Abstract
Laromustine is a sulfonylhdrazine alkylator with significant antileukemia activity. An international, randomized (2:1), double-blind, placebo-controlled study was conducted to compare complete remission (CR) rates and overall survival (OS) in patients with first relapse acute myeloid leukemia (AML) treated with laromustine and high-dose cytarabine (HDAC) versus HDAC/placebo. Patients received 1.5 g/m2 per day cytarabine continuous infusion for 3 days and laromustine 600 mg/m2 (n = 177) or placebo (n = 86) on day 2. Patients in CR received consolidation with laromustine/HDAC or HDAC/placebo as per initial randomization. After interim analysis at 50% enrollment, the Data Safety Monitoring Board (DSMB) expressed concern that any advantage in CR would be compromised by the observed on-study mortality, and enrollment was held. The CR rate was significantly higher for the laromustine/HDAC group (35% vs 19%, P = .005). However, the 30-day mortality rate and median progression-free survival were significantly worse in this group compared with HDAC/placebo (11% vs 2%; P = .016; 54 days vs 34; P = .002). OS and median response durations were similar in both groups. Laromustine/HDAC induced significantly more CR than HDAC/placebo, but OS was not improved due to mortality associated with myelosuppression and its sequelae. The DSMB subsequently approved a revised protocol with laromustine dose reduction and recombinant growth factor support. The study was registered as NCT00112554 at http://www.clinicaltrials.gov.
Introduction
The majority of patients who respond to chemotherapy for acute myeloid leukemia (AML) will relapse.1 Relapse therapy is inadequate, and factors such as age, comorbidities, and duration of first complete response (CR) are often factored into therapy choices.2 Cytosine arabinoside (ara-C) is the most effective single agent in the treatment of AML and is often used at various doses either alone or in combination regimens at time of relapse.2 Second CRs are achieved with combination regimens containing ara-C at various doses; however, median durations of response and overall survival are within the range of single-agent trials, and significant myelosuppression and sepsis are increased, emphasizing the unmet need for treatment in the relapsed AML population.1-3
Laromustine (Onrigin, VNP40101M; Vion Pharmaceuticals Inc) is a sulfonylhdrazine alkylating agent selected for clinical development based on its broad antitumor activity in preclinical models.4-6 Activated, it forms a chloroethylating group that preferentially targets the O6 over the N7 position of guanine, resulting in interstrand DNA cross-links.7 In vitro, it produces more cross-links and fewer DNA single-strand nicks compared with carmustine and lomustine.4 Laromustine is initially activated to yield 90CE {1,2-bis(methylsulfonyl)-1-(2-chloroethyl)hydrazine} and methylisocyanate. 90CE rapidly produces an alkylating, chloroethylating species, similar to the species generated by carmustine.4,5 Carmustine and laromustine produce different decomposition products as laromustine does not yield hydroxyethylating, vinylating, or aminoethylating species. Laromustine yields more than twice the molar yield of DNA cross-links than do the nitrosoureas. The chlorethylating species responsible for laromustine's alkylation is relatively specific to the O6 position of guanine, whereas carmustine (BCNU) and other alkylating agents, unlike laromustine, also alkylate the N7 position of guanine.4,5 Drugs that cause primarily N7 alkylations exhibit one-thirtieth of the anticancer activity and the same mutagenic potential as their counterparts that form both N7 alkylations and cross-links.8-11 Laromustine does not generate hydroxyethyl alkylations of the O6 position of guanine—these lesions are considered to be carcinogenic but therapeutically unimportant.5,11 Laromustine also inhibits the nucleotidyl-transferase activity of purified human DNA polymerase beta, a principal enzyme of DNA base excision repair.12 Alkylated DNA is often repaired via base excision repair in vivo. Inhibition of the polymerase activity of polymerase beta may account for some of the synergy between laromustine's 2 reactive subspecies (chloroethylating and methyl isocyanate) in cytotoxicity assays. Laromustine has significant activity against hematologic malignancy–derived cell lines, including those that are resistant to other alkylating agents.13,14
Solid tumor phase 1/2 studies of laromustine established the maximum tolerated dose (MTD) with myelosuppression as the dose-limiting toxicity and no significant extramedullary toxicity.15-17 A phase 1 study of single-agent laromustine in patients with advanced hematologic malignancies established 600 mg/m2 as the MTD with prolonged myelosuppression as the dose-limiting toxicity, again with no significant extramedullary adverse events.18 In a phase 2 study at 600 mg/m2, laromustine produced a CR rate of 28% in elderly patients with previously untreated AML or myelodysplastic syndrome.19 In poor-prognosis patients with AML in first relapse after an initial CR duration of less than 12 months, laromustine was associated with a 4% CR rate, which is within the range as an array of other myelosuppressive single agents studied in this patient population.20 Because of the significant activity observed in the single-agent trial in patients with AML and poor risk features,19 a phase 1 study was conducted in patients with refractory hematologic malignancies using a dose escalation of laromustine with high-dose ara-C (HDAC).21 The MTD was 600 mg/m2 laromustine combined with ara-C 1.5 mg/m2 per day for 3 days, and CR was attained in 7 (22%) of 32 patients with AML.21
The primary objective of the subsequent randomized, double-blind, placebo-controlled phase 3 clinical study presented here was to determine whether the combination of laromustine/HDAC produced a higher CR rate than placebo/HDAC in adults with first-relapse AML. Secondary objectives were to compare the 2 regimens with respect to time-to-progression, death from any cause, duration of response, survival, and toxicity.
Methods
This study was approved by the institutional review boards of all participating institutions. All participants signed informed consent and were aware of the investigational nature of the study.
Patients
Patients were eligible if they were 18 years or older, had a diagnosis of AML by World Health Organization (WHO) criteria (except promyelocytic leukemia), and were in first relapse after prior CR, including CR with incomplete platelet recovery (CRp), with a bone marrow containing 10% or more blasts. The duration of first CR must have been 3 or more months but less than 24 months. There was no restriction on the number of regimens or type of treatment required to induce first CR or administered for consolidation. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0 to 2, serum creatinine of 152.5 μM (2.0 mg/dL) or less, and total bilirubin and aspartate aminotransferase of 1.5 times or less and 3 times or less the upper limits of normal, respectively. Patients were excluded if they had uncontrolled active infection, clinical evidence of an active second malignancy, myocardial infarction within the prior 3 months, arrhythmia not controlled by medication, or uncontrolled congestive heart failure. Patients were not to have received any treatment other than hydroxyurea during first relapse, and if used, it had to have been discontinued at least 12 hours before initiation of protocol treatment. Because the formulation of laromustine contains 30% alcohol, patients were not allowed to receive disulfiram or metronidazole during the infusion.
Treatment and study design
All patients received ara-C 1.5 g/m2 per day as a continuous intravenous infusion (CIV) over 24 hours on days 1 to 3. Laromustine (600 mg/m2) or placebo was administered intravenously over 30 to 60 minutes on day 2, at least 12 hours after the start of the ara-C infusion. Patients received 50 mg methylprednisolone intravenously before ara-C. Antiemetics and antihistamines were administered on day 2 to avoid infusion-related flushing, headache, and hypotension, which had been noted in phase 1 studies of laromustine.18,21 Patients without evidence of clinical progression, who had not achieved at least a CRp after the first induction, were eligible to receive a second induction cycle no earlier than day 35, and no later than day 60. Patients who attained at least a CRp after the first or second induction cycle were eligible for 1 cycle of consolidation, beginning no later than 6 weeks after initial documentation of CR and, if CRp, after platelet counts had reached plateau. Patients received ara-C 1.5 g/m2 per day continuous intravenous infusion days 1 through 3 and 400 mg/m2 laromustine (or placebo). Patients received supportive care including antimicrobial therapy, blood transfusions, and recombinant growth factors as per institutional policy. Toxicity was graded according to National Cancer Institute (NCI) Common Toxicity Criteria for Adverse Events (CTCAE) V3.0 (http://ctep.info.nih.gov). All treated patients were included in safety assessments, and adverse events were reported for at least 30 days from any study treatment, and followed to resolution if considered at least possibly related to treatment.
Response criteria
CR was defined as the absence of leukemic blasts in the peripheral blood and a bone marrow with less than 5% blasts, absence of blasts with Auer rods, no extramedullary disease, an absolute granulocyte count greater than 109/L, a platelet count greater than 100 × 109/L, and transfusion independence. CRp was defined as CR except with a platelet count less than 100 × 109/L but maintained at 20 × 109/L or greater without platelet transfusions for at least 7 consecutive days. Recurrence was recorded when marrow blasts were greater than 5% or blasts appeared in peripheral blood. Response duration was calculated from the date that objective criteria for CR or CRp were met until the date of relapse plus 1 day. Time-to-progression or death was calculated from randomization date to date of documentation of recurrence or death plus 1 day. Overall survival (OS) was calculated from the date of randomization to death as a result of any cause.
Statistical analysis
The study was initially designed to include 420 patients, randomized in a 2:1 schema (laromustine/HDAC:HDAC/placebo). After a planned interim analysis of safety and efficacy data from the first 210 patients enrolled, the Data Safety Monitoring Board (DSMB) expressed concern that any advantage in CR rates might be compromised by an imbalance in the observed on-study mortality. No additional patients were enrolled or treated with study drug after the DSMB reported their concerns. Planned analyses were carried out on the as-treated population. Covariates included randomization stratification factors of age (< or ≥ 60 years) and duration of the first CR/CRp (> 3 months and < 12 months, or ≥ 12 months and < 24 months). Observations were censored if patients were alive at last follow-up visit and for patients who received other investigational therapy. Kaplan-Meier estimates were used for all time-to-event analyses. Descriptive statistics were used to summarize incidence of adverse events and deaths on study. Statistical analyses were generated using SAS Version 8.0 or higher.
Results
Between March 2005 and May 2007, a total of 268 patients at 57 North American and international sites were randomized (Figure 1); included were the 210 initial patients evaluated by the DSMB and the additional 58 patients randomized while the DSMB evaluation was ongoing and before the DSMB report was available. Of the total patients enrolled, 178 were randomized to receive laromustine/HDAC (177 patients treated), and 90 patients (86 patients treated) were randomized to receive HDAC/placebo. Baseline characteristics of the 263 treated patients are described in Table 1. Patients were evenly distributed between treatment groups for demographic variables; the median age was 59 years (range, 22-84 years), and the median duration of first CR or CRp was 9.7 months (range, 2.6-75.1 months). The majority of patients (89%) had an Eastern Cooperative Oncology Group performance status of 0 or 1. Although the group as a whole was evenly distributed by age older than and younger than 60 years, approximately two-thirds of patients had relapsed within 12 months of first documented CR.
Baseline patient characteristics
| Characteristic . | Laromustine/HDAC, n = 177 . | HDAC/placebo, n = 86 . | All patients, N = 263 . |
|---|---|---|---|
| Age, y | |||
| Median (range) | 59 (22-82) | 60 (25-84) | 59 (22-84) |
| Ethnicity, no. (%) | |||
| White | 151 (85) | 78 (91) | 229 (87) |
| Hispanic | 11 (6) | 4 (5) | 15 (6) |
| Black | 8 (5) | 0 | 8 (3) |
| Asian | 6 (3) | 4 (5) | 10 (4) |
| Other | 1 (1) | 0 | 1 (1) |
| ECOG status, no. (%) | |||
| 0 | 78 (44) | 31 (36) | 109 (41) |
| 1 | 78 (44) | 47 (55) | 125 (48) |
| 2 | 19 (11) | 8 (9) | 27 (10) |
| Missing | 2 (1) | 0 | 2 (1) |
| Duration of first CR/CRp, mo, median (minimum, maximum) | 9.6 (2.6, 23.6) | 9.74 (2.9, 75.1) | 9.7 (2.6, 75.1) |
| Duration of AML, mo,* median (minimum, maximum) | 12.1 (3.9, 30.9) | 11.7 (5.2, 76.7) | 12.0 (3.9, 76.7) |
| Age category, no. (%) | |||
| Younger than 60 y | 92 (52) | 42 (49) | 134 (51) |
| 60 y or older | 85 (48) | 44 (51) | 129 (49) |
| Duration of first CR/CRp, category, no. (%) | |||
| Less than 12 mo | 112 (63) | 52 (61) | 164 (62) |
| 12 mo or longer | 65 (37) | 34 (39) | 99 (37) |
| Stratum/duration of first CR or CRp, no. (%) | |||
| Younger than 60 y/less than 12 mo | 61 (35) | 26 (30) | 87 (33) |
| Younger than 60 y/12 or more mo | 31 (18) | 16 (19) | 47 (18) |
| 60 y or older/less than 12 mo | 51 (28) | 26 (30) | 77 (29) |
| 60 y or older/12 or more mo | 34 (19) | 18 (21) | 52 (20) |
| AML by WHO classification, no. (%) | |||
| AML with recurrent genetic abnormalities | 15 (9) | 4 (5) | 19 (7) |
| AML with multilineage dysplasia | 40 (23) | 21 (24) | 61 (23) |
| AML therapy-related | 1 (1) | 1 (1) | 2 (1) |
| AML, not otherwise categorized | 121 (68) | 60 (70) | 181 (69) |
| Characteristic . | Laromustine/HDAC, n = 177 . | HDAC/placebo, n = 86 . | All patients, N = 263 . |
|---|---|---|---|
| Age, y | |||
| Median (range) | 59 (22-82) | 60 (25-84) | 59 (22-84) |
| Ethnicity, no. (%) | |||
| White | 151 (85) | 78 (91) | 229 (87) |
| Hispanic | 11 (6) | 4 (5) | 15 (6) |
| Black | 8 (5) | 0 | 8 (3) |
| Asian | 6 (3) | 4 (5) | 10 (4) |
| Other | 1 (1) | 0 | 1 (1) |
| ECOG status, no. (%) | |||
| 0 | 78 (44) | 31 (36) | 109 (41) |
| 1 | 78 (44) | 47 (55) | 125 (48) |
| 2 | 19 (11) | 8 (9) | 27 (10) |
| Missing | 2 (1) | 0 | 2 (1) |
| Duration of first CR/CRp, mo, median (minimum, maximum) | 9.6 (2.6, 23.6) | 9.74 (2.9, 75.1) | 9.7 (2.6, 75.1) |
| Duration of AML, mo,* median (minimum, maximum) | 12.1 (3.9, 30.9) | 11.7 (5.2, 76.7) | 12.0 (3.9, 76.7) |
| Age category, no. (%) | |||
| Younger than 60 y | 92 (52) | 42 (49) | 134 (51) |
| 60 y or older | 85 (48) | 44 (51) | 129 (49) |
| Duration of first CR/CRp, category, no. (%) | |||
| Less than 12 mo | 112 (63) | 52 (61) | 164 (62) |
| 12 mo or longer | 65 (37) | 34 (39) | 99 (37) |
| Stratum/duration of first CR or CRp, no. (%) | |||
| Younger than 60 y/less than 12 mo | 61 (35) | 26 (30) | 87 (33) |
| Younger than 60 y/12 or more mo | 31 (18) | 16 (19) | 47 (18) |
| 60 y or older/less than 12 mo | 51 (28) | 26 (30) | 77 (29) |
| 60 y or older/12 or more mo | 34 (19) | 18 (21) | 52 (20) |
| AML by WHO classification, no. (%) | |||
| AML with recurrent genetic abnormalities | 15 (9) | 4 (5) | 19 (7) |
| AML with multilineage dysplasia | 40 (23) | 21 (24) | 61 (23) |
| AML therapy-related | 1 (1) | 1 (1) | 2 (1) |
| AML, not otherwise categorized | 121 (68) | 60 (70) | 181 (69) |
HDAC indicates high-dose cytarabine; ECOG, Eastern Cooperative Oncology Group; CR, complete response; CRp, CR except for a platelet count less than 10×109/L but maintained at 20×109/L or more without platelet transfusions for at least 7 consecutive days; AML, acute myelogenous leukemia; and WHO, World Health Organization.
Duration of AML was the number of days from initial AML diagnosis to date of randomization.
Response rates by stratum and for all patients are described in Table 2. The overall CR rate (CR including CRp) was significantly greater in the laromustine/HDAC group than in the HDAC/placebo (35% vs 19%; P = .005). Within the laromustine/HDAC arm, the highest CR rate was seen in patients who were 60 years or older and had first CR 12 months or longer, whereas in the placebo/HDAC group, the greatest CR rate was seen in patients who were younger than 60 years and had first CR 12 months or longer. Combining all strata, similar proportions of each treatment group had a best response of CR (20% in the laromustine/HDAC group vs 16% in the HDAC/placebo group). The proportion of patients with a best response of CRp was significantly higher in the laromustine/ara-C arm compared with the HDAC/placebo arm (14.7% vs 2.3%; P = .001). Postresponse survival (median, 6 months and 12 months), was equivalent in those with CR or CRp. In the laromustine/HDAC arm, 19 (11%) of 177 patients underwent allogeneic stem cell transplantation (SCT) after study treatment. Before SCT, 10 patients had achieved a CR (including 6 with CRp), 5 patients had marrow remissions, 2 were not evaluable, and 2 were treatment failures. In the HDAC/placebo arm, 13 (15%) of 86 patients underwent allogeneic SCT, of whom 6 were in CR and 7 were treatment failures. Thus, because transplantation was performed in patients with treatment failure, relatively more patients in the HDAC/placebo arm underwent SCT.
Response rates by patient stratum
| . | Complete response . | Complete response with inadequate platelet recovery . | Total complete response*† . | |||
|---|---|---|---|---|---|---|
| Laromustine/HDAC, no. (%) . | HDAC/placebo, no. (%) . | Laromustine/HDAC, no. (%) . | HDAC/placebo, no. (%) . | Laromustine/HDAC, no. (%) . | HDAC/placebo, no. (%) . | |
| All patients. Laromustine/HDAC, n = 179; HDAC/placebo, n = 86 | 36 (20) | 14 (16) | 26 (15) | 2 (2) | 62 (35)* | 16 (19) |
| Stratum 1: Younger than 60 y, CR less than 12 mo. Laromustine/HDAC, n = 61; HDAC/placebo, n = 26 | 7 (11) | 4 (15) | 9 (15) | 0 | 16 (26) | 4 (15) |
| Stratum 2: Younger than 60 y, CR 12 or more mo. Laromustine/HDAC, n = 31; HDAC/placebo, n = 16 | 7 (22) | 7 (44) | 3 (10) | 0 | 10 (32) | 7 (44) |
| Stratum 3: 60 y or older, CR less than 12 mo. Laromustine/HDAC, n = 51; HDAC/placebo, n = 26 | 14 (28) | 1 (4) | 4 (8) | 0 | 18 (35) | 1 (4) |
| Stratum 4: 60 y or older, CR 12 or more mo. Laromustine/HDAC, n = 34; HDAC/placebo, n = 18 | 8 (24) | 2 (11) | 10 (29) | 2 (11) | 18 (53) | 4 (22) |
| . | Complete response . | Complete response with inadequate platelet recovery . | Total complete response*† . | |||
|---|---|---|---|---|---|---|
| Laromustine/HDAC, no. (%) . | HDAC/placebo, no. (%) . | Laromustine/HDAC, no. (%) . | HDAC/placebo, no. (%) . | Laromustine/HDAC, no. (%) . | HDAC/placebo, no. (%) . | |
| All patients. Laromustine/HDAC, n = 179; HDAC/placebo, n = 86 | 36 (20) | 14 (16) | 26 (15) | 2 (2) | 62 (35)* | 16 (19) |
| Stratum 1: Younger than 60 y, CR less than 12 mo. Laromustine/HDAC, n = 61; HDAC/placebo, n = 26 | 7 (11) | 4 (15) | 9 (15) | 0 | 16 (26) | 4 (15) |
| Stratum 2: Younger than 60 y, CR 12 or more mo. Laromustine/HDAC, n = 31; HDAC/placebo, n = 16 | 7 (22) | 7 (44) | 3 (10) | 0 | 10 (32) | 7 (44) |
| Stratum 3: 60 y or older, CR less than 12 mo. Laromustine/HDAC, n = 51; HDAC/placebo, n = 26 | 14 (28) | 1 (4) | 4 (8) | 0 | 18 (35) | 1 (4) |
| Stratum 4: 60 y or older, CR 12 or more mo. Laromustine/HDAC, n = 34; HDAC/placebo, n = 18 | 8 (24) | 2 (11) | 10 (29) | 2 (11) | 18 (53) | 4 (22) |
Complete response plus complete response with inadequate platelet recovery.
P = .005 comparing the overall response rate between treatment arms using the Chochran-Mantel-Haenszal test stratified by age group and duration of first CR/CRp. Difference (95% confidence interval) between all strata of each treatment group (laromustine/HDAC–HDAC/placebo) was 16% (56%-27%).
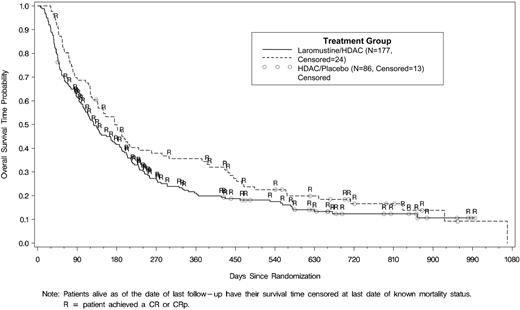
Survival is illustrated in Figure 2. Median survival time (95% confidence interval [CI]) was 128 (104-170) days versus 176 (136-270) days in the 177 and 86 patients in the laromustine/HDAC and placebo/HDAC groups, respectively (log-rank, P = .087). Increased response in the laromustine/HDAC group did not correlate with increased survival due to a relative excess of early deaths in the laromustine/HDAC group. In the 62 and 16 responders in the laromustine/HDAC and HDAC/placebo groups, median survival times (95% CI) were 264 (230-333) days versus 451 (386, not estimable) days, respectively (log-rank, P = .572). For all patients, median (95% CI) progression-free survival was significantly greater for laromustine/HDAC than HDAC/placebo patients (54 [45-71] days vs 34 [32-39] days; log rank, P = .002). Median response duration was similar: 275 (191-910) days versus 332 (238-783) days, respectively (log-rank, P = .640), for the 62 laromustine/HDAC and 16 HDAC/placebo responders.
Table 3 summarizes grades 3/4 potentially treatment-related adverse events occurring in 5% or more of patients in either treatment arm. Significantly more patients in the laromustine/HDAC group than in the HDAC/placebo group experienced serious adverse events (SAEs; 74% vs 51%, respectively; P < .001). Significantly more patients in the laromustine/HDAC group than HDAC/placebo group experienced infectious and respiratory SAEs (45% vs 27% [P = .005] and 21% vs 7% [P = .004], respectively), however, hematologic SAEs occurred at similar rates (25% and 23%, respectively). Of all grades of adverse events, those suggestive of pulmonary toxicity were more frequent in the laromustine/HDAC than HDAC/placebo patients (34% vs 17%, respectively; P = .006)
NCI-CTCAE grades 3/4 potentially related adverse events in 5% or more of patients
| Adverse event . | Laromustine/HDAC, n = 177, no. (%) . | HDAC/placebo, n = 86, no. (%) . |
|---|---|---|
| Febrile neutropenia | 81 (46) | 42 (49) |
| Neutropenic infection | 15 (8) | 7 (8) |
| Pneumonia | 15 (8) | 4 (5) |
| Neutropenic sepsis | 11 (6) | 5 (6) |
| Bacteremia | 15 (8) | 4 (5) |
| Sepsis | 8 (5) | 2 (2) |
| Dyspnea | 26 (15) | 4 (5) |
| Hypoxia | 19 (11) | 2 (2) |
| Acute respiratory distress syndrome | 5 (3) | 0 |
| Pyrexia | 14 (8) | 4 (5) |
| Fatigue | 13 (7) | 1 (1) |
| Hypotension | 15 (9) | 4 (5) |
| Diarrhea | 12 (7) | 1 (1) |
| Hypokalemia | 25 (14) | 6 (7) |
| Hyperglycemia | 9 (5) | 5 (6) |
| Adverse event . | Laromustine/HDAC, n = 177, no. (%) . | HDAC/placebo, n = 86, no. (%) . |
|---|---|---|
| Febrile neutropenia | 81 (46) | 42 (49) |
| Neutropenic infection | 15 (8) | 7 (8) |
| Pneumonia | 15 (8) | 4 (5) |
| Neutropenic sepsis | 11 (6) | 5 (6) |
| Bacteremia | 15 (8) | 4 (5) |
| Sepsis | 8 (5) | 2 (2) |
| Dyspnea | 26 (15) | 4 (5) |
| Hypoxia | 19 (11) | 2 (2) |
| Acute respiratory distress syndrome | 5 (3) | 0 |
| Pyrexia | 14 (8) | 4 (5) |
| Fatigue | 13 (7) | 1 (1) |
| Hypotension | 15 (9) | 4 (5) |
| Diarrhea | 12 (7) | 1 (1) |
| Hypokalemia | 25 (14) | 6 (7) |
| Hyperglycemia | 9 (5) | 5 (6) |
NCI-CTCAE indicates National Cancer Institute Common Toxicity Criteria for Adverse Events.
Nineteen (11%) of 177 patients in the laromustine/HDAC group and 2 (2%) of 86 patients in the HDAC/placebo group (P = .016) died within 30 days of the first ara-C infusion for induction (Table 4). Within 30 days of last infusion for second induction, 3 (43%) of 7 and none of 3 laromustine/HDAC and HDAC/placebo patients, respectively, died; within 30 days of consolidation, 5 (15%) of 34 and none of 12 laromustine/HDAC and HDAC/placebo patients, respectively, died. Overall, during treatment and follow-up, 154 (87%) of 177 and 74 (86%) of 86 laromustine/HDAC and HDAC/placebo patients, respectively, died. Fifteen patients treated with laromustine/HDAC died from acute respiratory distress, respiratory failure, and other respiratory disorders, and 12 patients died from pneumonia. The combined categories of sepsis, pneumonia, and infection accounted for 69% of the adverse events associated with a fatal outcome of laromustine/HDAC therapy.
Causes of death within 30 days from day 1 of ara-C therapy
| Patient ID . | Age, y . | CR1 duration . | Cytogenetic category . | Adverse event (study day of death) . |
|---|---|---|---|---|
| Laromustine/HDAC, n = 19 | ||||
| 010-004 | 29 | 11.8 | Not available | Multiorgan failure (19); acute respiratory distress (19) |
| 010-008 | 58 | 3.8 | Not available | Grade 3/4 neutropenic sepsis (30); progressive AML (30) |
| 015-001 | 56 | 8.1 | Unfavorable | Diarrhea (9); acute respiratory distress (9) |
| 122-004 | 55 | 9.1 | Unfavorable | Grade 3/4 neutropenic sepsis with pulmonary aspergillosis (17) |
| 001-025 | 49 | 18.6 | Favorable | Pneumonia (25) |
| 121-003 | 38 | 19.5 | Unfavorable | Grade 3/4 neutropenic sepsis (24) |
| 133-002 | 54 | 20.9 | Not available | Grade 3/4 neutropenic sepsis (23) |
| 152-016 | 66 | 11.8 | Intermediate | Cerebral vascular accident (29) |
| 010-003 | 71 | 7.7 | Intermediate | Typhilis (31); grade 3/4 neutropenic sepsis (31) |
| 013-003 | 70 | 6.5 | Intermediate | Sepsis (16) |
| 031-010 | 61 | 7.1 | Unfavorable | Sepsis (23) |
| 033-001 | 68 | 9.6 | Intermediate | Sepsis (30) |
| 129-002 | 80 | 5 | Intermediate | Grade 3/4 neutropenic sepsis (30) |
| 152-008 | 60 | 9.2 | Intermediate | Acute respiratory distress (16) |
| 012-003 | 67 | 6.9 | Intermediate | Septic shock (6) |
| 152-001 | 72 | 11.6 | Intermediate | Grade 3/4 neutropenic sepsis (28) |
| 016-002 | 60 | 13 | Intermediate | Sepsis (20) |
| 094-001 | 73 | 16.4 | Unfavorable | Grade 3/4 neutropenic sepsis with pneumonia (19) |
| 130-005 | 70 | 14 | Intermediate | Grade 3/4 neutropenic sepsis with pneumonia (25) |
| HDAC/placebo, n = 2 | ||||
| 008-012 | 77 | 6.4 | Unfavorable | Supraventricular tachycardia (30) |
| 095-001 | 64 | 9.1 | Unfavorable | AML progression (31) |
| Patient ID . | Age, y . | CR1 duration . | Cytogenetic category . | Adverse event (study day of death) . |
|---|---|---|---|---|
| Laromustine/HDAC, n = 19 | ||||
| 010-004 | 29 | 11.8 | Not available | Multiorgan failure (19); acute respiratory distress (19) |
| 010-008 | 58 | 3.8 | Not available | Grade 3/4 neutropenic sepsis (30); progressive AML (30) |
| 015-001 | 56 | 8.1 | Unfavorable | Diarrhea (9); acute respiratory distress (9) |
| 122-004 | 55 | 9.1 | Unfavorable | Grade 3/4 neutropenic sepsis with pulmonary aspergillosis (17) |
| 001-025 | 49 | 18.6 | Favorable | Pneumonia (25) |
| 121-003 | 38 | 19.5 | Unfavorable | Grade 3/4 neutropenic sepsis (24) |
| 133-002 | 54 | 20.9 | Not available | Grade 3/4 neutropenic sepsis (23) |
| 152-016 | 66 | 11.8 | Intermediate | Cerebral vascular accident (29) |
| 010-003 | 71 | 7.7 | Intermediate | Typhilis (31); grade 3/4 neutropenic sepsis (31) |
| 013-003 | 70 | 6.5 | Intermediate | Sepsis (16) |
| 031-010 | 61 | 7.1 | Unfavorable | Sepsis (23) |
| 033-001 | 68 | 9.6 | Intermediate | Sepsis (30) |
| 129-002 | 80 | 5 | Intermediate | Grade 3/4 neutropenic sepsis (30) |
| 152-008 | 60 | 9.2 | Intermediate | Acute respiratory distress (16) |
| 012-003 | 67 | 6.9 | Intermediate | Septic shock (6) |
| 152-001 | 72 | 11.6 | Intermediate | Grade 3/4 neutropenic sepsis (28) |
| 016-002 | 60 | 13 | Intermediate | Sepsis (20) |
| 094-001 | 73 | 16.4 | Unfavorable | Grade 3/4 neutropenic sepsis with pneumonia (19) |
| 130-005 | 70 | 14 | Intermediate | Grade 3/4 neutropenic sepsis with pneumonia (25) |
| HDAC/placebo, n = 2 | ||||
| 008-012 | 77 | 6.4 | Unfavorable | Supraventricular tachycardia (30) |
| 095-001 | 64 | 9.1 | Unfavorable | AML progression (31) |
HDAC indicates high-dose cytarabine; and AML, acute myelogenous leukemia.
During the first induction cycle, patients in the laromustine/HDAC and HDAC/placebo groups had equivalent times to absolute neutrophil count nadir and time to absolute neutrophil count recovery of 0.5 × 109/L or higher. The groups had equivalent times to platelet nadir but time to recovery to a platelet count of 100 × 109/L or higher was longer with laromustine/HDAC (median, 41 vs 25 days; P < .002). Overall, 115 (65%) of 177 and 28 (33%) of 86 (P < .001) patients in the laromustine/HDAC and HDAC/placebo groups, respectively, received granulocyte colony-stimulating factors.
After interim analysis at 50% enrollment, the Data Safety Monitoring Board (DSMB) expressed concern that any advantage in CR rates seen in the laromustine/HDAC arm would be compromised by the observed on-study mortality, and study enrollment was held. After a detailed data review and consultation with regulatory authorities, the DSMB subsequently approved a revised protocol with laromustine dose reduction and mandatory recombinant growth factor support.
Discussion
In this international randomized, double-blind, placebo-controlled study of patients with first relapse AML, the combination of laromustine and HDAC produced a CR rate of 35%, significantly greater than the CR rate of 19% associated with HDAC alone. Superior efficacy did not translate into better OS because of excess deaths associated with the combination therapy. Most deaths occurred early during the study (within 30 days of initiation of first induction); the combined categories of sepsis, pneumonia, and infection accounted for the majority of the adverse events associated with death in patients receiving laromustine/HDAC. It is likely that the longer duration of neutropenia observed in patients treated with laromustine/HDAC compared with HDAC/placebo contributed to the excess of infection/death.
A greater proportion of patients treated with laromustine/HDAC in the current study experienced grade 3/4 noninfectious respiratory events than those treated with HDAC/placebo. Pulmonary toxicity has long been established as a consequence of alkylating agent therapy, and, with widely varied recommendations on appropriate therapeutic approaches, corticosteroid therapy is most commonly suggested.22,23 Amplified pulmonary toxicity from combinations of agents known to have the potential for lung toxicity as single agents, has been documented.22 Lung toxicities, particularly noncardiogenic pulmonary edema, have been reported in 12% to 20% of AML patients treated with medium- to high-dose ara-C,24 and more commonly among relapsed patients.25 Noninfectious pulmonary disorders have been reported in 6% of previously untreated older patients with AML treated with laromustine alone.19 In a study of single-agent laromustine using the currently reported study regimen of 600 mg/m2 in a cohort of 85 patients 60 years or older with previously untreated AML and multiple baseline comorbidities, including severe pulmonary dysfunction, the overall response rate was 34% and was associated with a 30-day mortality rate of 14%, with only 1 of 12 deaths attributed to respiratory failure.26 Other grade 3/4 toxicities noted in the laromustine/HDAC arm of the current study (gastrointestinal, constitutional symptoms) are similar to those reported in previous laromustine studies.18,20,21
The preclinical development of laromustine was focused on the synthesis of an agent that generated an alkylating species that demonstrated antitumor activity with relatively less toxicity to normal tissues.5,7-10,27,28 The clinical development of laromustine in AML has evolved from a consistent demonstration, in both adult and pediatric patients with a variety of refractory solid tumors or hematologic malignancies, that myelosuppression is the dose-limiting toxicity, occurring at doses that incur relatively low extramedullary toxicity.16-21 In 2002, after analysis of 31 trials of at least 20 patients each, Leopold and Willemze concluded that no chemotherapy regimen had been shown to be more effective than another for patients with relapsed AML.2 More recently, review of various combination chemotherapeutic regimens based on HDAC described a range of second CR of 10% to 70%.29 An attempt to prolong second remission in selected patients with AML using SCT after second CR has resulted in an estimated 10-year survival of 32%, progression-free survival of 28%, and a relapse rate of 57%.30 The median patient age in the current study was higher than in the majority of other studies reported for relapsed AML, and older patients with longer first CRs on laromustine/HDAC were not observed to have excess deaths compared with younger patients. If a benefit can be demonstrated in older patients with a modified laromustine/HDAC regimen, this would offer a distinct advantage over current options. Effective treatment for patients with first relapse AML has proven difficult to develop. The laromustine/HDAC regimen was significantly more effective in producing second CR than HDAC/placebo. This enhanced efficacy justifies continued exploration of alternative doses or schedules of this combination accompanied by measures to improve the risk-benefit ratio of treatment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: All authors designed and conducted research, analyzed data, and wrote and approved the manuscript.
Conflict-of-interest disclosure: F.G. is a consultant to Vion Pharmaceuticals and receives research funding. A.C. is an employee of Vion Pharmaceuticals. All other authors receive research funding from Vion Pharmaceuticals.
Correspondence: Francis J. Giles, CTRC at the UT Health Science Center at San Antonio, 7979 Wurzbach Rd, Mail Code 8026, Urschel Tower, Suite 600, San Antonio, TX 78229; e-mail: frankgiles@aol.com.