In this issue of Blood, Kleinman and colleagues report that blood components containing low levels of B19V are unlikely to transmit infection, and that screening donated blood with sensitive B19V DNA assays would provide little benefit to blood recipients at a cost of excluding many safe blood components.1
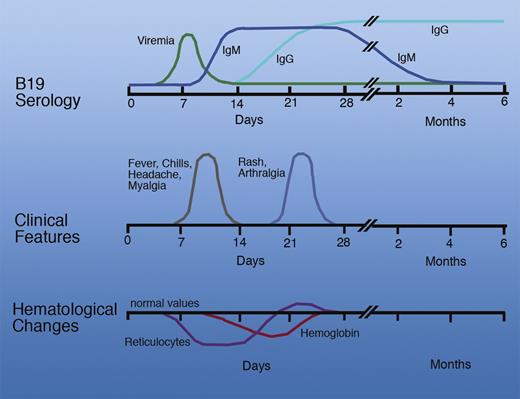
Parvovirus B-19 (B19V), a small, single-stranded, highly infectious DNA virus, has long been associated with a common, relatively benign, droplet-transmitted childhood exanthem, termed “fifth disease” (see figure). B19V, as it is now known, can cause serious illness, including transient aplastic crises in patients with chronic hemolysis, acquired aplastic anemia in immunocompromised patients, and hydrops fetalis when the midtrimester fetus is infected.2 B19V can be transmitted by transfusion.3 The prevalence of B19V DNA detected in donated blood, albeit at low levels, approaches 1%.4 This represents persistent low-level viremia in the presence of specific IgG antibody, the significance of which for transfusion-transmitted disease continues to ignite debate.5 However asymptomatic, newly infected blood donors may develop extremely high levels of B19V, up to 1012 IU/mL. Plasma derivatives, such as clotting factor concentrates, prepared from large plasma pools containing such high-titer units present substantial risk to the recipients. B19V is highly resistant to all commonly used inactivation methods.6 The potential risks seem like sufficient reasons to screen blood donors for B19V. However, low levels of virus in plasma pools do not seem to cause disease, possibly because of neutralization by B19V antibody contributed by other donations in the plasma pool. These observations have led plasma fractionators to test plasma donations (and pools) for levels of B19V that ensure the load of B19V DNA in the manufacturing pools does not exceed 104 IU/mL.7
Time course of events following B19V infection. Professional illustration by Marie Dauenheimer.
Time course of events following B19V infection. Professional illustration by Marie Dauenheimer.
In contrast to the experience with plasma fractions, information regarding B19V transmission by individual blood components has been confined to a handful of case reports. Until now, no studies have been sufficiently robust to calculate the rate of transmission by B19V DNA–positive components or to determine the risk of low levels of viral DNA in these units. In this issue of Blood, Kleinman et al have applied sensitive, carefully validated DNA detection methods to investigate the risk of low to moderate levels of B19V transmitted by single blood components to seronegative susceptible transfusion recipients. These investigators took advantage of a large repository of linked samples representing 13 201 donation specimens and 3575 recipients from 7 geographically dispersed US sites collected at intervals of 6 to 12 months before and after transfusion in a case-control study. The results are reassuring. Of 105 DNA-positive donations and 112 positive components transfused to 107 distinct recipients, no clinical disease related to B19V was observed. Of the 24 serologically susceptible recipients and 42 susceptible controls selected to measure the background rate of new infection, no B19V infections were detected. The large majority of DNA-positive donations had low or very low DNA concentrations. These findings confirm the long-held suspicion that the risk of disease from transfusion-transmitted B19V from single blood components with a low inoculating dose must be extremely low.
These results do not imply that red cells, plasma, and platelets pose zero risk of B19V-related disease. The rate of high-titer DNA (> 106 IU/mL) was still 1:6000. The authors report that these recipients were not likely to have been immunocompromised, and although that fact does not likely affect the rate of viral transmission, it might well reduce the frequency of clinical illness. Furthermore, 6 of the 7 components with the highest DNA concentrations were transfused to serologically nonsusceptible recipients. All DNA-containing components also contained B19V-specific IgG, which may well have played a role in neutralizing free virus. All recipients likely received other blood components containing B19V-specific IgG, and the interval between sample collections is such that evidence of transmission could have been missed unless persistent infection developed. Finally, as the authors emphasize, a transmission rate of 0.0% yields an upper 95% confidence interval of 11.7%, still far lower than what has been reported for HIV and the hepatitis viruses.
This study provides new and important information for making rational decisions regarding screening of donated blood. Although the authors studiously avoid making recommendations regarding the wisdom of screening blood for B19V, they sensibly advise that if such screening were undertaken, sensitivity of the screening test should be adjusted to retain the large number of units that present little if any risk to the patient.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■