Abstract
Damage to the integrity of the vessel wall leads to exposure of the subendothelial extracellular matrix (ECM), triggering platelet activation and aggregation. This process is essential for primary hemostasis but it may also lead to arterial thrombosis. Although the mechanisms underlying platelet activation on the ECM are well explored, it is less clear which receptors mediate cellular activation in a growing thrombus. Here we studied the role of the recently identified C-type lectin-like receptor 2 (CLEC-2) in this process. We show that anti–CLEC-2 antibody treatment of mice leads to complete and highly specific loss of CLEC-2 in circulating platelets for several days. CLEC-2–deficient platelets displayed normal adhesion under flow, but subsequent aggregate formation was severely defective in vitro and in vivo. As a consequence, CLEC-2 deficiency was associated with increased bleeding times and profound protection from occlusive arterial thrombus formation. These results reveal an essential function of CLEC-2 in hemostasis and thrombosis.
Introduction
Platelet aggregation is a key mechanism for normal hemostasis limiting blood loss after tissue trauma,1 but at sites of atherosclerotic plaque rupture it may also lead to arterial occlusion and embolism causing myocardial infarction or stroke.2,3 Therefore, the inhibition of platelet function has become an important strategy for prevention and treatment of ischemic cardiovascular and cerebrovascular events.4
Platelet plug formation is a multistep process that involves the concerted action of multiple membrane receptors and intracellular signaling pathways. Under conditions of elevated shear as found in arterioles or stenosed arteries, the rapid onset of interaction between glycoprotein Ib-V-IX (GPIb-V-IX) and von Willebrand factor immobilized on collagen is crucial for the initial tethering of flowing platelets.5 This interaction is, however, not stable and must be followed by the stimulation of platelet receptors that mediate cellular activation resulting in calcium mobilization, shape change, up-regulation of integrin affinity, release of secondary agonists, and coagulant activity of the cells.2,6,7 Two major classes of activatory receptors exist in platelets. Soluble agonists such as thrombin, adenosine diphosphate (ADP), and thromboxane A2 (TxA2) stimulate receptors that couple to heterotrimeric G proteins (Gq, G12/13, Gi/z) and activate downstream effectors.8 The other pathway is similar to that used by immunoreceptors and involves sequential activation of Src and Syk family tyrosine kinases that orchestrate a downstream signaling cascade that is regulated through the interaction of several adaptor proteins, including LAT and SLP-76, and leads to activation of effector enzymes, including phosphatidylinositol-3-kinases and phospholipase Cγ2. This pathway is triggered by the major activatory platelet collagen receptor, GPVI, which signals via the immunoreceptor tyrosine-based activation motif (ITAM)–bearing Fc receptor γ-chain9,10 or by CLEC-2, where signaling is initiated by tyrosine phosphorylation of a single YXXL motif in its cytoplasmic tail.11,12
CLEC-2 is a C-type lectin-like type II transmembrane receptor that was originally identified in immune cells13,14 and only recently revealed to be expressed in platelets where it serves as the receptor for rhodocytin, a very potent platelet-activating protein isolated from the Malayan pit viper Calloselasma rhodostoma.11 Based on the observation that rhodocytin-induced platelet activation shares some similarities with the responses elicited by collagen, integrin α2β1 and GPIbα were initially proposed to be targeted by the protein,15-17 but this was shortly later disproven by the finding that rhodocytin fully activated platelets in the absence of these receptors.18 Due to its impressive activatory potential, it has been speculated that CLEC-2 might become a target for antithrombotic agents,19 although its physiologic function has remained elusive.
Recent data indicate that CLEC-2 might be involved in hematogenous tumor metastasis as the receptor mediates tumor cell–induced platelet activation, a process known to significantly promote tumor cell spreading20,21 by interacting with the type I transmembrane sialomucin-like glycoprotein podoplanin (aggrus) on the tumor cell surface.22 In addition, CLEC-2 has been identified as an attachment factor for human immunodeficiency virus type 1 (HIV-1) that mediates the capture and transfer of infectious HIV-1 by platelets, making it a potentially important determinant of virus spread in infected humans.23 In addition, the receptor has been reported to play a role in phagocytic activity of neutrophils.24 However, the importance of CLEC-2 for platelet activation during hemostasis and in the course of thrombotic events is not defined.19
Methods
Mice
Specific-pathogen-free male NMRI mice 4 to 10 weeks of age were obtained from Harlan Winkelmann (Borchen, Germany) and kept in our animal facilities. Animal studies were approved by the district government of Lower Franconia (Bezirksregierung Unterfranken).
Chemicals and antibodies
Anesthetic drugs, medetomidine (Pfizer), midazolam (Roche Pharma AG), fentanyl (Janssen-Cilag GmbH), and antagonists, atipamezol (Pfizer), flumazenil, and naloxon (both from Delta Select GmbH), were used according to the regulation of the local authorities. ADP (Sigma-Aldrich), U46619 (Alexis Biochemicals), thrombin (Roche Diagnostics), and collagen (Kollagenreagents Horm; Nycomed) were purchased. Mouse α-rat IgG (Dianova) and α-rat fluorescein isothiocyanate (FITC; DAKO Cytomation) were purchased. Rhodocytin was isolated as described.18 Monoclonal antibodies conjugated to FITC, phycoerythrin (PE), or DyLight-488 were from Emfret Analytics. The JON/A-PE antibody preferentially binds to the high-affinity conformation of mouse αIIbβ3 integrin.25
Production of mAbs
Female Wistar rats, 6 to 8 weeks of age, were immunized repeatedly with mouse platelets. The rat spleen cells were then fused with mouse myeloma cells (Ag14.653) and hybridomas were selected in HAT medium. Hybridomas secreting monoclonal antibodies (mAbs) directed against platelet receptors were identified by flow cytometry. Briefly, a 1:1 mixture of resting and thrombin-activated platelets (106 cells) was incubated with 100 μL supernatant for 30 minutes at room temperature, washed with PBS (1300g, 10 minutes), and stained with FITC-labeled rabbit anti–rat Ig for 15 minutes. The samples were analyzed on a FACSCalibur (Becton Dickinson) in the setup mode. Platelets were gated by FSC/SSC characteristics. Positive hybridomas were subcloned twice before large-scale production.
Modification of antibodies
Fab fragments from INU1 were generated by 12-hour incubation of 10 mg/mL mAb with immobilized papain (Pierce), and the preparations were then applied to an immobilized protein A column followed by an immobilized protein G column (Biorad and GE Healthcare) to remove Fc fragments and any undigested IgG. The purity of the Fab fragments was checked by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
mCLEC-2-Fc fusion protein
For cloning of the extracellular domain of mouse CLEC-2, RNA from mouse bone marrow was isolated using the RNeasy Mini Kit (QIAGEN) according to the manufacturer's protocol and reverse transcription was performed (Titan One Tube RT-PCR-Kit; Roche). RNA (100 ng) was used as the template and the oligonucleotides AA CTC GAG ACA CAG CAA AAG TAT CTA (with the XhoI site underlined) and AA GGA TCC AGC AGT TTG TCC ACT CTT (with the BamHI site underlined) were used as the forward and reverse primer, respectively. The polymerase chain reaction product was purified using a QIAquick gel extraction kit (QIAGEN) digested with XhoI and BamHI, purified again, and ligated to the Signal plg plus vector containing the human immunoglobulin Fc-domain. The ligation mixture was transformed into Escherichia coli DH5α. The obtained construct was verified by restriction enzyme digestion and DNA sequencing. Human embryonic kidney 293 cells were transfected with the construct using the calcium phosphate method. Stable cell lines expressing recombinant mCLEC-2-Fc were selected in medium containing 700 μg/mL geneticin. The mCLEC-2-Fc protein was purified on a protein G–Sepharose column.26
Immunoprecipitation
Washed platelets (108) were surface labeled with EZ-Link sulfo-NHS-LC-biotin (Pierce; 100 μg/mL in PBS) and subsequently solubilized in 1 mL lysis buffer (Tris-buffered saline containing 20 mM Tris/HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, 0.5 μg/mL leupeptin, and 0.5% Nonidet P-40; all from Boehringer Mannheim). Cell debris was removed by centrifugation (15 000g, 10 minutes). After preclearing (8 hours), 10 μg mAb was added along with 25 μL protein G–Sepharose and precipitation took place overnight at 4°C. Samples were separated on a 12% SDS-polyacrylamide gel (PAGE) along with a molecular weight marker and transferred onto a polyvinylidene difluoride membrane. The membrane was incubated with streptavidin–horseradish peroxidase (1 μg/mL) for 1 hour after blocking. After extensive washing, biotinylated proteins were visualized by enhanced chemiluminescence (ECL; Amersham, GE Healthcare).
Platelet aggregation and flow cytometry
Platelet aggregation.
Washed platelets (200 μL with 0.25 × 106 platelets/μL) were analyzed in the presence of 70 μg/mL human fibrinogen. Transmission was recorded on a Fibrintimer 4-channel aggregometer (APACT Laborgeräte und Analysensysteme) for 10 minutes, and was expressed in arbitrary units with buffer representing 100% transmission.
Flow cytometry.
Washed blood was diluted 1:20 and incubated with appropriate fluorophore-conjugated monoclonal antibodies for 15 minutes at room temperature and analyzed on a FACSCalibur instrument (Becton Dickinson).
Measurement of ATP release
Washed platelets were adjusted to a concentration of 0.4 × 106/μL. Platelets were activated with the indicated agonists for 2 minutes at 37°C under stirring conditions (1000 rpm). After activation, EDTA (3 mM final concentration) and formaldehyde (0.1% final concentration) were added and platelets were fixed for 2 hours. The platelets were then centrifuged for 1 minute at 15 000g, and 100 μL supernatant was added to 100 μL absolute ethanol. Samples were stored at −20°C until measuring. Levels of ATP in 12.5-μL sample were quantified using a bioluminescence assay kit (Roche Diagnostics) and a Fluostar Optima luminometer (BMG Lab Technologies).
Adhesion under flow conditions
Rectangular coverslips (24 × 60 mm) were coated with 0.2 mg/mL fibrillar type I collagen (Nycomed) for 1 hour at 37°C and blocked with 1% BSA. Heparinized whole blood was labeled with a Dylight-488–conjugated anti-GPIX Ig derivative at 0.2 μg/mL and perfusion was performed as described.27 Image analysis was performed off-line using Metavue software (Visitron). Thrombus formation was expressed as the mean percentage of total area covered by thrombi, and as the mean integrated fluorescence intensity per square millimeter as described.28 For flow adhesion studies under nonanticoagulated conditions, blood was taken into 1:10 volume 129 mM trisodium citrate and coinfused with CaCl2/MgCl2 buffer as described.29
Bleeding time
Mice were anesthetized and a 1-mm segment of the tail tip was removed with a scalpel. Tail bleeding was monitored by gently absorbing blood with filter paper at 20-second intervals, without making contact with the wound site. When no blood was observed on the paper, bleeding was determined to have ceased. Experiments were stopped after 20 minutes.
Intravital microscopy of thrombus formation in FeCl3-injured mesenteric arterioles
Mice (4-5 weeks of age) were anesthetized, and the mesentery was exteriorized through a midline abdominal incision. Arterioles (35-60 μm diameter) were visualized with a Zeiss Axiovert 200 inverted microscope (× 10) equipped with a 100-W HBO fluorescent lamp source, and a CoolSNAP-EZ camera (Visitron). Digital images were recorded and analyzed off-line using Metavue software. Injury was induced by topical application of a 3-mm2 filter paper saturated with FeCl3 (20%). Adhesion and aggregation of fluorescently labeled platelets (Dylight-488 conjugated anti-GPIX Ig derivative) in arterioles were monitored for 40 minutes or until complete occlusion occurred (blood flow stopped for > 1 minute).
Statistics
Results from at least 3 experiments per group are presented as mean plus or minus SD. Differences between control and INU1-treated groups were assessed by the Mann-Whitney U test. P values less than .05 were considered statistically significant.
Results
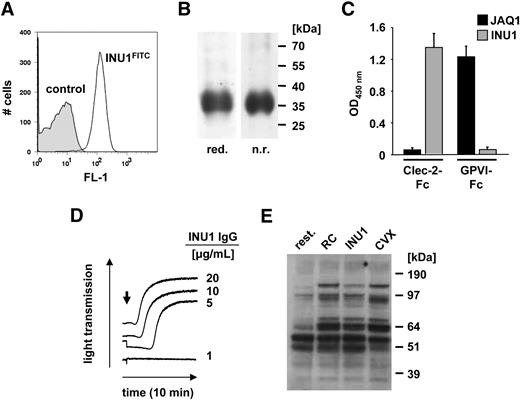
A new rat monoclonal antibody (mAb) against mouse CLEC-2 (INU1, rat IgG1) was generated. INU1 bound to mouse platelets (Figure 1A) and precipitated a protein of an apparent molecular weight of approximately 32 to 38 kDa under reducing and nonreducing conditions (Figure 1B), demonstrating that the apparent molecular weight of mouse CLEC-2 is similar to its human homologue.11,30 The antigenic specificity of INU1 for CLEC-2 was confirmed by enzyme-linked immunosorbent assay (ELISA) where it bound to the extracellular domain of mouse CLEC-2 fused to the Fc-portion of human IgG1 (mCLEC-2-Fc), but not to mGPVI-Fc.26 In contrast, the anti–mouse GPVI antibody, JAQ1,31 bound to mGPVI-Fc, but not to mCLEC-2-Fc (Figure 1C). INU1 did not recognize CLEC-2 in Western blot analysis of platelet lysates, indicating that the antibody binds to a 3-dimensional epitope on the receptor that is lost under the denaturing conditions during SDS-PAGE (not shown).
INU1 recognizes murine CLEC-2. (A) Flow cytometric detection of CLEC-2 on mouse platelets. Platelets were incubated with INU1-FITC (solid line) or an irrelevant rat IgG-FITC (shaded area) at saturating concentrations for 15 minutes at room temperature and analyzed directly. (B) Immunoprecipitation of CLEC-2 from surface-biotinylated mouse platelets. NP-40 lysates were incubated with 10 μg/mL INU1, followed by protein G–Sepharose. Proteins were separated on a 12% SDS-PAGE gel under reducing (red.) or nonreducing (n.r.) conditions, transferred onto a polyvinylidene difluoride membrane, and detected by streptavidin-HRP and ECL. (C) Binding of INU1 to mCLEC-2-Fc or mGPVI-Fc was tested by ELISA. The anti–mouse GPVI antibody, JAQ1, was used as a control (n = 3). Results are expressed as mean OD450 nm ± SD. (D) Washed platelets from control mice were incubated with the indicated concentrations of INU1, and light transmission was recorded on a Fibrintimer 4-channel aggregometer. The results are representative of 4 individual experiments. (E) Washed platelets were stimulated with vehicle (rest.), 0.24 μg/mL rhodocytin (RC), 20 μg/mL INU1, or 1 μg/mL convulxin (CVX), lysed after 90 seconds, and probed with the phosphotyrosine-specific antibody 4G10 and ECL.
INU1 recognizes murine CLEC-2. (A) Flow cytometric detection of CLEC-2 on mouse platelets. Platelets were incubated with INU1-FITC (solid line) or an irrelevant rat IgG-FITC (shaded area) at saturating concentrations for 15 minutes at room temperature and analyzed directly. (B) Immunoprecipitation of CLEC-2 from surface-biotinylated mouse platelets. NP-40 lysates were incubated with 10 μg/mL INU1, followed by protein G–Sepharose. Proteins were separated on a 12% SDS-PAGE gel under reducing (red.) or nonreducing (n.r.) conditions, transferred onto a polyvinylidene difluoride membrane, and detected by streptavidin-HRP and ECL. (C) Binding of INU1 to mCLEC-2-Fc or mGPVI-Fc was tested by ELISA. The anti–mouse GPVI antibody, JAQ1, was used as a control (n = 3). Results are expressed as mean OD450 nm ± SD. (D) Washed platelets from control mice were incubated with the indicated concentrations of INU1, and light transmission was recorded on a Fibrintimer 4-channel aggregometer. The results are representative of 4 individual experiments. (E) Washed platelets were stimulated with vehicle (rest.), 0.24 μg/mL rhodocytin (RC), 20 μg/mL INU1, or 1 μg/mL convulxin (CVX), lysed after 90 seconds, and probed with the phosphotyrosine-specific antibody 4G10 and ECL.
In line with previous observations with (polyclonal) anti–CLEC-2 antibodies in human platelets,11 INU1 induced aggregation of mouse platelets (Figure 1D). This response occurred in a dose-dependent manner, evident as a decrease in the delay before the onset of aggregation rather than an increase in the maximal aggregation response. A similar effect can be seen with rhodocytin on human platelets11 and mouse platelets (not shown). INU1-induced activation was associated with changes in the tyrosine phosphorylation patterns that were comparable with those induced by rhodocytin (Figure 1E).
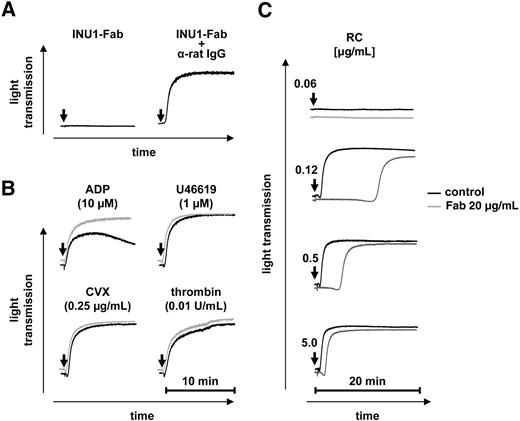
In contrast to INU1-IgG, monovalent Fab fragments of the antibody did not induce aggregation at concentrations up to 20 μg/mL (Figure 2A), suggesting that receptor dimerization/clustering is a critical prerequisite for CLEC-2–mediated platelet activation. This was confirmed by cross-linking of the bound Fab fragments by a secondary antibody (rabbit anti–rat IgG) that induced robust aggregation (Figure 2A). INU1-Fab (20 μg/mL) had no effect on the aggregation response to the stable TxA2 analog U46619, the GPVI agonist convulxin (CVX), or thrombin, but ADP-induced aggregation was consistently increased in the presence of the antibody fragment (83.53% ± 4.57% vs 58.76% ± 5.12% in control; Figure 2B). In contrast, INU1-Fab had a significant inhibitory effect on rhodocytin-induced platelet aggregation at low and intermediate agonist concentrations (visible as a delay in the onset of aggregation) that was, however, overcome at high rhodocytin concentrations (Figure 2C). These results suggested that INU1 binds to an epitope on CLEC-2 that is at least partially overlapping with the rhodocytin binding site on the receptor.
INU1-Fab partially inhibits CLEC-2 function in vitro. (A) INU1-Fab (20 μg/mL) induces aggregation of washed control platelets only when cross-linked (20 μg/mL anti–rat IgG). (B-C) Washed control platelets were incubated with vehicle (black) or INU1-Fab (20 μg/mL, gray) for 5 minutes at 37°C and then stimulated with the indicated agonists under stirring conditions. Light transmission was recorded on a Fibrintimer 4-channel aggregometer. The results are representative of 4 individual measurements.
INU1-Fab partially inhibits CLEC-2 function in vitro. (A) INU1-Fab (20 μg/mL) induces aggregation of washed control platelets only when cross-linked (20 μg/mL anti–rat IgG). (B-C) Washed control platelets were incubated with vehicle (black) or INU1-Fab (20 μg/mL, gray) for 5 minutes at 37°C and then stimulated with the indicated agonists under stirring conditions. Light transmission was recorded on a Fibrintimer 4-channel aggregometer. The results are representative of 4 individual measurements.
INU1-induced loss of CLEC-2 in circulating platelets
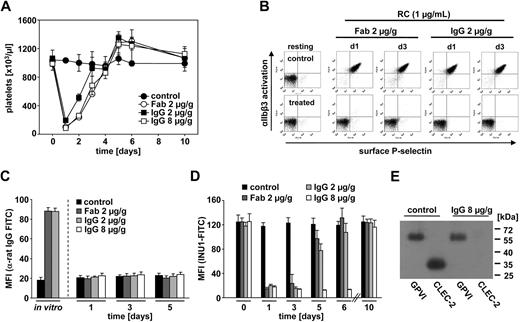
To study the effect of INU1 on platelets in vivo, mice received 2 μg/g body weight of the antibody intravenously, and circulating platelets were studied ex vivo at different time points after injection. INU1 treatment induced transient thrombocytopenia with a maximum drop of platelet counts more than 85% on day 1 and a return to normal or in some animals slightly increased platelet counts after 3 to 4 days where they remained for at least 6 more days (Figure 3A). This effect was not Fc dependent or caused by antibody-induced receptor dimerization, as it was also seen with Fab fragments of INU1 (2 μg/g body weight; Figure 3A). INU1-treated mice did not develop spontaneous bleeding for at least 3 weeks (not shown). Free INU1 IgG was detectable by ELISA for approximately 2 to 3 days in the plasma of the animals (data not shown).
INU1 induces the loss of CLEC-2 in circulating platelets in vivo. (A) Mice received the indicated amounts of purified IgG or Fab fragments of INU1 intravenously in 200 μL sterile PBS, and platelet counts were determined by flow cytometry at the indicated time points. Results are expressed as the mean platelet count ± SD for 4 mice per group and are representative of 3 individual experiments. Parallel analyses on an automated cell analyser (Sysmex) yielded similar results (not shown). (B) Two-color flow cytometric analysis of integrin αIIbβ3 activation (JON/A-PE) and P-selectin exposure (FITC) in platelets from mice 1 or 3 days after INU1-IgG or INU1-Fab injection. Diluted whole blood was stimulated with 1 μg/mL rhodocytin (RC). The data are representative of 8 mice per group. (C) Mice were injected with the indicated amounts INU1-IgG or INU1-Fab, and surface-bound IgG or Fab was detected by flow cytometry using anti–rat IgG-FITC. Maximal binding was determined by incubating platelets from untreated mice with INU1-IgG or INU1-Fab (10 μg/mL) in vitro. (D) INU1-FITC binding to platelets from the indicated mice. Results are expressed as mean fluorescence intensity (MFI) ± SD (C-D). (E) Immunoprecipitation of CLEC-2 and GPVI from surface-biotinylated platelets from control and INU1 (8 μg/g)–treated mice on day 5 under reducing conditions.
INU1 induces the loss of CLEC-2 in circulating platelets in vivo. (A) Mice received the indicated amounts of purified IgG or Fab fragments of INU1 intravenously in 200 μL sterile PBS, and platelet counts were determined by flow cytometry at the indicated time points. Results are expressed as the mean platelet count ± SD for 4 mice per group and are representative of 3 individual experiments. Parallel analyses on an automated cell analyser (Sysmex) yielded similar results (not shown). (B) Two-color flow cytometric analysis of integrin αIIbβ3 activation (JON/A-PE) and P-selectin exposure (FITC) in platelets from mice 1 or 3 days after INU1-IgG or INU1-Fab injection. Diluted whole blood was stimulated with 1 μg/mL rhodocytin (RC). The data are representative of 8 mice per group. (C) Mice were injected with the indicated amounts INU1-IgG or INU1-Fab, and surface-bound IgG or Fab was detected by flow cytometry using anti–rat IgG-FITC. Maximal binding was determined by incubating platelets from untreated mice with INU1-IgG or INU1-Fab (10 μg/mL) in vitro. (D) INU1-FITC binding to platelets from the indicated mice. Results are expressed as mean fluorescence intensity (MFI) ± SD (C-D). (E) Immunoprecipitation of CLEC-2 and GPVI from surface-biotinylated platelets from control and INU1 (8 μg/g)–treated mice on day 5 under reducing conditions.
On days 1 and 3, platelets from INU1-IgG– or INU1-Fab–treated mice were refractory toward rhodocytin (1 μg/mL) as revealed by flow cytometry (Figure 3B), whereas the response was partially restored on day 5 and back to normal on day 7 (not shown). Notably, this inhibitory effect was not due to blockade of the receptor by INU1, as the antibody (or Fab fragment) was not detectable by a FITC-conjugated anti–rat IgG antibody on the surface of the cells on days 1 and 3 after injection, whereas control platelets incubated with INU1-IgG or INU1-Fab in vitro yielded strong signals (Figure 3C). INU1-FITC did not bind to platelets from INU1-IgG– or INU1-Fab–treated mice on days 1 and 3, whereas a subpopulation of the cells was stained on day 5 and more than 90% were stained on day 6 (Figure 3D). These results indicated that INU1 treatment induced the loss of CLEC-2 in circulating platelets within 24 hours and that this effect was overcome by the production of new platelets.
To study the functional consequences of CLEC-2 deficiency in more detail and to test whether a prolonged loss of the receptor can be achieved, mice received 8 μg/g body weight INU1 intravenously. In these animals, free INU1 IgG was detectable by ELISA for 5 to 8 days (data not shown). The resulting thrombocytopenia and the kinetics of CLEC-2 loss were comparable with the effects caused by 2 μg/g body weight (Figure 3A), but CLEC-2 was not detectable on the surface of the cells for at least 6 days (Figure 3C-D). Immunoprecipitation experiments performed on day 5 after antibody injection confirmed the complete loss of CLEC-2, whereas other receptors such as GPVI were not affected (Figure 3E). This was also shown by flow cytometric analysis of basal surface expression levels of GPVI, GPIb-V-IX, CD9, and integrins αIIbβ3 and α2β1 on day 5 (Table 1). Hematocrits and white blood cell counts were not significantly different between control and INU1-treated mice (Table 1).
Hematology and platelet glycoprotein expression in control and INU1-treated mice
| . | Control . | INU1 . |
|---|---|---|
| HCT, % | 0.53 ± 0.02 | 0.49 ± 0.04 |
| WBC, ×106 | 11.1 ± 1.2 | 11.0 ± 1.7 |
| CLEC-2 | 119 ± 10 | 14 ± 1 |
| GPIb | 398 ± 5 | 391 ± 19 |
| GPV | 354 ± 5 | 364 ± 14 |
| GPIX | 550 ± 13 | 563 ± 20 |
| CD9 | 1496 ± 27 | 1338 ± 66 |
| GPVI | 61 ± 3 | 56 ± 3 |
| α2 | 76 ± 5 | 69 ± 1 |
| β1 | 173 ± 11 | 165 ± 12 |
| αIIbβ3 | 888 ± 40 | 854 ± 51 |
| . | Control . | INU1 . |
|---|---|---|
| HCT, % | 0.53 ± 0.02 | 0.49 ± 0.04 |
| WBC, ×106 | 11.1 ± 1.2 | 11.0 ± 1.7 |
| CLEC-2 | 119 ± 10 | 14 ± 1 |
| GPIb | 398 ± 5 | 391 ± 19 |
| GPV | 354 ± 5 | 364 ± 14 |
| GPIX | 550 ± 13 | 563 ± 20 |
| CD9 | 1496 ± 27 | 1338 ± 66 |
| GPVI | 61 ± 3 | 56 ± 3 |
| α2 | 76 ± 5 | 69 ± 1 |
| β1 | 173 ± 11 | 165 ± 12 |
| αIIbβ3 | 888 ± 40 | 854 ± 51 |
Hematocrit (HCT) in percentage and white blood cell counts (WBCs) per milliliter for control and INU1-treated mice (day 5) were analyzed with an automated blood analyzer (Sysmex; n=6 mice per group). Expression of glycoproteins on the platelet surface was determined by flow cytometry. Diluted whole blood from the indicated mice was incubated with fluorescein isothiocyanate–labeled antibodies at saturating concentrations for 15 minutes at reverse transcription and platelets were analyzed directly. Results are expressed as mean fluorescence intensity ± SD for 6 mice per group.
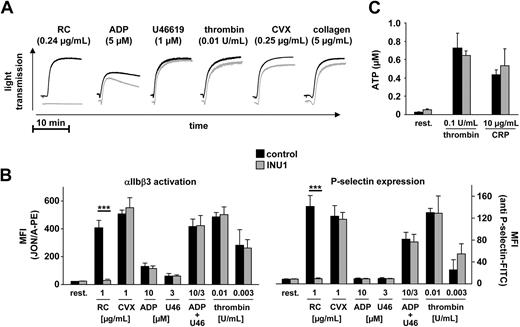
Standard aggregometry revealed that platelets from mice on day 5 after INU1 injection (8 μg/g body weight) were completely resistant to activation with rhodocytin at any concentration tested (up to 10 μg/mL), whereas responses to ADP, U46619, CVX, collagen, and thrombin were normal (Figure 4A). Flow cytometric analyses of integrin αIIbβ3 activation and degranulation-dependent P-selectin exposure confirmed that INU1-induced CLEC-2 deficiency had no significant effect on platelet activation by ADP, U46619, CVX, or thrombin, whereas responses to rhodocytin were blunted (Figure 4B). Furthermore, thrombin- and CRP-induced ATP release from dense granules was not significantly different in platelets from INU1-treated mice compared with controls, confirming that the antibody treatment does not have a general effect on the degranulation machinery (Figure 4C).
CLEC-2–deficient platelets show abolished response to rhodocytin but normal responses to classic agonists. Mice were treated with vehicle or 8 μg/g INU1 and analyzed on day 5. (A) Washed platelets from control (black) or INU1-treated (gray) mice were stimulated with the indicated agonists, and light transmission was recorded on a Fibrintimer 4-channel aggregometer. The results are representative of 6 individual experiments. (B) Flow cytometric analysis of αIIbβ3 integrin activation (binding of JON/A-PE, left panel) and degranulation-dependent P-selectin exposure (right panel) in response to the indicated agonists from control or INU1-treated mice. U46 indicates U46619. Results are mean fluorescent intensities (MFI) ± SD of 6 mice per group. (C) Measurement of released ATP. Washed platelets were incubated for 2 minutes at 37°C with the indicated agonists and fixed. ATP present in the supernatant was measured using a luminometric assay. Results are given as mean ATP concentration (μM) ± SD (n = 6 per group).
CLEC-2–deficient platelets show abolished response to rhodocytin but normal responses to classic agonists. Mice were treated with vehicle or 8 μg/g INU1 and analyzed on day 5. (A) Washed platelets from control (black) or INU1-treated (gray) mice were stimulated with the indicated agonists, and light transmission was recorded on a Fibrintimer 4-channel aggregometer. The results are representative of 6 individual experiments. (B) Flow cytometric analysis of αIIbβ3 integrin activation (binding of JON/A-PE, left panel) and degranulation-dependent P-selectin exposure (right panel) in response to the indicated agonists from control or INU1-treated mice. U46 indicates U46619. Results are mean fluorescent intensities (MFI) ± SD of 6 mice per group. (C) Measurement of released ATP. Washed platelets were incubated for 2 minutes at 37°C with the indicated agonists and fixed. ATP present in the supernatant was measured using a luminometric assay. Results are given as mean ATP concentration (μM) ± SD (n = 6 per group).
On day 10 after INU1 injection, CLEC-2 expression (Figure 3D) and rhodocytin-induced responses (not shown) were fully restored in all tested animals. Together, these results demonstrated that INU1-induced CLEC-2 deficiency very specifically abolished one activation pathway in platelets while leaving other ones fully intact.
CLEC-2 is required for stable thrombus formation under flow
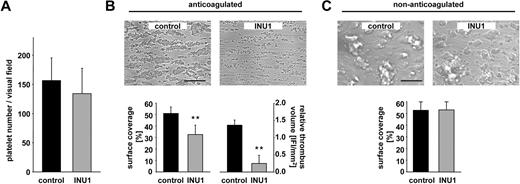
At sites of vascular injury, the signals generated by multiple platelet receptor-ligand interactions are integrated to ensure efficient platelet attachment and thrombus formation under flow conditions.7 A possible function of CLEC-2 in this process has not been assessed to date. Therefore, we analyzed the ability of CLEC-2–deficient platelets to form thrombi on collagen-coated surfaces in a whole blood perfusion system.27 Under high shear conditions (1700 s−1), control platelets adhered to collagen fibers and formed aggregates within 2 minutes that consistently grew into large thrombi by the end of the perfusion period of 4 minutes (supplemental Video 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In marked contrast, although CLEC-2–deficient platelets exhibited unaltered adhesion to collagen under flow (Figure 5A), the subsequent formation of 3-dimensional aggregates was severely impaired (Figure 5B). During the entire perfusion time, adherent platelets recruited numerous new platelets from the blood flow, but these were consistently unable to firmly attach and were released after a few seconds (supplemental Video 2). As a consequence, the surface area covered by platelets and the total thrombus volume at the end of the experiment were reduced by 36% and 82%, respectively (Figure 5B). Similar results were obtained at intermediate shear rates (1000 s−1, data not shown). These findings demonstrated that CLEC-2–dependent processes are essential for stable aggregate formation under flow, whereas the receptor is not required for the adhesion process on collagen.
CLEC-2–deficient platelets fail to form stable aggregates under flow. Whole blood from control or INU1-treated mice (8 μg/g, day 5) was perfused over a collagen-coated surface (0.2 mg/mL) at a shear rate of 1700s−1. (A) Platelet adhesion on collagen after 30 seconds, indicated as number of platelets per visual field. (B) Aggregate formation on collagen after 4-minute perfusion time under anticoagulated conditions. (Top) Representative phase-contrast images. Bar represents 100 μm. (Bottom) Mean surface coverage (left) and relative thrombus volume expressed as integrated fluorescence intensity (IFI) per square millimeter (right) ± SD of 6 mice per group; **P < .01. (C) Aggregate formation on collagen after 4-minute perfusion time under nonanticoagulated conditions. (Top) Representative phase-contrast images. Bar represents 100 μm. (Bottom) Mean surface coverage ± SD of 6 mice per group.
CLEC-2–deficient platelets fail to form stable aggregates under flow. Whole blood from control or INU1-treated mice (8 μg/g, day 5) was perfused over a collagen-coated surface (0.2 mg/mL) at a shear rate of 1700s−1. (A) Platelet adhesion on collagen after 30 seconds, indicated as number of platelets per visual field. (B) Aggregate formation on collagen after 4-minute perfusion time under anticoagulated conditions. (Top) Representative phase-contrast images. Bar represents 100 μm. (Bottom) Mean surface coverage (left) and relative thrombus volume expressed as integrated fluorescence intensity (IFI) per square millimeter (right) ± SD of 6 mice per group; **P < .01. (C) Aggregate formation on collagen after 4-minute perfusion time under nonanticoagulated conditions. (Top) Representative phase-contrast images. Bar represents 100 μm. (Bottom) Mean surface coverage ± SD of 6 mice per group.
To test whether the thrombus instability was based on impaired platelet activation, we performed flow adhesion studies under nonanticoagulated conditions to allow thrombin generation. Under these conditions, both control and CLEC-2–deficient platelets formed large stable thrombi (Figure 5C), indicating that in the presence of high amounts of thrombin, platelet activation through CLEC-2 is not essential for thrombus stabilization in this flow-dependent system. Similarly, coinfusion of ADP (10 μM) and U46619 (1 μM) into anticoagulated blood shortly before entering the flow chamber likewise resulted in the formation of large and stable aggregates in both control and CLEC-2–deficient blood (data not shown). Together, these data indicate that CLEC-2 functions as an activatory receptor in platelets that is required for thrombus stabilization under conditions where other agonists are not present in concentrations sufficient to fully activate the cells.
Increased bleeding times and defective arterial thrombus formation in CLEC-2–deficient mice
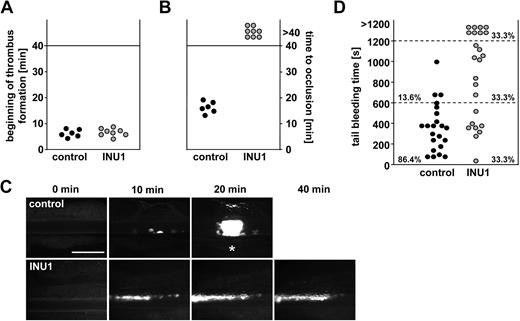
As platelet aggregation is a major pathomechanism in acute ischemic cardiovascular events, we studied the effects of CLEC-2 deficiency on pathologic occlusive thrombus formation by in vivo fluorescence microscopy after ferric chloride–induced mesenteric arteriole injury. In all control mice, the formation of small aggregates was observed approximately 5 to 7 minutes after injury, with progression to complete vessel occlusion within 20 minutes (mean occlusion time: 16.4 ± 2.2 minutes; Figure 6A-C). In contrast, whereas initial adhesion and formation of small aggregates occurred with similar kinetics in CLEC-2–deficient mice (6.9 ± 1.5 minutes vs 6.5 ± 1.5 minutes in control, P > .05), progression to stable large thrombi was almost completely abrogated. This defect was to a great extent caused by the release of individual platelets from the thrombus surface, but also embolization of small thrombus fragments was observed (supplemental Videos 3–4). Consequently, blood flow was maintained throughout the 40-minute observation period in all CLEC-2–deficient mice, revealing a crucial role for CLEC-2 during occlusive thrombus formation.
Defective thrombus formation and prolonged bleeding times in CLEC-2–deficient mice. Mice received vehicle or INU1 (8 μg/g) and were analyzed after 5 days. (A-C) Mesenteric arterioles were injured with FeCl3, and adhesion and thrombus formation of fluorescently labeled platelets were monitored in vivo by fluorescence microscopy. (A) Time to appearance of first thrombus larger than 10 μm and (B) time to vessel occlusion are shown. Each symbol represents 1 individual. (C) Representative images are depicted. Bar represents 50 μm. Each symbol represents 1 individual. * indicates occlusion of the vessel. (D) Tail bleeding times in control and CLEC-2–deficient mice. Each symbol represents 1 individual.
Defective thrombus formation and prolonged bleeding times in CLEC-2–deficient mice. Mice received vehicle or INU1 (8 μg/g) and were analyzed after 5 days. (A-C) Mesenteric arterioles were injured with FeCl3, and adhesion and thrombus formation of fluorescently labeled platelets were monitored in vivo by fluorescence microscopy. (A) Time to appearance of first thrombus larger than 10 μm and (B) time to vessel occlusion are shown. Each symbol represents 1 individual. (C) Representative images are depicted. Bar represents 50 μm. Each symbol represents 1 individual. * indicates occlusion of the vessel. (D) Tail bleeding times in control and CLEC-2–deficient mice. Each symbol represents 1 individual.
To test whether the INU1-induced CLEC-2 deficiency has an impact on hemostasis, we measured tail bleeding times. Whereas bleeding stopped in all (22/22) control mice during the 20-minute observation period (mean bleeding time: 6.1 ± 3.9 minutes), bleeding times were increased in INU1-treated mice, with 8 (33.3%) of 24 mice bleeding for more than 20 minutes and a mean bleeding time of 10.8 plus or minus 6.0 minutes for the other animals (P < .05; Figure 6D). These results show that CLEC-2 plays a significant role in normal hemostasis.
Discussion
Here we have demonstrated that the recently identified platelet membrane glycoprotein CLEC-2 plays a fundamental role in thrombus formation in hemostasis and thrombosis. Despite the previously known activatory potential of the receptor11,18 these results were unanticipated, as the only known endogenous ligand of the receptor, podoplanin, is expressed mainly in epithelial and tumor cells and can therefore not account for CLEC-2 activation at sites of vascular injury.19,22
Thrombus formation under flow conditions in vitro and also in vivo is severely defective in the antibody-induced absence of CLEC-2. This strongly suggests that the ligand(s) of the receptor is present in plasma or may be presented on the surface of or released by (activated) platelets. CLEC-2 ligands could also be present at the injured vessel wall, but this may not be crucial for attachment of the first layer of platelets as indicated by unaltered formation of small aggregates at sites of injury in CLEC-2–deficient mice in vivo (Figure 6). This notion is also supported by the observation that CLEC-2–deficient platelets can attach normally to collagen under high shear flow conditions (Figure 5A, supplemental Video 2), a process known to be driven mainly by GPIb, GPVI, and integrins α2β1 and αIIbβ3.6,7,32 Rather, CLEC-2–dependent signaling appears to be required for establishing (activation-dependent) stable platelet-platelet contacts under flow conditions as revealed by the defective transition of newly recruited platelets to firm adhesion on the surface of collagen-adherent platelets (supplemental Video 2). This process also appears to be crucial for stable thrombus formation in vivo as revealed by the constant release of individual platelets and frequent embolization of small aggregates from the surface of the developing thrombus in CLEC-2–deficient mice (supplemental Video 4). As a consequence, no occlusion was seen in these animals, thus identifying CLEC-2 as an interesting potential target for antithrombotic therapy.
We show that CLEC-2 can indeed be specifically targeted and functionally inactivated by the antibody INU1 in vivo. Remarkably, this in vivo inhibition by far exceeded the inhibitory potential of the antibody in vitro, as it was not based on pure blockade of the receptor. Rather, INU1 induced the irreversible loss of CLEC-2 from circulating platelets, a process previously reported only for GPVI in mouse and human platelets.33,34 The complete loss of functional CLEC-2 in platelets of INU1-treated mice was confirmed by different approaches. First, these platelets were completely refractory to activation with rhodocytin (Figures 3–4), whereas Fab fragments of the antibody had only a limited inhibitory potential in vitro (Figure 2). Second, flow cytometric analyses confirmed the complete loss of CLEC-2 from the surface of circulating platelets within 24 hours after INU1 injection, and third, immunoprecipitation confirmed a prolonged absence of the protein for at least 5 days (Figure 3).
We were not able to identify the route of CLEC-2 down-regulation, but mClec-2A expressed in human embryonic kidney 293T cells has been shown to be proteolytically cleaved to produce a soluble extracellular fragment.35 This indicates that the loss of CLEC-2 could occur through ectodomain shedding, but also internalization/degradation might contribute to this process. We have previously demonstrated that antibody-induced down-regulation of GPVI can occur through both of these routes and that these are regulated by diverging signaling pathways downstream of the receptor in vivo.36 These are, however, difficult to assess as neither GPVI33,34,37,38 nor CLEC-2 (not shown) can be down-regulated in murine platelets by antibodies in vitro. This indicates that additional signals or a certain environment may be required for this process to occur that is absent in our in vitro assays. It is clear, however, that the Fc part of the antibody or receptor dimerization is not required for CLEC-2 down-regulation in vivo, as Fab fragments are equally efficient compared with the intact IgG.
Monovalent INU1-Fab fragments amplified ADP-induced aggregation, indicating that occupancy of the INU1 binding epitope on CLEC-2 induces subliminal signaling independently of receptor clustering. This notion may also be supported by the observation that INU1-Fab induced transient thrombocytopenia to the same extent as the intact IgG. It is not clear whether the transient drop in platelet counts is mechanistically linked to the loss of CLEC-2, but it is feasible to speculate that INU1-opsonized platelets might become (partially) activated and transiently trapped in the reticuloendothelial system where the actual loss of CLEC-2 occurs. In the case of JAQ-induced GPVI down-regulation, shedding of the receptor is associated with transient thrombocytopenia, whereas internalization/degradation can occur independently thereof.36
Upon INU1 injection (8 μg/g), CLEC-2 was not detectable in platelets for approximately 7 days, although the normal life span of mouse platelets is only approximately 4 to 5 days,39 indicating that the antibody may also affect megakaryocytes. Indeed, INU1 is detectable on megakaryocytes in spleen and bone marrow 90 minutes after antibody injection (data not shown), and preliminary data indicate that a second injection of INU1 on day 6 has no effect on circulating (CLEC-2–deficient) platelets but prolongs the absence of CLEC-2 (data not shown). Similar effects can be seen in JAQ1-treated mice where the “knockout-like” phenotype does, however, last for 2 weeks upon a single injection of 100 μg antibody per mouse.33,37,40 This indicates that INU1 may affect only those megakaryocytes that produce the very next generation of platelets, but further studies will be required to test this hypothesis.
Irrespective of the underlying mechanism, platelets from INU1-treated mice showed a specific loss of CLEC-2 activity that translated into a severe defect in thrombus growth and stabilization under flow conditions in vitro and in vivo (Figures 5–6). Similarly impaired aggregate stabilization can also be seen in mice lacking functional stromal interaction molecule 1 (STIM1), an essential regulator of Ca2+ signaling in platelets.28,41 Such platelets display a selective defect in ITAM/YXXL-dependent activation, indicating that this signaling route is of importance not only for platelet activation on the ECM via GPVI but also for thrombus growth where collagen/GPVI does not play a role.28,41 In light of the data reported here, it is tempting to speculate that the thrombus instability observed in the absence of STIM1 may be caused to a great extent by impaired CLEC-2 signaling. Similarly, the thrombus formation defects seen in mice lacking critical molecules of ITAM-signaling pathway, such as LAT42 or phospholipase Cγ2,43 might also be related to defective CLEC-2 signaling.
Our in vivo studies show that treatment of mice with an antibody against CLEC-2 induces a specific CLEC-2 deficiency in platelets for a prolonged period of time that is associated with profound protection of the animals from occlusive thrombus formation. On the other hand, lack of CLEC-2 is associated with a significant increase in bleeding times (Figure 6D), but compared with bleeding time prolongations induced by integrin αIIbβ3 or GPIbα inhibition,44 the effect is moderate. The high variability in bleeding times most likely reflects a rather mild hemostatic defect based on thrombus instability at the site of the tail wound. Based on this assumption, one may speculate that the lack of CLEC-2 signaling is to a certain extent compensated for by other mechanisms (ADP, TxA2, thrombin, etc) that may vary between individuals, for example, because of slight differences in the injury. In line with this, a similar variability in tail bleeding times has previously been reported for other mouse lines with deficiencies in activatory platelet receptors, such as P2Y145 or the α2A adrenergic receptor.46 Although bleeding times do not allow reliable prediction of a potential bleeding risk,47 one may speculate on the grounds of these results that anti–CLEC-2 therapy might be associated with a relatively low risk of clinical hemorrhage. CLEC-2–deficient humans have not been reported to date, and it is not clear whether mutations in the CLEC-2 gene affect development in humans or mice. However, it can be anticipated that patients with an acquired CLEC-2 deficiency exist, caused by autoantibody-induced CLEC-2 clearing in platelets as previously reported for GPVI deficiencies.34,48 The identification of such patients might help to define the role of CLEC-2 in the physiology of human platelets.
Taken together, our results demonstrated that CLEC-2 is an essential mediator of platelet activation in vitro and in vivo and that it represents an interesting antithrombotic target that can be functionally inactivated in vivo. These findings may serve as a basis for development of a new generation of powerful, yet safe, agents for prophylaxis and treatment of ischemic cardiovascular events.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Stefanie Hartmann and Juliana Goldmann for excellent technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 688, TP A1 [B.N.] and Sonderforschungsbereich 815, TP A6 [J.E.]).
Authorship
Contribution: F.M., I.H., I.P., M.B., T.V., and M.E. performed experiments and analyzed data; J.E. provided vital reagents and contributed to writing the paper; and B.N. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernhard Nieswandt, Chair of Vascular Medicine, Rudolf Virchow Center, DFG Research Center for Experimental Biomedicine, University Clinic Würzburg, Zinklesweg 10, 97078 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal