No statistically significant differences in compliance between both treatment arms were assessed at all time points. The clinical parameters performance status, incidence of comorbidities, age, sex, incidence of side effects, or response to treatment were well balanced between patients completing the questionnaires or not as well as in patients with additional follow-up questionnaires. At study entry, the global health status was statistically significantly lower in the fludarabine arm by coincidence (mean value 49 vs 58; P = .05). Comparing differences in health-related quality of life (HRQOL) between baseline values and scores after 6, 12, and 24 months, fludarabine-treated patients showed a significant improvement in global health status and fatigue (Figure 6A-C). In both arms, global health status was impaired dramatically after 24 months. This phenomenon is related to the fact that 54% of these patients had a relapse within 6 months before they filled in the questionnaire. Two other patients (8%) had a severe infection at this time point. No significant differences in HRQOL were observed between responders and nonresponders (Figure 7). In comparing HRQOL between sexes, men had a better physical function at baseline (81 vs 64; P < .001) as well as a better role function and social function after 24 months (75 vs 50, P = .01; and 83 vs 64, P = .03, respectively).
Time to treatment failure. Seventy-three of 89 (82%) patients initially treated with fludarabine had a treatment failure in comparison with 86 of 98 patients (88%) who received chlorambucil in first-line therapy (P = .004).
Time to treatment failure. Seventy-three of 89 (82%) patients initially treated with fludarabine had a treatment failure in comparison with 86 of 98 patients (88%) who received chlorambucil in first-line therapy (P = .004).
Differences in HRQOL between baseline and follow-up. (A-C) Differences in global health status (GH) as well as 5 functioning scales [physical functioning (PF), role functioning (RF), emotional functioning (EF), cognitive functioning (CF), social functioning (SF)] and fatigue between baseline and month 6 (A), baseline and month 12 (B), as well as baseline and month 24 (C). Differences in linear analog scales (Δ) of mean HRQOL values for chlorambucil- versus fludarabine-treated patients between baseline and month 6 (Figure 6A), baseline and month 12 (Figure 6B), and baseline and month 24 (Figure 6C).  marks a significant difference between the Δ of mean scores. Baseline questionnaire and follow-up questionnaires were available in 65 patients (28 for chlorambucil, 37 for fludarabine) at 6 months, 51 (27 for chlorambucil, 24 for fludarabine) at 12 months, and 24 (13 for chlorambucil, 11 for fludarabine) at 24 months.
marks a significant difference between the Δ of mean scores. Baseline questionnaire and follow-up questionnaires were available in 65 patients (28 for chlorambucil, 37 for fludarabine) at 6 months, 51 (27 for chlorambucil, 24 for fludarabine) at 12 months, and 24 (13 for chlorambucil, 11 for fludarabine) at 24 months.
Differences in HRQOL between baseline and follow-up. (A-C) Differences in global health status (GH) as well as 5 functioning scales [physical functioning (PF), role functioning (RF), emotional functioning (EF), cognitive functioning (CF), social functioning (SF)] and fatigue between baseline and month 6 (A), baseline and month 12 (B), as well as baseline and month 24 (C). Differences in linear analog scales (Δ) of mean HRQOL values for chlorambucil- versus fludarabine-treated patients between baseline and month 6 (Figure 6A), baseline and month 12 (Figure 6B), and baseline and month 24 (Figure 6C).  marks a significant difference between the Δ of mean scores. Baseline questionnaire and follow-up questionnaires were available in 65 patients (28 for chlorambucil, 37 for fludarabine) at 6 months, 51 (27 for chlorambucil, 24 for fludarabine) at 12 months, and 24 (13 for chlorambucil, 11 for fludarabine) at 24 months.
marks a significant difference between the Δ of mean scores. Baseline questionnaire and follow-up questionnaires were available in 65 patients (28 for chlorambucil, 37 for fludarabine) at 6 months, 51 (27 for chlorambucil, 24 for fludarabine) at 12 months, and 24 (13 for chlorambucil, 11 for fludarabine) at 24 months.
Discussion
The results of this study indicate that first-line therapy with fludarabine is not superior to chlorambucil with regard to progression-free survival and overall survival, although it induces higher response rates and a longer time to treatment failure in CLL patients older than 65 years. This result is likely to have some impact on the general practice of CLL therapy. To our knowledge, this trial is the largest randomized study in an elderly CLL patient cohort. The median age of 70 years in this study is approximately 5 years older than in most previous clinical trials, thus representing a different patient population in CLL.
This study confirms the results of a phase III trial of the British LRF CLL4 trial comparing chlorambucil versus fludarabine versus fludarabine plus cyclophosphamide (FC),5 including a significant proportion of patients older than 70 years. The United Kingdom trial also demonstrated better response rates with fludarabine in comparison with chlorambucil; similarly, no difference in progression-free survival or overall survival was observed.5 In contrast, the American intergroup study showed that fludarabine was superior to chlorambucil with regard to response rates and progression-free survival.1 However, this study also failed to show any difference in overall survival, which was explained by the crossover design in this trial.
The better overall response rate and complete remission rate with fludarabine did not translate into longer progression-free survival. However, when analyzing the time to treatment failure, patients receiving fludarabine performed significantly better, because there was a significant proportion of chlorambucil-treated patients with stable disease receiving second-line therapy before they became apparently progressive.
There exist several potential explanations why fludarabine was not as effective as expected: First, fludarabine might have been used at a lower dose, because it was more frequently dose reduced than in a similar trial conducted by the GCLLSG in younger patients at the same time.6 Another explanation is that a larger proportion of patients did not receive any relapse treatment after initial therapy with fludarabine. This failure was probably due to the lack of treatment alternatives at the time the study was conducted.
In our study, the median administered chlorambucil dose was relatively low with 0.5 mg/kg but still resulted in satisfactory response outcomes. In the previously published Intergroup trial,1 a chlorambucil dose of 40 mg/m2 given every 28 days was chosen, which represents a similar dose intensity compared with our study (using an estimated 70 mg per month at 70 kg of BW). Within the United Kingdom trial, all patients received a higher dose of chlorambucil per protocol with 70 mg/m2 per month.5 Although the 72% overall response rate in the chlorambucil arm of the United Kingdom trial was higher than in the Intergroup study or in our trial, chlorambucil was still not superior to fludarabine.
Fludarabine was generally well tolerated in our study. The incidence of low and severe grade infections was not different between either arm. A phase II trial on 32 elderly patients with a median age of 67 years reported a 22% rate of severe infections after fludarabine therapy.19 The impaired tolerability of the chemotherapy in that study was probably due to the pretreatment of all included patients.
The analysis of several prognostic factors showed that elevated β2-microglobulin and the concomitance of 2 or more comorbidities were the only significant prognostic factors predicting very poor progression-free and overall survival. Our data regarding the role of β2-microglobulin are consistent with previously published data.20-23 Because data of previous publications suggest that the prognostic impact of del(11q) is diminished in CLL patients above the age of 55 years,24 β2-microglobulin might be one of the most important prognostic factors in the elderly CLL population.
When evaluating HRQOL, the overall compliance rate in the elderly was similar to younger patients5,25 but showed inferior rates in the later follow-up. In comparing the differences between baseline values and values obtained during follow-up, a small, but significant, improvement in global health status of fludarabine-treated patients was observed in the first year of follow-up. Global health status impaired dramatically after 2 years of follow-up, which is consistent with the median time of relapse. As shown previously, quality of life in elderly patients decreases very fast as soon as they become more symptomatic.26
Fludarabine-based combination regimens have become the new standard in younger patients or elderly patients who are still physically fit and have a low number of comorbidities. The CLL4 trial of the British study group showed that FC was superior to fludarabine and chlorambucil in all patients, including the subgroup of patients above the age of 70 years.5 Because the elderly patients included in British trial were selected to have very good physical condition and low comorbidity, an Italian monocentric phase II trial evaluated a dose-reduced FC regimen in elderly patients with CLL.27 Using a low-dose FC regimen for first-line therapy in 26 elderly patients (median age 71) resulted in an overall response rate of 92%, with 46% complete responses. Similarly promising data were obtained by a chemoimmunotherapy regimen consisting of pentostatin, cyclophosphamide and rituximab.28 However, these chemo(immuno)therapy regimens need to be tested in larger multicenter trials in elderly patients.
Taken together, the study provides support that chlorambucil may be used further on for treatment of CLL in patients with decreased physical fitness and relevant comorbidities. The study is in agreement with findings in younger patients with CLL that failed to show a survival benefit for fludarabine monotherapy.1-5 This has been confirmed by different meta-analyses.29,30 Moreover, the results emphasize the need for further phase III trials for comorbid patients to determine the role of novel treatment modalities in this patient population, especially of chemoimmunotherapy regimens.28 These studies should include concepts for supportive care, an assessment of the quality of life, and recommendations for second-line therapies, which seem to be or primordial importance in this group of patients.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Results from an interim analysis were presented, in part, at the 45th Annual Meeting of the American Society of Hematology, San Diego, CA, December 6-9, 2003. Preliminary results from the final analysis were presented, in part, at the 14th International CLL Workshop, London, United Kingdom, September 14-16, 2007, as well as at the 49th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 8-11, 2007.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by a grant from the Deutsche Krebshilfe (grant no. 70-2353), Bonn, by the Competence Network Lymphoma Bundesministerium für Bildung und Forschung (BMBF), Cologne, as well as by Medac Schering Onkologie, Munich.
Authorship
Contribution: B.F.E. supervised and monitored the trial, made the analysis, and wrote the report; R.B. was responsible for the study design and made the analysis of the trial; S.S. and H.D. were responsible for the genetic analysis; H.D. was also responsible for study design; S.S., M.S., M.B., M.R., N.K., R.R., U.S., O.B., C.W., H.D., and M.H. were responsible for patient accrual, monitoring, and management of the clinical data at their referring center; A.W., V.G., C.S., K.F., and A.M.F. monitored the study; B.F.E., C.W., G.B., H.D., and B.E. contributed to the design of the study; V.G., S.S., H.D., C.W., and M.H. contributed to the analysis; and M.H. designed and supervised the trial, acted as the sponsor and principal investigator, and wrote the report; and all authors critically contributed to the final preparation of the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of German CLL Study Group participants, see the supplemental Appendix.
Correspondence: Barbara F. Eichhorst, MD, Department I of Internal Medicine, Centre of Integrated Oncology Köln Bonn, University of Cologne, Kerpener Str 62, D-50924 Köln, Germany; e-mail: barbara.eichhorst@uk-koeln.de.



![Figure 7. Differences in HRQOL between baseline and follow-up. (A-C) Differences in global health status (GH) as well as 5 functioning scales [physical functioning (PF), role functioning (RF), emotional functioning (EF), cognitive functioning (CF), social functioning (SF)] and fatigue between baseline and month 6 (A), baseline and month 12 (B), as well as baseline and month 24 (C). Differences in linear analog scales (Δ) of mean HRQOL values for chlorambucil- versus fludarabine-treated patients between baseline and month 6 (Figure 6A), baseline and month 12 (Figure 6B), and baseline and month 24 (Figure 6C). marks a significant difference between the Δ of mean scores. Baseline questionnaire and follow-up questionnaires were available in 65 patients (28 for chlorambucil, 37 for fludarabine) at 6 months, 51 (27 for chlorambucil, 24 for fludarabine) at 12 months, and 24 (13 for chlorambucil, 11 for fludarabine) at 24 months.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/16/10.1182_blood-2009-02-206185/4/m_zh89990941510007.jpeg?Expires=1769091944&Signature=c-H1zh3GhT6ueVnKav5Rk-zYRjL0LufUcUKmXQv-ZFZCz~JHsjq~kq6VFlmswgdnEGiGDm6Lm9WdFcZQpH~L7mtvnYLeHE5ozfmZXmnbF72~N7V71B5EmeE2wvhG-s7lvaKBN83wmomKUw3kY~Rq0UIN5U41DloyTR3FOdQLGvlLm5oMAfO9Y8Ee9BcR9XsPzJOmQ9NBsIWXjAIRqmtcss5TVRX~YmqRMDzidK8rz~yZJRcxi5fEQ3ZG8EsGtRK71N3HMz28-w~D~E1ZS1TiAgSoZb8~8KazouWqm1QrbWYs2k7vbbqqPvX-vcZNTehMG19ptgtp4nNVffsZE2aBog__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal