Abstract
Romiplostim is a thrombopoietin receptor agonist that increases platelet counts in patients with chronic immune thrombocytopenia (ITP). Thrombopoietin receptor agonists are reported to increase the risk for reticulin fiber deposition within bone marrow. This report describes bone marrow findings from romiplostim-treated rats, a retrospective analysis of reticulin observed in romiplostim ITP clinical trials, and a prospective clinical study of the effects of romiplostim on bone marrow morphology. In rats, romiplostim produced a dose-dependent increase in bone marrow fibrosis that resolved after treatment withdrawal. Of 271 ITP patients in romiplostim clinical trials, 10 were reported to have reticulin deposition; reticulin grade was increased in 4 of 5 patients with both pretreatment and on-treatment bone marrow results. Reticulin grade often decreased soon after romiplostim discontinuation. In the prospective study, reticulin grade during romiplostim treatment remained within the normal range for all patients and was increased in only 1 of 6 patients with pretreatment and on-treatment bone marrow results. This report suggests that romiplostim produces reversible, dose-dependent bone marrow changes in rats and produces modest increases in bone marrow reticulin in some ITP patients that decrease when therapy is discontinued. These studies were registered at www.clinicaltrials.gov as #NCT00102323, #NCT00102336, #NCT00861224, and #NCT00116688.
Introduction
Chronic immune thrombocytopenia (ITP) is an autoimmune disorder characterized by antibody-mediated platelet destruction1-6 and insufficient platelet production.7,8 Romiplostim is a novel thrombopoiesis-stimulating protein (peptibody) that mimics endogenous thrombopoietin (TPO) by binding to the human TPO receptor, leading to activation of downstream signaling pathways that results in increased platelet production.9,10 Romiplostim has been shown to improve platelet counts during both short- and long-term use in adult patients with chronic ITP.9,11,12 In both preclinical studies and clinical trials with TPO mimetics (romiplostim and eltrombopag) in patients with ITP, a small number of cases of bone marrow fibrosis (reticulin and collagen) have been noted.12-15
Reticulin is a normal component of the bone marrow stroma and can be detected with a reticulin stain in 73% to 81% of healthy subjects.16-19 Increased reticulin staining (reticulin fibrosis) is associated with many benign conditions as well as some malignant diseases.19 Fully reversible increases in reticulin have also been seen in patients with acute myeloid leukemia who were treated with recombinant human TPO to aid postchemotherapy platelet recovery.20 In contrast, increased trichrome staining (collagen fibrosis) is less common, less likely to be reversible, and is primarily associated with myeloproliferative diseases and metastatic solid tumors.19
Bone marrow fibrosis (reticulin and collagen) can be quantified using several different grading schemes.16,17,21,22 In a study of bone marrow biopsy specimens from healthy subjects analyzed using the modified Bauermeister scheme, which grades the degree of reticulin/collagen on a scale from 0 to 4,16,17,19,22 19% were found to be grade 0 (no reticulin fibers), 76% were grade 1 (occasional fine fibers), 5% were grade 2 (fine fibers throughout section), and none was grade 3 (diffuse fiber network, scattered coarse fibers) or grade 4 (same as grade 3 but with areas of collagenization [positive trichrome stain]).16 In a retrospective analysis of bone marrow biopsies from 40 patients with ITP (no prior treatment with TPO mimetics), the distribution of reticulin grades was similar to healthy subjects: 38% were grade 0, 50% were grade 1, 13% were grade 2, and none was grades 3 or 4.23
The purpose of the present report is to provide a detailed description of the currently available results regarding the relationship between bone marrow fibrosis (reticulin and collagen) and romiplostim. These results include the bone marrow findings from an animal study, reports of bone marrow reticulin in clinical trials of romiplostim in patients with chronic ITP, and the results of a small prospective clinical study of the effects of romiplostim on bone marrow morphology in chronic ITP patients.
Methods
Animal study
The toxicity and toxicokinetics of romiplostim were evaluated in an animal study conducted according to a protocol approved by the Institutional Animal Care and Use Committee at Covance that satisfied all Association for Assessment and Accreditation of Laboratory Animal Care International specifications. Male and female Crl:CD(SD)IGS BR rats (Charles River Laboratories) 45 to 50 days of age were supplied with food and water ad libitum. An equal number of male and female animals were distributed among 4 treatment groups (20-30 animals per group) and were balanced with respect to weight. Animals were given a subcutaneous injection once daily of 10, 30, or 100 μg/kg romiplostim or placebo, 3 days a week for 4 weeks (ie, on days 1, 3, 5, 8, 10, 12, 15, 17, 19, 22, 24, and 26). Injections were rotated among 3 subcutaneous sites. Eight to 10 animals of each gender and group were killed on day 29 (week 5) to examine the effects of romiplostim treatment on bone marrow morphology. To assess the persistence, reversibility, or delayed onset of romiplostim effects, 20 animals were treated from weeks 1 to 4 with placebo or 100 μg/kg romiplostim subcutaneously (10 animals/group) and then maintained without further dosing for 4 more weeks until death on day 58 (week 9).
Femurs and sternum from each animal were embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined microscopically. In addition, smears were prepared from femoral bone marrow and stained with Wright-Giemsa stain. Trichrome and reticulin staining were not performed; therefore, it was not possible to distinguish between reticulin and collagen fibers. Fibrosis was evaluated at Covance Laboratories in Madison, WI and graded as minimal, slight, moderate, or severe based on the hematoxylin and eosin features. The use of specific stains in addition to hematoxylin and eosin is not essential to evaluate fibrosis in rat bone marrow because of the distinctive morphologic presentation.24-26
Clinical studies
Two sets of bone marrow data were evaluated. The first set included all reports of elevated reticulin identified during a retrospective analysis of all patients who participated in clinical trials of romiplostim in ITP from June 2002 to February 2008 (total of 11 studies).27 The second set included patients who participated in a prospective bone marrow morphology study in which both baseline (pretreatment) and follow-up (on-treatment and posttreatment) samples were taken (www.clinicaltrials.gov; #NCT00861224). The objective of this prospective study was to describe changes from baseline in bone marrow morphology during long-term treatment with romiplostim. The studies received approval from the Institutional Review Board at each participating study site, and participants' informed consent was obtained in accordance with the Declaration of Helsinki.
The prospective bone marrow morphology study was conducted as an ancillary study to 2 phase 3, double-blind, randomized, placebo-controlled, 6-month, clinical trials of romiplostim in patients with ITP (www.clinicaltrials.gov; #NCT00102323 and #NCT00102336) and an open-label extension study of romiplostim in ITP patients who had completed an earlier romiplostim clinical trial (www.clinicaltrials.gov; #NCT00116688). The protocols for the 2 double-blind trials were identical except that 1 enrolled splenectomized patients, whereas the other enrolled nonsplenectomized patients.14 In the double-blind studies, patients were randomized to receive weekly subcutaneous injections of placebo or romiplostim for 24 weeks (romiplostim dose adjusted to maintain platelet counts of 50-200 × 109/L).14 On entering the extension study, patients who had been treated with romiplostim during the previous clinical trial started with the same weekly dose they received at the end of that study. Patients who had received placebo during the previous clinical trial started the extension study with 1 μg/kg per week. Dose adjustments were allowed to achieve a target platelet count of 50 to 250 × 109/L.12
Patient bone marrow assessments
Patients in the prospective study had a baseline bone marrow biopsy that was taken up to 1 year before enrollment and were assigned to have follow-up biopsies taken after 3, 6, and 9 months of romiplostim treatment. Except for those performed as part of the prospective study, bone marrow biopsies were not required during romiplostim clinical trials and were only performed at the investigator's discretion. A biopsy could be obtained for any reason but was recommended if abnormalities in the peripheral blood smear (eg, nucleated red blood cells) were observed or there was loss of treatment response despite increasing doses of romiplostim. In the prospective study, both baseline and follow-up bone marrow samples were sent to a central laboratory for staining and initial assessment.
Bone marrow assessments were conducted by an external panel of expert hematologists and hematopathologists (D.J.K., R.P.H., B.J.B., and G.J.M.). For the retrospective study, assessments were made from aspirate smears, core biopsies, reticulin stains, trichrome stains, and/or written descriptions. The data from the prospective study were available in the form of photomicrographs only; no actual bone marrow slides were provided to the panel for evaluation. Photomicrographs were taken by the central laboratory assigned to the respective clinical study, using the same methodology for each patient, including a subjective assessment of a representative area. Samples sent to the central laboratory were blinded with regard to treatment group.
Reticulin was graded according to a modified Bauermeister scale as follows: 0 (absent), 1 (fine fibers), 2 (diffuse fine fiber network), 3 (diffuse fiber network with scattered coarse fibers), and 4 (areas of collagen on trichrome stain).19 The review of bone marrow samples for all patients was unblinded with regard to treatment assignment and medical history.
Results
Animal study
Romiplostim-treated rats had higher platelet counts than did placebo-treated animals (Table 1). At day 10, mean plus or minus SD platelet counts were 2- to 4-fold higher in romiplostim-treated animals (10 μg/kg, 2481 ± 380 × 109/L; 30 μg/kg, 3438 ± 694 × 109/L; 100 μg/kg, 3749 ± 553 × 109/L) than controls (1108 ± 112 × 109/L), and this difference was also apparent at study week 5 (romiplostim: 10 μg/kg, 2467 ± 1017 × 109/L; 30 μg/kg, 1917 ± 1247 × 109/L; 100 μg/kg, 1981 ± 1008 × 109/L; controls, 1077 ± 81 × 109/L). In animals evaluated after a 4-week posttreatment recovery period (week 9), platelet counts were similar between placebo and romiplostim-treated animals.
Effect of romiplostim on bone marrow in rats
| . | Week 5 (date of death) . | Week 9 (date of death) . | ||||
|---|---|---|---|---|---|---|
| 0 μg/kg romiplostim (n = 20) . | 10 μg/kg romiplostim (n = 18) . | 30 μg/kg romiplostim (n = 20) . | 100 μg/kg romiplostim (n = 17) . | 0 μg/kg romiplostim (n = 10) . | 100 μg/kg romiplostim (n = 10) . | |
| Femur, n (%) | ||||||
| Fibrosis | 0 | 1 (6) | 4 (20) | 9 (53) | 0 | 0 |
| Megakaryocyte hyperplasia | 0 | 9 (50) | 8 (40) | 11 (65) | 0 | 0 |
| Hyperostosis | 0 | 0 | 4 (20) | 12 (71) | 0 | 0 |
| Sternum, n (%) | ||||||
| Fibrosis | 0 | 0 | 1 (5) | 9 (53) | 0 | 0 |
| Megakaryocyte hyperplasia | 0 | 8 (44) | 8 (40) | 10 (59) | 0 | 0 |
| Hyperostosis | 0 | 0 | 0 | 3 (18) | 0 | 0 |
| Mean platelet count, ×109/L | 1077 | 2467 | 1917 | 1981 | 1165 | 1017 |
| . | Week 5 (date of death) . | Week 9 (date of death) . | ||||
|---|---|---|---|---|---|---|
| 0 μg/kg romiplostim (n = 20) . | 10 μg/kg romiplostim (n = 18) . | 30 μg/kg romiplostim (n = 20) . | 100 μg/kg romiplostim (n = 17) . | 0 μg/kg romiplostim (n = 10) . | 100 μg/kg romiplostim (n = 10) . | |
| Femur, n (%) | ||||||
| Fibrosis | 0 | 1 (6) | 4 (20) | 9 (53) | 0 | 0 |
| Megakaryocyte hyperplasia | 0 | 9 (50) | 8 (40) | 11 (65) | 0 | 0 |
| Hyperostosis | 0 | 0 | 4 (20) | 12 (71) | 0 | 0 |
| Sternum, n (%) | ||||||
| Fibrosis | 0 | 0 | 1 (5) | 9 (53) | 0 | 0 |
| Megakaryocyte hyperplasia | 0 | 8 (44) | 8 (40) | 10 (59) | 0 | 0 |
| Hyperostosis | 0 | 0 | 0 | 3 (18) | 0 | 0 |
| Mean platelet count, ×109/L | 1077 | 2467 | 1917 | 1981 | 1165 | 1017 |
Romiplostim or placebo was administered subcutaneously 3 times per week for 4 weeks. Bone marrow samples were taken from animals killed either 3 days after the last dose of romiplostim (week 5) or after a 4-week recovery period (week 9).
By week 5, the mean platelet volume showed a dose-dependent increase from 4.9 plus or minus 0.26 fL in placebo-treated rats to 5.2 plus or minus 0.36 fL, 5.7 plus or minus 0.74 fL, and 6.7 plus or minus 1.87 fL for the 10, 30, and 100 μg/kg romiplostim treatment groups, respectively. Erythrocyte effects included lower red blood cell counts (day 29 only), hemoglobin, and hematocrit; transiently lower mean corpuscular volume; and higher mean corpuscular hemoglobin concentration (day 10 only). The findings for red blood cell count, hemoglobin, and hematocrit were relatively mild. There were no significant between-group differences in white blood cell counts except between the placebo group (9.6 ± 2.1 × 109/L) and both the 100 μg/kg (17.2 ± 3.8 × 109/L) and 30 μg/kg (14.7 ± 3.3 × 109/L) romiplostim groups on day 10 (both P < .001).
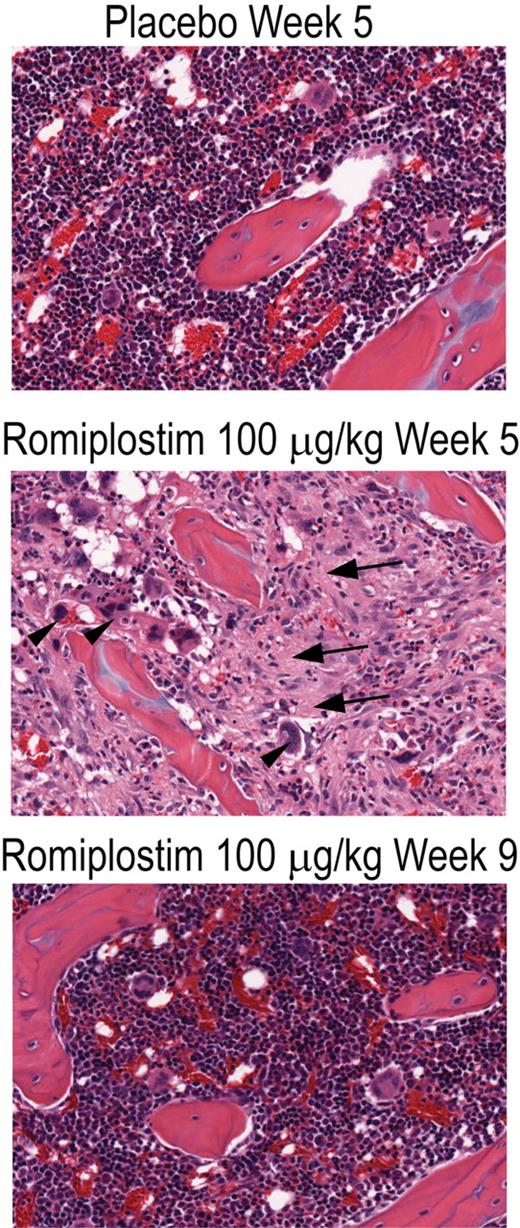
The bone marrow from placebo-treated animals showed no megakaryocyte hyperplasia, hyperostosis, or bone marrow fibrosis (Figure 1; Table 1). Bone marrow from animals in each of the romiplostim treatment groups killed at the end of the 4-week treatment period had greater bone marrow fiber content and hyperostosis than did the bone marrow of control animals (Figure 1), and the extent of these changes was dose-dependent (Table 1). Bone marrow cavities from romiplostim-treated animals exhibited variably increased numbers of megakaryocytes that were somewhat pleomorphic, relative to control animals, ranging from smaller, round cells similar to those in control animals to larger cells with indented or irregular cell-outlines and enlarged nuclei.
Romiplostim produced a dose-dependent increase in bone marrow fibrosis in rats. Photomicrograph of hematoxylin and eosin–stained bone marrow sections (original magnification ×20) of rat femurs from placebo-treated animals (Placebo Week 5), from animals treated for 4 weeks with romiplostim (Romiplostim 100 μg/kg Week 5), and from animals treated for 4 weeks with romiplostim followed by 4 weeks without treatment (Romiplostim 100 μg/kg Week 9). Areas of increased collagen fibrosis (arrows) and increased megakaryocytes (arrowheads) are indicated.
Romiplostim produced a dose-dependent increase in bone marrow fibrosis in rats. Photomicrograph of hematoxylin and eosin–stained bone marrow sections (original magnification ×20) of rat femurs from placebo-treated animals (Placebo Week 5), from animals treated for 4 weeks with romiplostim (Romiplostim 100 μg/kg Week 5), and from animals treated for 4 weeks with romiplostim followed by 4 weeks without treatment (Romiplostim 100 μg/kg Week 9). Areas of increased collagen fibrosis (arrows) and increased megakaryocytes (arrowheads) are indicated.
Fibrosis in the marrow cavities of affected femurs was characterized by fibrous, wavy connective tissue that distorted or replaced the normal architecture, a typical characteristic of collagen fibrosis seen using the hematoxylin and eosin stain (Figure 1). The fibrous stroma was well vascularized and contained abundant fibroblasts. In some cases, the fibrosis occurred as irregular to coalescing foci randomly scattered through the diaphyseal and/or epiphyseal cavities, or as diffuse accumulations, which almost filled the diaphyseal cavity. These foci often contained entrapped megakaryocytes and were often surrounded or infiltrated by large clusters of granulocytes and their precursors. Some of these fibrotic foci also contained closely spaced, narrow, pale eosinophilic bone trabeculae or angular clumps of pale eosinophilic, fibrillar osteoid-like material. At week 5, the proportion of animals with femoral and sternal bone marrow fibrosis was greater in the higher romiplostim dose groups (Table 1). None of the animals evaluated after a 4-week posttreatment recovery period (week 9) had any sign of bone marrow fibrosis, megakaryocyte hyperplasia, or hyperostosis (Table 1).
Retrospective analysis of reticulin observed in romiplostim clinical trials
Between June 2002 and February 2008, a total of 271 ITP patients had been treated with romiplostim in 11 different clinical trials. Other than those required as part of the prospective study, only 11 of these patients had bone marrow biopsies performed. Ten of these biopsies were positive for reticulin and are the subject of this analysis. Biopsies were recommended for specific reasons as outlined in “Patient bone marrow assessments.”
The positive reticulin biopsies were all from 3 romiplostim ITP clinical trials: one of the double-blind, randomized, placebo-controlled studies (www.clinicaltrials.gov; #NCT00102323) and 2 ongoing open-label studies. The open-label studies included an extension study of romiplostim that enrolled patients who had completed a previous clinical trial of romiplostim in ITP (www.clinicaltrials.gov; #NCT00116688) and an individual patient protocol study that provided open label use of romiplostim to refractory ITP patients who did not qualify for other romiplostim clinical trials (www.clinicaltrials.gov; #NCT00508820).
Bone marrow samples from 7 of the 10 patients identified in this retrospective analysis were available for review by the panel, but a full complement of slides (hematoxylin and eosin, reticulin stain, and trichrome stain) was not available for every patient. In 2 cases, reticulin content had to be evaluated on the basis of written descriptions only (cases 7 and 9) and 1 case (case 10) was reported after the external expert panel had met. The latter case was therefore not graded by the panel, and only the assessments provided by the study investigator and the central laboratory are reported here.
Clinical characteristics of the patients with reports of bone marrow reticulin during romiplostim ITP clinical trials are described in Table 2. Most of these patients had been splenectomized and had received a large number of other treatments for ITP before entering a romiplostim study. The maximum dose of romiplostim received ranged from 5 to 18 μg/kg with 6 of 10 patients receiving doses more than 10 μg/kg. The duration of romiplostim exposure before the detection of bone marrow reticulin ranged from 5 to 123 weeks with 9 of 10 patients being treated for 26 weeks or more. Most patients (9 of 10) had experienced increases in platelet counts in response to romiplostim treatment. Three patients had conditions in their baseline medical history that could have confounded the assessment of the cause of their bone marrow reticulin findings (cases 1 and 9 had a cytogenetic abnormality and case 7 had a history of systemic lupus erythematosus).
Clinical characteristics of patients from the retrospective analysis of reports of bone marrow reticulin in romiplostim clinical trials
| Patient ID . | Age, y/sex . | Duration of ITP, y . | Splenectomy . | No. of ITP treatments before romiplostim . | Maximum dose of romiplostim, μg/kg . | Duration of romiplostim exposure, wk* . | Platelet count nearest to bone marrow examination, ×109/L . | Maximum platelet count before report of reticulin, ×109/L . | Relevant medical history . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 40/M | 15 | Yes | 6 | 9 | 5 | 9 | 10 | Bone marrow hypocellularity; chromosomal abnormality (inversion 12) |
| 2 | 27/F | 12 | Yes | 8 | 11 | 40 | 15 | 565 | — |
| 3 | 60/F | 23 | Yes | 8 | 11 | 45 | 25 | 157 | — |
| 4 | 31/M | 2 | Yes | 3 | 18 | 26 | 33 | 523 | — |
| 5 | 53/M | 7 | Yes | 9 | 8 | 35 | 86 | 88 | — |
| 6 | 58/M | 6 | Yes | 8 | 15 | 31 | 58 | 70 | — |
| 7 | 54/F | 3 | No† | 4 | 13 | 27 | 4 | 71 | Systemic lupus erythematosus |
| 8 | 37/M | 3 | Yes | 8 | 9 | 26 | 4 | 221 | — |
| 9 | 63/M | 15 | No | NA | 11 | 32 | 47 | 72 | Clonal hematologic disorder (20q deletion) |
| 10 | 44/F | 8 | Yes | 4 | 5 | 123 | 82 | 728 | — |
| Patient ID . | Age, y/sex . | Duration of ITP, y . | Splenectomy . | No. of ITP treatments before romiplostim . | Maximum dose of romiplostim, μg/kg . | Duration of romiplostim exposure, wk* . | Platelet count nearest to bone marrow examination, ×109/L . | Maximum platelet count before report of reticulin, ×109/L . | Relevant medical history . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 40/M | 15 | Yes | 6 | 9 | 5 | 9 | 10 | Bone marrow hypocellularity; chromosomal abnormality (inversion 12) |
| 2 | 27/F | 12 | Yes | 8 | 11 | 40 | 15 | 565 | — |
| 3 | 60/F | 23 | Yes | 8 | 11 | 45 | 25 | 157 | — |
| 4 | 31/M | 2 | Yes | 3 | 18 | 26 | 33 | 523 | — |
| 5 | 53/M | 7 | Yes | 9 | 8 | 35 | 86 | 88 | — |
| 6 | 58/M | 6 | Yes | 8 | 15 | 31 | 58 | 70 | — |
| 7 | 54/F | 3 | No† | 4 | 13 | 27 | 4 | 71 | Systemic lupus erythematosus |
| 8 | 37/M | 3 | Yes | 8 | 9 | 26 | 4 | 221 | — |
| 9 | 63/M | 15 | No | NA | 11 | 32 | 47 | 72 | Clonal hematologic disorder (20q deletion) |
| 10 | 44/F | 8 | Yes | 4 | 5 | 123 | 82 | 728 | — |
NA indicates not available; and —, not applicable.
Exposure to romiplostim until the time of the first follow-up biopsy.
Patient underwent a splenectomy approximately 1 week after the reticulin-positive biopsy.
The bone marrow findings for all patients with reports of increased bone marrow reticulin during romiplostim ITP clinical trials are summarized in Table 3. Of the 8 patients for whom reticulin grading could be performed by the expert panel, 1 exhibited a peak reticulin grade of 1, 2 attained grade 2, 4 attained grade 2-3 or 3, and 1 attained grade 4 (presence of collagen). In 1 patient for whom only a written description was available, the reticulin was described as moderate (case 9); in the patient who was not evaluated by the external panel (case 10), the reticulin was described by the investigator as mild. An increase in bone marrow reticulin grade was seen in 4 of the 5 patients for whom both baseline and on-treatment bone marrow biopsies were available. In addition to showing increased reticulin, the bone marrow samples from some patients exhibited hypercellularity, emperipolesis, increased megakaryocyte numbers, megakaryocyte clustering, hyperchromatic megakaryocyte nuclei, and nuclear hyperlobation. None showed hyperostosis or increased osteoid. In patients for whom both on-treatment and follow-up biopsies were available, reticulin and the other bone marrow findings often appeared to improve or disappear with time, typically soon after romiplostim was discontinued (Tables 2–3). An example of this can be seen in Figure 2.
Bone marrow findings of patients from the retrospective analysis of reports of bone marrow reticulin in romiplostim clinical trials
| Patient ID . | Reasons for bone marrow biopsy . | Event led to treatment discontinuation, yes/no . | Peripheral blood abnormalities . | Reticulin grade assessment (weeks after initiating romiplostim) . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (months before romiplostim) . | During romiplostim treatment . | After romiplostim discontinuation . | |||||||
| First BM . | Second BM . | Third BM . | First BM . | Second BM . | |||||
| 1 | NA | Yes | Transient increased blood blasts | 0-1, focal 2 (month −4) | 3 (week 5) | — | — | 1-2 (week 17) | — |
| 2 | Vaginal bleeding | No* | — | NA | 2+ (week 40) | — | — | 2+ (week 108) | — |
| 3 | ↑ teardrop cells | Yes | — | NA | 1, focal 2 (week 45) | — | — | — | — |
| 4 | ↑ peripheral nucleated RBCs and blasts | Yes | Transient increased blood blasts | 0 (month −33) | 2-3 (week 26) | — | — | 1-2 (week 34) | 1 (week 46) |
| 5 | Prospective study protocol | No | — | 0-1 (month −6) | 0-1† (week 35) | — | — | — | — |
| 6 | ↑ nucleated RBCs | No | — | 0-1 (month −24) | 1-3‡ (week 31) | 2-3 (week 51) | 1-2 (week 67) | — | — |
| 7 | NA | No | — | NA | — | — | — | 2-3 (week 30) | — |
| 8 | NA | No | — | 1 (month −47) | 4§ (week 26) | — | — | 1-2 (week 38) | — |
| 9 | Splenomegaly | Yes | — | Positive (month −2) | Moderate (week 32) | — | — | — | — |
| 10 | NA | No | — | NA | Mild (week 135) | — | — | — | — |
| Patient ID . | Reasons for bone marrow biopsy . | Event led to treatment discontinuation, yes/no . | Peripheral blood abnormalities . | Reticulin grade assessment (weeks after initiating romiplostim) . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (months before romiplostim) . | During romiplostim treatment . | After romiplostim discontinuation . | |||||||
| First BM . | Second BM . | Third BM . | First BM . | Second BM . | |||||
| 1 | NA | Yes | Transient increased blood blasts | 0-1, focal 2 (month −4) | 3 (week 5) | — | — | 1-2 (week 17) | — |
| 2 | Vaginal bleeding | No* | — | NA | 2+ (week 40) | — | — | 2+ (week 108) | — |
| 3 | ↑ teardrop cells | Yes | — | NA | 1, focal 2 (week 45) | — | — | — | — |
| 4 | ↑ peripheral nucleated RBCs and blasts | Yes | Transient increased blood blasts | 0 (month −33) | 2-3 (week 26) | — | — | 1-2 (week 34) | 1 (week 46) |
| 5 | Prospective study protocol | No | — | 0-1 (month −6) | 0-1† (week 35) | — | — | — | — |
| 6 | ↑ nucleated RBCs | No | — | 0-1 (month −24) | 1-3‡ (week 31) | 2-3 (week 51) | 1-2 (week 67) | — | — |
| 7 | NA | No | — | NA | — | — | — | 2-3 (week 30) | — |
| 8 | NA | No | — | 1 (month −47) | 4§ (week 26) | — | — | 1-2 (week 38) | — |
| 9 | Splenomegaly | Yes | — | Positive (month −2) | Moderate (week 32) | — | — | — | — |
| 10 | NA | No | — | NA | Mild (week 135) | — | — | — | — |
NA indicates not available; BM, bone marrow examination; and —, not applicable.
Discontinued for reason other than reticulin deposition.
This patient was also evaluated in the prospective analysis.
A focal area of possible collagen deposition was seen on trichrome stain that may have been confounded by the presence of crush artifact.
Minimal collagen deposition in this sample; none on follow-up after treatment discontinuation.
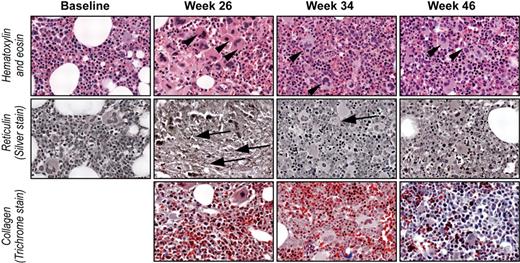
Improvement in bone marrow reticulin after cessation of romiplostim treatment. Photomicrographs (original magnification ×20) of bone marrow biopsies from patient 4 before romiplostim treatment (Baseline), during treatment (Week 26), and after discontinuation of treatment (Week 34 and Week 46). Sections stained with hematoxylin and eosin, reticulin, and trichrome stains as indicated. Megakaryocytes (arrowheads) were increased at baseline, markedly increased by week 26, and returned to baseline number by weeks 34 and 46. Reticulin (arrows) increased from grade 0 at baseline to grade 2/3 by week 26 followed by a decrease to grade 1 by week 46. Collagen fibrosis (trichrome stain) was not detected at any time. Blasts were not increased at any time.
Improvement in bone marrow reticulin after cessation of romiplostim treatment. Photomicrographs (original magnification ×20) of bone marrow biopsies from patient 4 before romiplostim treatment (Baseline), during treatment (Week 26), and after discontinuation of treatment (Week 34 and Week 46). Sections stained with hematoxylin and eosin, reticulin, and trichrome stains as indicated. Megakaryocytes (arrowheads) were increased at baseline, markedly increased by week 26, and returned to baseline number by weeks 34 and 46. Reticulin (arrows) increased from grade 0 at baseline to grade 2/3 by week 26 followed by a decrease to grade 1 by week 46. Collagen fibrosis (trichrome stain) was not detected at any time. Blasts were not increased at any time.
Some patients (all splenectomized) had circulating nucleated erythroid precursors. Increased peripheral blood blasts were observed in 2 patients (both splenectomized), but this was not seen in blood samples taken after discontinuation of romiplostim. Furthermore, neither patient showed increased blasts in the bone marrow or marrow hypercellularity. Trichrome-stained samples were available for 7 patients. In 1 of these patients (case 8), minimal collagen deposition was observed during romiplostim treatment but was not seen in a follow-up biopsy taken after treatment discontinuation; in another of these patients (case 6), the biopsy contained a focal area of possible collagen deposition, but the expert panel could not reach a consensus on the nature of the finding. Neither patient subsequently showed clinical features of any myeloproliferative disorder; follow-up periods were 3 months after study discontinuation for the first patient and 30 months on-study up to the data cut-off for the second patient.
Prospective study of the effects of romiplostim on bone marrow morphology
The prospective study evaluated patients who enrolled between August 2005 and October 2006. The clinical characteristics of patients from the prospective study are described in Table 4. Most of these patients had been splenectomized and had received several prior treatments for ITP before entering the study. The maximum dose of romiplostim ranged from 2 to 10 μg/kg with 7 of 10 patients receiving doses less than or equal to 5 μg/kg. The duration of romiplostim exposure ranged from 9 to 37 weeks. The 4 patients treated with placebo in the phase 3 studies before receiving romiplostim in the extension study were treated for a median of 89 days (range, 83-98 days); the 5 patients treated with romiplostim in both the phase 3 and extension studies were treated for a median of 251 days (range, 63-259 days). All of the patients in the prospective study were identified as treatment responders (platelet count rose to > 50 × 109/L) during either double-blind or open-label treatment (or both) with romiplostim. Three patients had other medical conditions in their baseline medical history that might confound an assessment of their bone marrow findings (case 13, HIV infection; case 16, B-cell lymphoma; and case 19, multiple myeloma).
Clinical characteristics of patients from prospective study of romiplostim on bone marrow morphology
| Patient ID . | Age, y/sex . | Duration of ITP, y . | Splenectomy . | No. of ITP treatments before romiplostim . | Maximum dose of romiplostim, μg/kg . | Duration of romiplostim exposure, wk* . | Platelet count nearest to bone marrow examination, ×109/L . | Maximum platelet count before report of reticulin, ×109/L . | Relevant medical history . |
|---|---|---|---|---|---|---|---|---|---|
| 11 | 70/F | 31 | Yes | 4 | 7 | 14 | 53 | 88 | — |
| 12 | 43/F | 20 | Yes | 5 | 2 | 35 | 80 | 132 | — |
| 13 | 42/F | 6 | Yes | 5 | 4 | 12 | 36 | 108 | HIV infection† |
| 14 | 55/F | 6 | Yes | 6 | 3 | 36 | 93 | 182 | — |
| 15 | 83/F | 13 | No | 2 | 2 | 12 | 137 | 235 | — |
| 16 | 48/F | 0.1 | No | 7 | 4 | 9 | 170 | 281 | Lymphadenopathy, bone marrow lymphoid aggregates†; B-cell lymphoma‡ |
| 5 | 53/M | 7 | Yes | 9 | 7 | 35 | 86 | 105 | — |
| 17 | 46/F | 25 | Yes | 3 | 5 | 37 | 119 | 179 | — |
| 18 | 64/M | 15 | Yes | 5 | 3 | 37 | 139 | 374 | — |
| 19 | 88/F | 12 | No | 5 | 10 | 13 | 138 | 208 | Anemia†; multiple myeloma‡ |
| Patient ID . | Age, y/sex . | Duration of ITP, y . | Splenectomy . | No. of ITP treatments before romiplostim . | Maximum dose of romiplostim, μg/kg . | Duration of romiplostim exposure, wk* . | Platelet count nearest to bone marrow examination, ×109/L . | Maximum platelet count before report of reticulin, ×109/L . | Relevant medical history . |
|---|---|---|---|---|---|---|---|---|---|
| 11 | 70/F | 31 | Yes | 4 | 7 | 14 | 53 | 88 | — |
| 12 | 43/F | 20 | Yes | 5 | 2 | 35 | 80 | 132 | — |
| 13 | 42/F | 6 | Yes | 5 | 4 | 12 | 36 | 108 | HIV infection† |
| 14 | 55/F | 6 | Yes | 6 | 3 | 36 | 93 | 182 | — |
| 15 | 83/F | 13 | No | 2 | 2 | 12 | 137 | 235 | — |
| 16 | 48/F | 0.1 | No | 7 | 4 | 9 | 170 | 281 | Lymphadenopathy, bone marrow lymphoid aggregates†; B-cell lymphoma‡ |
| 5 | 53/M | 7 | Yes | 9 | 7 | 35 | 86 | 105 | — |
| 17 | 46/F | 25 | Yes | 3 | 5 | 37 | 119 | 179 | — |
| 18 | 64/M | 15 | Yes | 5 | 3 | 37 | 139 | 374 | — |
| 19 | 88/F | 12 | No | 5 | 10 | 13 | 138 | 208 | Anemia†; multiple myeloma‡ |
—, not applicable.
Exposure to romiplostim until the time of the first follow-up biopsy.
Diagnosed pretreatment.
Diagnosed during phase 3 study.
The bone marrow findings for the prospective study are summarized in Table 5. The protocol required that all patients have bone marrow examinations both before and during romiplostim treatment, but not all marrow samples were of good enough quality to be evaluable by hematoxylin and eosin, reticulin, and trichrome staining. Except for the 1 patient who developed a B-cell lymphoma during the study (case 16), bone marrow cellularity was normal at baseline and did not change with romiplostim treatment. Of the 8 bone marrow samples with an adequate reticulin stain at baseline, all showed a normal reticulin grade of 0-1. Of the 8 bone marrow samples with an adequate reticulin stain obtained after romiplostim treatment of up to 8.6 months, only 2 had reticulin greater than grade 0-1. One of these patients (case 16) had extensive marrow infiltration (95% of the marrow cavity) by B-cell lymphoma (a known cause of increased reticulin formation) and grade 2 reticulin after 9 weeks of romiplostim treatment, but no evaluable baseline reticulin-stained bone marrow sample for comparison. Only 1 patient (case 11) had a clearly documented increase in reticulin between baseline and on treatment, although the amount of reticulin seen on treatment was still within the normal range (Table 5; Figure 3). Since the cutoff date for this report, the patient has continued on romiplostim for an additional 68 weeks with a median platelet count of 99 × 109/L and no further reports of blood or bone marrow abnormalities.
Bone marrow findings of patients from prospective study of romiplostim on bone marrow morphology
| Patient ID . | Cellularity, percentage . | Megakaryocytes per high power field . | Reticulin grade assessment . | |||
|---|---|---|---|---|---|---|
| Baseline . | On treatment . | Baseline . | On treatment . | Baseline . | On treatment . | |
| 11 | 40 | 60 | 5.3 | 10.6 | 0-1 | 1-2 |
| 12 | 60 | 50 | 5.6 | 8.8 | 0-1 | 0-1 |
| 13 | 50 | 50 | 7.0 | 5.8 | 0-1 | 1 |
| 14 | 30 | 25 | 1.7 | 2.7 | 0-1 | 0-1 |
| 15 | 25 | 50 | 3.0 | 2.7 | 0-1 | 0-1 |
| 16 | 50 | > 95 | 2.5 | 0.1 | NE | 2 |
| 5 | 60 | 60 | 12.0 | 21.1 | 0-1 | 0-1 |
| 17 | 50 | NA | 2.7 | NA | 0-1 | NE |
| 18 | 50 | 50 | 6.9 | 6.5 | NE | 1 |
| 19 | 60 | 75 | 3.8 | 12.0 | 0-1 | NE |
| Patient ID . | Cellularity, percentage . | Megakaryocytes per high power field . | Reticulin grade assessment . | |||
|---|---|---|---|---|---|---|
| Baseline . | On treatment . | Baseline . | On treatment . | Baseline . | On treatment . | |
| 11 | 40 | 60 | 5.3 | 10.6 | 0-1 | 1-2 |
| 12 | 60 | 50 | 5.6 | 8.8 | 0-1 | 0-1 |
| 13 | 50 | 50 | 7.0 | 5.8 | 0-1 | 1 |
| 14 | 30 | 25 | 1.7 | 2.7 | 0-1 | 0-1 |
| 15 | 25 | 50 | 3.0 | 2.7 | 0-1 | 0-1 |
| 16 | 50 | > 95 | 2.5 | 0.1 | NE | 2 |
| 5 | 60 | 60 | 12.0 | 21.1 | 0-1 | 0-1 |
| 17 | 50 | NA | 2.7 | NA | 0-1 | NE |
| 18 | 50 | 50 | 6.9 | 6.5 | NE | 1 |
| 19 | 60 | 75 | 3.8 | 12.0 | 0-1 | NE |
NE indicates specimen not evaluable for reticulin staining; and NA, not available.
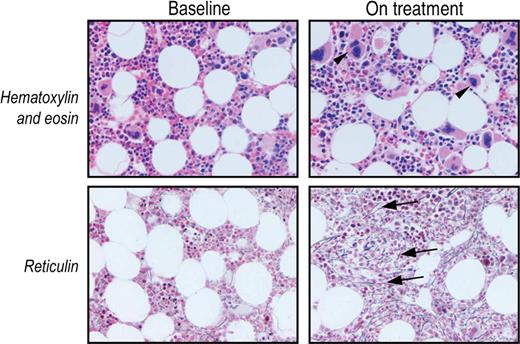
Bone marrow reticulin increased in one romiplostim-treated patient in the prospective study. Photomicrograph (original magnification ×20) of patient 11 before (Baseline) and after 3.2 months of romiplostim treatment (On treatment). The on-treatment bone marrow sample exhibited an increased number of megakaryocytes (arrowheads) and reticulin (grade 1-2; arrows) but no increase in cellularity.
Bone marrow reticulin increased in one romiplostim-treated patient in the prospective study. Photomicrograph (original magnification ×20) of patient 11 before (Baseline) and after 3.2 months of romiplostim treatment (On treatment). The on-treatment bone marrow sample exhibited an increased number of megakaryocytes (arrowheads) and reticulin (grade 1-2; arrows) but no increase in cellularity.
There was no evidence that a myeloid neoplasm developed in any patient: there were no ring sideroblasts and no increased blasts in any follow-up samples. Trichrome staining was negative for all 8 patients with evaluable biopsies. None showed hyperostosis or increased osteoid.
Discussion
Several studies have found that the overexpression of TPO can lead to bone marrow changes in animals and humans. For example, in mice the constitutive overexpression of TPO by transplanted transfected bone marrow cells may produce a fatal syndrome of megakaryocyte hyperproliferation, osteosclerosis, and marrow fibrosis (reticulin and collagen fibrosis) comparable with myeloproliferative disorders.28,29 Chronic overexpression of TPO to a lesser degree in mice (induced by other adenoviral or retroviral vectors) has been shown to lead to thrombocytosis, osteosclerosis, and reticulin and collagen fibrosis, but not to any fatal myeloproliferative disorders.28,30,31 However, chronic TPO pathway activation in humans carrying a mutation in the TPO receptor32 or the TPO gene33,34 has been shown to be associated with lifelong thrombocytosis but not with progression to myeloproliferative disorders, despite “increased” reticulin in 60% of such patients.35 These findings raise the concern that TPO receptor agonists (such as romiplostim and eltrombopag) might also promote bone marrow fibrosis. Because romiplostim binds with high affinity to the TPO receptor,36 resulting in megakaryocyte proliferation and differentiation and increased platelet production,37 it is important to investigate the potential effects of romiplostim on bone marrow.
In the animal study in the present report, rats were treated for 4 weeks with 3 to 30 times as much romiplostim per week as the maximum recommended dose for humans (based on 3 times per week dosing in the rats). At the doses tested, there was megakaryocyte hyperplasia and the platelet count increased but showed either a plateau effect (on megakaryocyte hyperplasia) or actual decline (in platelet count) with increasing dose. This biphasic response of the platelet count to extremely high doses of TPO has previously been described in animal models38 and has been attributed to inhibition of platelet shedding from megakaryocytes. Platelet shedding is thought to be triggered by apoptotic signals that are inhibited by the antiapoptotic effect of massive doses of TPO.39 Nonetheless, romiplostim produced a dose-dependent increase in bone marrow fibrosis and hyperostosis. Although staining specific for reticulin and collagen was not performed in the animal studies reported here, the features of fibrosis seen using the hematoxylin and eosin stain invariably correlate with collagen fibrosis seen with trichrome staining. However, after a posttreatment recovery period, animals administered even the highest romiplostim dose exhibited normal platelet counts, no megakaryocyte hyperplasia, no hyperostosis, and no bone marrow fibrosis, indicating full reversibility of fibrosis in these animal studies. This is consistent with other animal models that have evaluated the effect of TPO and TPO mimetics on bone marrow.40
The retrospective analysis included in the present report identified a total of 271 ITP patients who had been exposed to romiplostim in clinical trials between June 2002 and February 2008. Of these patients, bone marrow examinations were performed for a wide variety of reasons in only 11, of which 10 demonstrated some degree of reticulin staining. Of the 5 patients with bone marrow specimens evaluable both at baseline and on-treatment, the expert panel identified 4 with an increase in reticulin grade (including 1 with focal areas of collagen deposition). Discontinuation of romiplostim in 3 of the 4 patients exhibiting an increase in reticulin grade produced a 2-grade drop in reticulin; in the fourth patient, reticulin decreased (from grade 1-3 to grade 1-2) despite the continuation of romiplostim therapy. Common characteristics among these 10 patients were a high likelihood of splenectomy, a large number of prior therapies, and very low platelet counts, all characteristics of “severe” ITP. Moreover, most of these patients had received relatively high doses of romiplostim; 6 of 10 had received doses above the standard dose of 1 to 10 μg/kg now recommended in the prescribing information.41
Similar findings have been reported for rhTPO and the oral TPO receptor agonist eltrombopag. A study with rhTPO found that 8 of 9 patients with acute myeloid leukemia treated with rhTPO developed increased bone marrow reticulin, whereas only 2 of 6 patients who received placebo developed increased bone marrow reticulin.20 On discontinuation of rhTPO, the reticulin disappeared within an average of 30 days (range, 13-42 days).20 In a recent Food and Drug Administration report, 19 of 117 ITP patients exposed to eltrombopag had bone marrow examinations (after a median of 13 months of treatment) and 7 showed fibrosis (5 reticulin and 2 collagen).15
In animal and clinical studies of TPO administration, reversible increases of reticulin in the bone marrow have been reported in the literature and described in textbooks on bone marrow pathology.20,22,40,42 This observation has been identified as a reversible, expected outcome of stimulation with TPO. Increased reticulin formation may be the result of cytokines (eg, transforming growth factor-β) or other factors that are elaborated by megakaryocytes40,43,44 and that are increased in the bone marrow as a result of TPO stimulation.19,20 Increased reticulin has also been observed on megakaryocyte stimulation by treatment with interleukin-3 and interleukin-11.19
The small prospective bone marrow study included in the present report collected evaluable baseline and on treatment sets of bone marrow stains for 6 of the 10 enrolled patients. Of these, 5 showed no changes in reticulin and 1 showed a 1-grade increase that was still within the normal range. Whether this single increase reflects a sampling variation or a treatment-related increase in reticulin cannot be established.
The studies included in the present report have some distinct limitations. For example, the interpretation of the results of both of the clinical studies is limited by the small sample sizes. Except for the prospective study, bone marrow biopsies were not required as part of any study protocol, and the evaluation of material from patients in the retrospective analysis was limited to what each individual investigator had collected during the study and was able to provide to the expert panel. Moreover, for those patients for whom bone marrow biopsies were performed, only half had an evaluable bone marrow biopsy performed both before and after romiplostim treatment. This means that the results of the retrospective study and the prospective study do not provide an adequate sample size to calculate confidence limits for the frequency of reticulin increase after exposure to romiplostim. In addition, only photomicrographs of biopsies from the prospective study were available for review by the panel, which does not allow an assessment of the potential heterogeneity of reticulin staining in these samples. Lastly, it should also be noted that several patients had conditions in their medical history that could have confounded the assessment of the cause of their bone marrow changes.
Despite these limitations, the data described here indicate that romiplostim is capable of increasing bone marrow reticulin/collagen in animals and has the potential to increase reticulin and possibly collagen in some humans with ITP. It should be kept in mind, however, that reticulin can also increase as a result of a variety of inflammatory disorders. Moreover, the results from the clinical studies support earlier findings that the increased reticulin produced by exposure to romiplostim decreased after discontinuation of the therapy. No myeloid neoplasms have yet been noted even after up to 4 years of treatment.45 In the 2 patients with reports of some peripheral blood blasts, neither showed increased bone marrow blasts or left-shifted myeloid precursors. When increased, the reticulin was usually not associated with cytopenias or splenomegaly (although it should be noted that most patients in the present report had been splenectomized). Follow-up or serial bone marrow examinations in the present study suggested that any increase in reticulin during romiplostim treatment is probably reversible on withdrawal of treatment in both animals and humans. This finding is consistent with the results of earlier studies.19
In conclusion, the present report suggests that romiplostim produces reversible, dose-dependent bone marrow changes in animals as well as relatively benign increases in bone marrow reticulin in some ITP patients, which decrease when therapy is discontinued. Ongoing postapproval patient monitoring and a prospective bone marrow study in a larger number of patients will provide a clearer view of the frequency, reversibility, and clinical consequences of bone marrow changes associated with romiplostim treatment in ITP patients. In the meantime, the present results support the generally accepted practice of always using the minimally effective dose of romiplostim in the clinical setting.
An Inside Blood analysis of this article appears at the front of this issue.
Presented in abstract form at the 50th annual meeting of the American Society of Hematology, San Francisco, CA, December 8, 2008.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the following investigators for providing patient biopsies for bone marrow analysis: Louis Aledort, Mount Sinai Hospital, New York, NY; James Bussel, New York Presbyterian Hospital, New York, NY; Solomon Hamburg, Tower Cancer Research Foundation, Los Angeles, CA; Craig Kessler, Georgetown University Medical Center, Washington, DC; Howard Liebman, University of Southern California, Keck School of Medicine, Los Angeles, CA; Roger Lyons, Cancer Care Centers South Texas/US Oncology, San Antonio, TX; Jean-François Viallard, Medecine Interne-Maladies Infectieuses, Pessac, France; Magaral Murali, Central Indiana Cancer Centers-Rama, Indianapolis, IN; and Jeffrey Wasser, DeQuatro Community Cancer Center, Manchester, CT.
This work was supported by Amgen Inc. In collaboration with the investigators, Amgen designed the studies and conducted statistical analyses. Writing assistance was provided by Amy Lindsay, who was funded by Amgen Inc. Animal pathology specimens were analyzed by Margarita Gruebbel, Experimental Pathology Laboratories Inc.
Authorship
Contribution: The expert panel consisted of D.J.K., R.P.H., B.J.B., and G.J.M.; and all authors were responsible for writing the article and approving the final version.
Conflict-of-interest disclosure: D.J.K. has served as a consultant and received research funding from Amgen Inc, Glaxo-Smith-Kline, Ligand, and MGI Pharma. G.J.M. has received honoraria from Amgen Inc, Celgene, Johnson and Johnson, and Novartis, participated on an advisory committee for Novartis and Amgen Inc, and is the member of a speakers bureau for Amgen Inc. B.J.B. has served as a consultant for Amgen Inc. R.P.H. has served as a paid consultant to Amgen Inc. W.D. and M.R. are employees of and have equity ownership in Amgen Inc.
Correspondence: David J. Kuter, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114; e-mail: kuter.david@MGH.harvard.edu.