Stem cell gene therapy was previously shown to lead to high levels of gene correction and immune reconstitution in chronic granulomatous disease1 and X-linked severe combined immunodeficiency.2 However, genotoxicity of the gammaretroviral vectors resulted in clonal dominance and T-cell leukemia, respectively. In contrast, as described by Cassani and colleagues in this issue of Blood, stable polyclonal long-term gene correction was achieved using retrovirus vectors to transduce CD34+ cells from patients with adenosine deaminase deficient–severe combined immunodeficiency.3
Integrating viral vectors, derived from gammaretroviruses (eg, murine leukemia virus) or lentiviruses (eg, HIV), are generally used to stably express therapeutic genes in highly proliferating cell populations, such as hematopoietic stem cells/hematopoietic progenitor cells (HSCs/HPCs) or mature T lymphocytes. However, retroviral vector integration into the target cell genome can lead to transactivation or disruption of cellular gene expression in the vicinity of the retroviral integration site (RIS) and thereby increase or decrease the cell's fitness. Again, this can result in expansion or contraction of a gene-modified cell clone, leading to clonal dominance and eventually the development of hematologic malignancies, where RIS are generally found near prominent proto-oncogenes. The mechanisms underlying clonal dominance and leukemogenesis driven by retroviral integrations have been studied extensively in mouse models4 and now have been described in several (X-linked severe combined immunodeficiency [X-SCID],2 chronic granulomatous disease [CGD]1 ), but not in all (eg, adenosine deaminase deficient [ADA]–SCID3,5,6 ) stem cell gene therapy trials in which substantial levels of gene correction were achieved.
Cassani et al performed a detailed clonal analysis of patients treated with gene-corrected autologous CD34+ HPC for ADA-SCID.3 Nine of the 10 treated children had immune reconstitution with high levels of gene correction in peripheral blood lymphocytes. The overall gene expression profile as determined by GeneChip analysis in ex vivo bulk T cells 10 to 30 months after transplantation did not differ from healthy controls. Analysis of individual T-cell clones from 2 selected patients revealed a broad clonal diversity with respect to the T-cell receptor (TCR) Vß repertoire and RIS. Although in roughly 20% of the clones genes in the vicinity of the RIS were moderately deregulated, no effect on cellular phenotype or in vitro function was observed.
A drawback of the Cassani study is that the clonal analysis was performed on T cells obtained only 18 months after transplantation.3 In the CGD and X-SCID trials, clonal dominance and leukemia developed somewhat later. However, a recent additional report on the ADA-SCID trial provides long-term follow-up data (up to 8 years) and shows that all patients maintained this highly diverse TCR Vß repertoire in peripheral blood T cells.6 Moreover, none of the patients has developed leukemia. Therefore, together with this second report,6 the Cassani study provides sound evidence that the stem cell gene therapy protocol used to treat children with ADA-SCID is safe and does not lead to clonal dominance or hematologic malignancy.
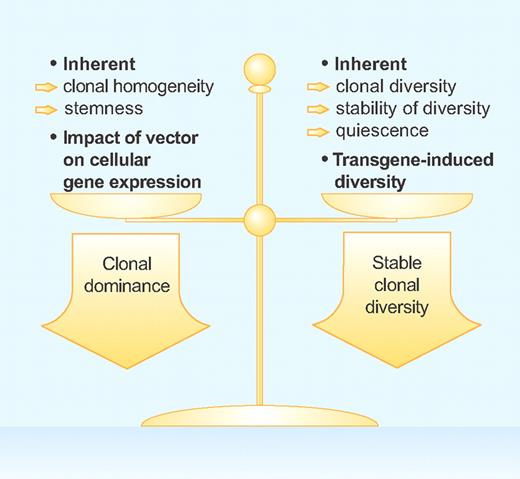
To design safe stem cell gene therapy protocols in the future, we must understand the exact conditions that favor clonal dominance as in the X-SCID and CGD trials and those that stabilize clonal diversity as in the ADA-SCID children. The basic factors that favor one condition over the other are summarized in the figure. The vector design is crucial and a vector configuration that has a strong impact on the expression of neighboring genes will favor clonal dominance. In addition, cell populations that have an inherent ability to self-renew (ie, stemness), such as HPCs and hepatocytes, are expected to more easily expand and give rise to dominant clones when mutagenized.4,7 On the other hand, dominant clones are obviously not likely to arise from highly differentiated cell populations with little inherent turnover.
Therapeutic gene transfer with integrating vectors can result in the outgrowth of dominant cells clones and the development of malignancies or in “peaceful coexistence” of gene-modified cell clones and stable clonal diversity. The factors that favor each of these 2 options are listed and discussed in the text in more detail. Professional illustration by Paulette Dennis.
Therapeutic gene transfer with integrating vectors can result in the outgrowth of dominant cells clones and the development of malignancies or in “peaceful coexistence” of gene-modified cell clones and stable clonal diversity. The factors that favor each of these 2 options are listed and discussed in the text in more detail. Professional illustration by Paulette Dennis.
In addition, Cassani et al postulate that the expression of the ADA transgene may have provided a stronger survival advantage than insertional activation of growth-promoting cellular genes.3 The level of expression of ADA, which is initially thought to have been highly diverse and slightly below levels found in healthy controls, thus becomes the major determinant of cell survival, whereby cell clones with high ADA expression are expected to prevail. Indeed, RIS that supported high levels of ADA expression, rather than clones that overexpress growth-promoting genes, dominated in the T lymphocytes of the ADA-SCID patients. Interestingly, in the X-SCID trial, transgene expression was homogeneous at levels comparable to those of healthy controls.2 In these patients, the level of transgene expression is therefore not expected to be the main determinant for survival.
An interesting aspect not discussed by the authors is the impact of the inherent diversity of a cell population in conjunction with the intrinsic mechanisms that preserve clonal diversity. This is particularly pronounced in mature T cells. The highly diverse T-cell repertoire is regularly perturbed during antigen encounter. However, homeostatic forces, most likely involving competition of T-cell clones for their cognate MHC/self-peptide complexes, preserve TCR diversity.8 These homeostatic mechanisms may well have additionally stabilized the clonal diversity of the mature T lymphocytes in the ADA-SCID patients, especially as an unexpectedly high number of RIS was found near potential proto-oncogenes in the CD34+ progenitors cells analyzed ex vivo 47 months after transplantation.5 Clonal homeostasis may also be the reason that retroviral gene transfer into mature T lymphocytes has never been reported to lead to clonal dominance or leukemia/lymphoma.8
In summary, the report of Cassani et al is expected to promote the development of novel stem cell gene therapy strategies, as it shows that genetic modification of HPC does not necessarily result in clonal dominance and hematologic malignancy, but can be safe and thus remains a promising option if the target disease, tools, and technologies are selected carefully.3 Future efforts should focus on understanding the mechanisms that determine whether therapeutic gene transfer using integrating vectors will result in peaceful clonal coexistence and stable clonal diversity or clonal dominance and malignant transformation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal