Abstract
Vaso-occlusive crisis (VOC) is the primary cause of hospitalization of patients with sickle-cell disease. Treatment mainly consists of intravenous morphine, which has many dose-related side effects. Nonsteroidal antiinflammatory drugs have been proposed to provide pain relief and decrease the need for opioids. Nevertheless, only a few underpowered trials of nonsteroidal antiinflammatory drugs for sickle-cell VOC have been conducted, and conflicting results were reported. We conducted a phase 3, double-blind, randomized, placebo-controlled trial with ketoprofen (300 mg/day for 5 days), a nonselective cyclooxygenase inhibitor, for severe VOC in adults. A total of 66 VOC episodes were included. The primary efficacy outcome was VOC duration. The secondary end points were morphine consumption, pain relief, and treatment failure. Seven VOC episodes in each group were excluded from the analysis because of treatment failures. No significant between-group differences were observed for the primary outcome or the secondary end points. Thus, although ketoprofen was well-tolerated, it had no significant efficacy as treatment of VOC requiring hospitalization. These findings argue against its systematic use in this setting.
Introduction
Pain is the most frequent manifestation of sickle-cell disease (SCD) and is thought to be a consequence of sickle-cell vaso-occlusion. Vaso-occlusive crisis (VOC) is the primary cause of hospitalization of patients with SCD and is among the main causes of death in affected adults.1,2 To date, no specific therapeutic agents have been approved for VOC, and treatment of uncomplicated VOC is merely symptomatic: bed rest, analgesics and hydration. According to American and British recommendations,3,4 severe pain of VOC that requires hospitalization should be treated with intravenous morphine. However, pain relief is not easily obtained during episodes of VOC, and morphine has many dose-related side effects, including sedation, nausea, constipation, pruritus, and hypoventilation. In addition, it might contribute to the development of acute chest syndrome.5 In light of these potentially severe adverse events, many physicians are still reluctant to administer high doses of potent narcotics, and many medical texts do not provide adequate information for the treatment of pain.6 A drug that would provide pain relief in patients with SCD and decrease the need for narcotics would be of obvious benefit.
According to the results of 2 trials,7,8 corticosteroids had a potentially beneficial effect on VOC by reducing the duration of analgesics, but corticosteroids have been shown to induce recurrent pain episodes.7-11 Pilot trials with inhaled nitric oxide12 and poloxamer 18813 yielded promising results but were experimental, and those treatments are not yet available for routine use.
Nonsteroidal antiinflammatory drugs (NSAIDs) are an effective treatment to decrease surgical and posttraumatic pain.14-21 NSAIDs and opioid analgesics have a synergic effect,22 thereby achieving better pain control and an opioid-sparing effect with fewer adverse events in those settings. Therefore, the use of NSAIDs also could be a good strategy for the treatment of VOC in patients with SCD. Furthermore, increased inflammation markers during VOC, including prostaglandin,23,24 argue for the use of NSAIDs. In an American Society of Hematology publication, Lottenberg and Hassell,25 who applied an evidence-based approach to the treatment of adults with SCD, concluded that NSAIDs may be beneficial for patients without contraindication but only at an evidence level of III. The Cochrane database systematic review on SCD pain management concluded that “there is some evidence that parenteral NSAIDs may be beneficial but studies have been underpowered.”26 Finally, the British guidelines4,27 proposed the use of NSAIDs to control mild-to-moderate pain and severe pain in combination with opioids, and several SCD therapeutic guidelines3,26,28 also have suggested NSAID use.
However, only 3 studies of adults and 1 of children compared NSAIDs versus placebo in the context of rescue analgesia with strong opioids.29-32 Those studies included small numbers of patients and obtained conflicting results. Moreover, the use of NSAIDs in patients with SCD raises other concerns. These agents often are responsible for adverse events that affect the kidney.33 Notably, the SCD population already experiences high rates of kidney complications, some chronic and sometimes requiring transplantation,34-43 and NSAIDs have been associated with acute renal failure in patients with SCD.44,45
To resolve these lingering questions and the efficacy and safety of NSAIDs in adult VOC requiring hospitalization, we undertook a prospective, double-blind, randomized, controlled trial in which we compared ketoprofen to placebo for adults with SCD and severe VOC requiring hospitalization.
Methods
Study population
This phase 3, monocenter, double-blind, randomized, placebo-controlled trial was conducted from August 2000 to March 2003. Homozygous SCD patients who were at least 15 years of age and had a severe VOC requiring hospitalization in the internal medicine department associated with the Adult Sickle-Cell Referral Center at our university hospital were eligible for inclusion. This study was approved by the local ethics committee (Comité de Protection des Personnes) and was conducted in accordance with the provisions of the Declaration of Helsinki, Good Clinical Practice guidelines, and local laws and regulations. Patients were enrolled after giving their written informed consent.
Severe VOC was defined as pain or tenderness, affecting at least one part of the body, including limbs, ribs, sternum, head (skull), spine, and/or pelvis, that required opioids and was not attributable to other causes. Patients could be enrolled in the trial more than once if their hospitalizations were separated by at least 1 month. Criteria for exclusion were as follows: VOC lasting longer than 72 hours or less than 24 hours; parenteral hydration longer than 24 hours; blood transfusion during the previous month; any NSAID intake during the previous 7 days; pregnancy; a history of drug abuse; hypertension; fever greater than 39°C; leukocyte count greater than 30 × 109/L or less than 4 × 109/L; the presence at inclusion of an acute chest syndrome, defined as the association of 2 criteria among chest pain, radiologic infiltrate and auscultatory abnormality; severe anemia requiring a blood transfusion at inclusion; a psychiatric disorder or progressive visceral disease; and ketoprofen allergy or NSAID contraindication, defined as abnormal renal function (creatininemia > 120 μmol/L) or hepatic insufficiency (prothrombin time < 60%) or peptic ulcer. Because of the increased risk of NSAID side effects, patients who had been treated with 1 of the following drugs the week before enrollment also were excluded: aspirin, valproic acid, macrolides, anti-H2, imidazole, rifampicin, phenobarbital, carbamazepine, phenytoin, heparin, vitamin K antagonist, ticlopidine, lithium, methotrexate, interferon-α, diuretics, and/or antihypertensive agents.
Study protocol
Patients were randomly assigned (1:1) to receive, in conjunction with intravenous morphine, a continuous intravenous infusion of ketoprofen (300 mg/day for 2 days) or physiologic saline as a placebo, with a programmable syringe pump, then 100 mg of oral ketoprofen (100 mg every 8 hours) or placebo for the next 3 days, thereby allowing us to compare ketoprofen with placebo over the course of 5 days. In both groups, adjunctive treatment was standardized, including bed rest, fluid replacement with 5% glucose infusion (50 mL/kg/day, < 3 L), oral alkaline water (1 L/day), folic acid (20 mg/day), and analgesia with morphine and intravenous proparacetamol (acetaminophen, 2 g every 8 hours for 48 hours, then 1 g every 8 hours). Morphine was administered at 0.1 mg/kg every 5 minutes until pain relief was achieved, followed by continuous morphine infusion at an initial dose of 2 mg/hour with repeated pulses until pain was well-controlled. Pulse frequencies and morphine-dose tapering were left to the discretion of the prescriber. Side effects were recorded daily, noted by a treating physician throughout the hospital stay and at the follow-up consultation 14 days after discharge.
Pain was assessed with the visual analog scale (VAS; 0 mm = no pain and 100 mm = worst possible pain) and a categorical pain score (CPS) ranging from 0 to 3 points (0 = no pain or residual pain without need for analgesia; 1 = mild pain, no pain increase upon mobilization; 2 = moderate pain, increased by mobilization; 3 = severe pain with disability), which was used by patients to grade their pain in 7 body sites (the 4 limbs, ribs and sternum, head, and spine and pelvis). VAS was recorded by a nurse every 4 hours, and the CPS was determined every 12 hours by a treating physician. VAS was noted as 0 when the patient was sleeping. For each study day, we recorded the number of pain sites, CPS for each site, the average CPS, and the average VAS.
It was calculated prospectively that 33 patients in each group would be able to detect a 2-day difference in the VOC duration between placebo and ketoprofen groups with 90% power and a 5% α-risk, based on a mean VOC duration of 5 days in a prospective, controlled trial13 and our clinical practice experience.
Response and toxicity criteria
The primary efficacy outcome was VOC duration from inclusion (hours). VOC was considered terminated when at least 3 of the following 4 criteria were met: absence of fever for 8 hours; absence of pain progression and no requirement of intravenous infusion of opioid analgesics for the last 8 hours; the patient was able to walk or move without pain; or absence of spontaneous pain with a CPS of 1 or less. The success rate was defined as the percentage of VOC terminated without recourse to transfusion or complications.
Secondary efficacy end points were as follows: morphine consumption during VOC; pain intensity evaluated with the VAS and the CPS; and treatment failure, defined as acute chest syndrome or the need for blood transfusion because of severe complication(s), uncontrolled anemia, uncontrolled VOC lasting longer than 5 days, or sepsis.46 Patients in whom treatment failed were secondarily censored; their data on pain were not taken into account for the analysis of the evolution of pain intensity, which was assessed in uncensored patients from day 1 to day 6. Total VAS and total CPS were the averages of the means of daily evaluations. Toxicity and side effects were reported on a standardized case report form.
Statistical analysis
Results are expressed as medians (interquartile range), means, numbers or percentages, as appropriate. The unit of analysis was the VOC. Quantitative parameters were compared between groups by use of a Mann-Whitney U nonparametric test. Categorical data were compared by the use of a χ2 or Fisher exact test where appropriate. The mean VAS and CPS were calculated for each day and were considered to be 0 after patients were discharged.
Two-way analysis of variance was applied to test the evolution (repeated measures) of VAS and CPS according to therapeutic group. Treatment failure and time to success were calculated according to the Kaplan-Meier method, and the curves were compared by the use of a log-rank test between the 2 treatment groups. P values of .05 or less were considered significant.
Results
Patients
A total of 102 patients with SCD were hospitalized for VOC during the trial; 36 of them had exclusion criteria, which are detailed in Figure 1. A total of 54 consecutive patients (34 men, 20 women) were included for 66 VOC episodes. Randomization assigned 33 patients each to receive ketoprofen or the placebo. The characteristics of the 2 groups at study entry were comparable (Table 1).
Characteristics of sickle-cell disease vaso-occlusive crises (VOCs) at inclusion, therapeutic impact, and adverse events as a function of the treatment-group assignment
| Characteristic . | Placebo group . | Ketoprofen group . |
|---|---|---|
| At inclusion, n | 33 | 33 |
| Age, y | 27 ± 7* | 26 ± 7 |
| Body mass index | 21 ± 3 | 21 ± 2 |
| VOC duration before inclusion, hours | 31 ± 13 | 29 ± 15 |
| Morphine dose before inclusion, mg | 44.2 ± 37 | 43.7 ± 32 |
| VAS score, mm | 71 ± 16 | 73 ± 16 |
| Categorical pain score, points | 5.7 ± 3.6 | 5.6 ± 2.9 |
| Number of pain sites | 2.5 ± 1.5 | 2.5 ± 1.3 |
| Maximal pain score for 1 site | 2.5 ± 0.6 | 2.6 ± 0.5 |
| Leukocyte count, 109/L | 14.4 ± 3.7 | 14.8 ± 4.2 |
| Hemoglobin value, g/dL | 9.2 ± 1.3 | 9.5 ± 1.1 |
| Platelet count, 109/L | 393.7 ± 102 | 373.9 ± 128 |
| Lactate dehydrogenase, IU/L | 357 ± 127 | 413 ± 190 |
| C-reactive protein, g/L | 63 ± 48 | 64 ± 69 |
| Treatment impact, n | 26† | 26† |
| Duration of VOC, median (IQR), h | 50 (36-103) | 51 (35.5-87) |
| Morphine dose, median (IQR), mg | 88 (52.5-262.5) | 110 (46-195) |
| Total CPS, median (IQR)‡ | 0.4 (0.2-0.7) | 0.4 (0.2-0.7) |
| Total VAS score, median (IQR), mm‡ | 9.6 (5.8-33.2) | 12.6 (4.8-23.2) |
| Adverse events, n | 33 | 33 |
| Abdominal pain | 1 | 0 |
| Infection (urinary tract and bronchitis) | 0 | 2 |
| Constipation | 2 | 0 |
| Epigastralgia | 2 | 1 |
| Facial edema | 1 | 0 |
| Fever | 5 | 5 |
| Hepatic cytolysis | 1 | 0 |
| Myocardial repolarization abnormality | 0 | 1 |
| Nausea/vomiting | 3 | 4 |
| Pruritus | 2 | 2 |
| Somnolence | 1 | 0 |
| Tachycardia | 1 | 0 |
| Urinary retention | 0 | 1 |
| Total of adverse events | 19 | 16 |
| Characteristic . | Placebo group . | Ketoprofen group . |
|---|---|---|
| At inclusion, n | 33 | 33 |
| Age, y | 27 ± 7* | 26 ± 7 |
| Body mass index | 21 ± 3 | 21 ± 2 |
| VOC duration before inclusion, hours | 31 ± 13 | 29 ± 15 |
| Morphine dose before inclusion, mg | 44.2 ± 37 | 43.7 ± 32 |
| VAS score, mm | 71 ± 16 | 73 ± 16 |
| Categorical pain score, points | 5.7 ± 3.6 | 5.6 ± 2.9 |
| Number of pain sites | 2.5 ± 1.5 | 2.5 ± 1.3 |
| Maximal pain score for 1 site | 2.5 ± 0.6 | 2.6 ± 0.5 |
| Leukocyte count, 109/L | 14.4 ± 3.7 | 14.8 ± 4.2 |
| Hemoglobin value, g/dL | 9.2 ± 1.3 | 9.5 ± 1.1 |
| Platelet count, 109/L | 393.7 ± 102 | 373.9 ± 128 |
| Lactate dehydrogenase, IU/L | 357 ± 127 | 413 ± 190 |
| C-reactive protein, g/L | 63 ± 48 | 64 ± 69 |
| Treatment impact, n | 26† | 26† |
| Duration of VOC, median (IQR), h | 50 (36-103) | 51 (35.5-87) |
| Morphine dose, median (IQR), mg | 88 (52.5-262.5) | 110 (46-195) |
| Total CPS, median (IQR)‡ | 0.4 (0.2-0.7) | 0.4 (0.2-0.7) |
| Total VAS score, median (IQR), mm‡ | 9.6 (5.8-33.2) | 12.6 (4.8-23.2) |
| Adverse events, n | 33 | 33 |
| Abdominal pain | 1 | 0 |
| Infection (urinary tract and bronchitis) | 0 | 2 |
| Constipation | 2 | 0 |
| Epigastralgia | 2 | 1 |
| Facial edema | 1 | 0 |
| Fever | 5 | 5 |
| Hepatic cytolysis | 1 | 0 |
| Myocardial repolarization abnormality | 0 | 1 |
| Nausea/vomiting | 3 | 4 |
| Pruritus | 2 | 2 |
| Somnolence | 1 | 0 |
| Tachycardia | 1 | 0 |
| Urinary retention | 0 | 1 |
| Total of adverse events | 19 | 16 |
VAS indicates visual analog scale; CPS, categorical pain score; and IQR, interquartile range.
No significant differences between treatment groups and no trends toward significance were found.
Values are means ± standard deviation.
Treatment failures (8 blood transfusions, 5 acute chest syndromes, and 1 sepsis) led to the exclusion of 7 patients in each group from the analysis.
Total VAS and total CPS are the averages of the patients' daily mean values for the 2 scores.
Primary outcome
Seven VOCs in each group were excluded from the analysis because of treatment failures, suggesting that the prevention of acute chest syndrome or need for blood transfusions as the result of ketoprofen administration is not supported by evidence (Figure 2A). For the 52 assessable VOCs, no significant difference (P = .61) was found for the primary outcome, with comparable median durations for the 2 groups (Table 1). The day-by-day success rates were also comparable for the 2 groups (Figure 2B).
Rates of SCD VOC treatment failures and successes as a function of treatment group. (A) Failure rate was defined as development of an acute chest syndrome or the need for blood transfusion because of a severe complication. (B) Success rate was defined as the percentage of VOC terminated without recourse to transfusion or complications.
Rates of SCD VOC treatment failures and successes as a function of treatment group. (A) Failure rate was defined as development of an acute chest syndrome or the need for blood transfusion because of a severe complication. (B) Success rate was defined as the percentage of VOC terminated without recourse to transfusion or complications.
Secondary end points
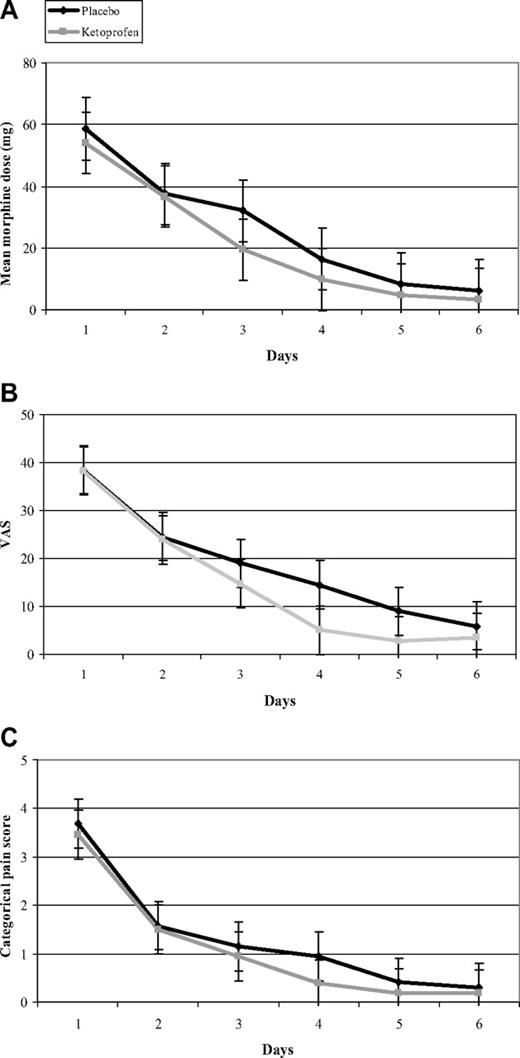
The administration of ketoprofen did not achieve any morphine-sparing or pain-intensity relief. Median total morphine consumption during the study was similar for the 2 groups (P = .64; Table 1), and we did not find any significant between-group difference (Figure 3A) in our day-by-day analysis. In addition, pain relief did not differ between the 2 groups, as assessed by the median total VAS (P = .50) and the median total CPS (P = .46). In addition, our day-by-day analysis of the mean of daily VAS and mean of daily CPS showed the similar trajectories of their curves (Figure 3B-C). Among the 52 of 66 VOCs discharged after VOC termination, 4 ketoprofen-group and 5 placebo-group patients were readmitted (P = 1).
Day-by-day treatment values. Mean values of morphine dose (A), visual analog score (B), and categorical pain score (C) according to treatment group for the 52 assessable VOCs are shown. No significant between-group differences were observed for the secondary end points.
Day-by-day treatment values. Mean values of morphine dose (A), visual analog score (B), and categorical pain score (C) according to treatment group for the 52 assessable VOCs are shown. No significant between-group differences were observed for the secondary end points.
The types and the frequencies of adverse events were similar for the 2 groups (Table 1). One patient in each group was censored because of sepsis without documented infection, associated with a transfusion for the ketoprofen-group patient.
Discussion
Numerous guidelines indicate that NSAIDs could be a good treatment strategy for SCD VOC, but they were mainly based on expert opinions rather than outcomes observed during clinical trials. Only 4 randomized studies29-32 on NSAIDs have been conducted on VOC, and the results of those trials were contradictory, with the evidence level of NSAID efficacy also low. The authors of 2 adult and 1 pediatric controlled study compared parenteral ketorolac to placebo29,31,32 and another diflunisal versus placebo.30 Two of those studies29,32 concerned single-dose administrations with short follow-up periods (4 and 6 hours, respectively), and no significant differences were found for the average opioid requirement or pain relief. In contrast, Perlin et al31 evaluated 21 hospitalized adults and found a significant one-third reduction of the meperidine dose and a shorter duration of hospitalization for the ketorolac group. However, the 2 groups of patients were not homogeneous, and too few patients were included to obtain sufficient statistical power. Finally, in the study comparing diflunisal versus placebo during 46 VOC,30 the same authors found no differences between the average numbers of hospital days and pain intensities, but only 37 patients were suitable for analysis (1 in the placebo group died, and data were incomplete for 8).
Our study was designed to ensure the capacity to detect a significant shortening of VOC duration and included the largest number of adult SCD patients enrolled in a controlled trial comparing an NSAID and placebo. We did not find any significant differences between the placebo and ketoprofen groups in terms of VOC duration, pain relief, or the morphine dose required. Moreover, C-reactive protein values, determined daily for 16 placebo-group patients and 18 ketoprofen-group patients for 6 consecutive days, did not differ between the 2 groups (P = .38). These findings argue against the use of NSAIDs in adults hospitalized for a VOC, in contrast to the recommendations of published guidelines.3,4,26,28 However, it must be kept in mind that our study focused exclusively on hospitalized patients. It is possible that earlier NSAID administration at home, as of VOC onset, could generate different results. Because the pathophysiology of pain in VOC is not well understood, earlier NSAID administration could perhaps control pain better. However, that possibility must be confirmed by a controlled study. In addition, we cannot assume that ketoprofen is not effective in children; therefore, a pediatric trial should also be undertaken to address this question.
Our objective of a 2-day shortening of VOC duration might seem too ambitious. However, in our opinion, the clinical benefit of using a potentially toxic drug in SCD must be obvious, and the 95% and 90% confidence intervals of the mean VOC duration difference between ketoprofen and placebo groups were −17 to 32 hours and −13 to 28 hours, respectively. Therefore, outcomes would probably be the same even with a goal of only a 1-day shorter episode. A statistical limitation of our study is the post-hoc β-risk of 18%, which is explained by the 7 treatment failures in each group. Thus, had there been a 2-day difference between groups, there is 82% likelihood that we would have found that difference with P less than .05. End-point medians were compared with nonparametric tests because normal distributions could not be assumed for a sample size less than 30, and those data had skewed distributions.
Unlike earlier studies, our trial included a homogeneous and well-selected SCD patient population, hospitalized exclusively in the internal medicine department associated with the Adult Sickle-Cell Referral Center. Pain evaluation was not limited to only the first few hours after NSAID administration and the primary outcome was VOC duration because we think that, from a clinical point of view, it is a more accurate objective. In addition, patients were systematically reevaluated 2 weeks after hospital discharge to detect potential late side effects or relapses, which had been observed with other VOC treatments, such as high-dose methylprednisolone. VAS is commonly used to evaluate the pain intensity. However, because of its subjectivity and the pain heterogeneity during SCD VOC, it might poorly estimate pain caused by SCD. To avoid this risk and to better characterize pain, we also used a CPS, based on 7 body sites, which is more precise in terms of location, and classifies pain intensity by its functional effect (impact of movement). The correlation between VAS and our CPS was excellent (P < .001; Pearson r2 = 0.7).
We administered NSAID parenterally at the beginning of VOC to overcome the possible inferiority of the oral formulation,47,48 and used a continuous infusion to obtain stable plasma ketoprofen levels. We considered that the absence of any benefit with an intravenous NSAID would mean that oral NSAIDs also would be ineffective. Continuous infusion of an NSAID, including ketoprofen, has been reported to be as effective as intermittent administration49-51 and excludes the bias caused by the differences between the time of injecting the pulse and the time when pain scores are noted. We chose ketoprofen because its efficacy has been proven in many other pain settings.14,15,17,19-21,51-54 Since its marketing in 1973 in Europe, ketoprofen, which belongs to the propionic acid class of NSAIDs, has been widely used in France and Europe. It is, like most NSAIDs (eg, ketorolac), a nonselective cyclooxygenase inhibitor.
According to guidelines for VOC therapy and for ethical reasons, morphine was rapidly combined with NSAID or placebo, in agreement with recommendations for opioid use to treat VOC.6 In addition, a synergistic effect had been demonstrated between NSAID and narcotic22 in patients with postoperative pain. It must be remembered that our patients very rapidly received morphine and the cyclooxygenase inhibitor paracetamol along with the assigned treatment, perhaps masking an effect of the NSAID alone. Indeed, in developing countries, where morphine is not readily available, VOCs often are treated with antiinflammatory drugs alone.55-57
Ketoprofen was well-tolerated and was not associated with more adverse events than the placebo. In addition, most of the adverse effects noted were attributable to morphine use rather than the NSAID, such as nausea, constipation, pruritus, and urine retention. However, it must be highlighted that our patients were carefully selected and did not have any NSAID contraindications, such as peptic ulcer or renal insufficiency. We cannot affirm that NSAID would be well tolerated in unselected SCD patients.
In conclusion, on the basis of our trial results and despite its being well-tolerated, ketoprofen had no significant efficacy in the treatment of SCD VOC requiring hospitalization. These results do not support its systemic use in this precise setting.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Janet Jacobson for editorial assistance.
Inserm was the trial promoter.
Authorship
Contribution: P.B. analyzed and interpreted data, conducted research, and wrote the manuscript; T.E.M. conducted research and collected data; F.R.-T. participated in research design and performed statistical analyses; A.H., A.S., B.R., V.N., D.B., and M.M. participated in research; F.G. designed research; and B.G. designed research, carried out research, collected data, and analyzed and interpreted data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bertrand Godeau, Service de Médecine Interne, Hôpital Henri-Mondor, 51 avenue du Maréchal-de-Lattre-de-Tassigny, 94010 Créteil Cedex, France; e-mail: bertrand.godeau@hmn.aphp.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal