Abstract
Interleukin-21 (IL-21) is an important promoter for differentiation of human B cells into immunoglobulin (Ig)–secreting cells. The objective of this study was to evaluate an IL-21–based approach to induce immunoglobulin production in B cells from patients with common variable immunodeficiency (CVID) or selective IgA deficiency (IgAD). We show that a combination of IL-21, IL-4, and anti-CD40 stimulation induces class-switch recombination to IgG and IgA and differentiation of Ig-secreting cells, consisting of both surface IgG+ (sIgG+) and sIgA+ B cells and CD138+ plasma cells, in patients with CVID or IgAD. Stimulation with IL-21 was far more effective than stimulation with IL-4 or IL-10. Moreover, spontaneous apoptosis of CD19+ B cells from patients with CVID or IgAD was prevented by a combination of IL-21, IL-4, and anti-CD40 stimulation. Analysis of IL-21 and IL-21 receptor (IL-21R) mRNA expression upon anti-CD3 stimulation of T cells, however, showed no evidence for defective IL-21 expression in CVID patients and sequencing of the coding regions of the IL21 gene did not reveal any mutations, suggesting a regulatory defect. Thus, our work provides an initial basis for a potential therapeutic role of IL-21 to reconstitute immunoglobulin production in CVID and IgAD.
Introduction
Selective IgA deficiency (IgAD) and common variable immunodeficiency (CVID) represent the most prevalent primary immunodeficiency diseases in whites, characterized by low or absent levels of serum IgA or by all switched immunoglobulin isotypes, respectively.1,2 The diagnosis of CVID is also based on an inability to mount protective antibody responses in the presence of normal numbers of circulating B cells and exclusion of other causes of antibody deficiency.2,3
Although many patients with IgAD are asymptomatic, some suffer from an increased susceptibility to infections of the respiratory and gastrointestinal tract. CVID shows a highly variable clinical presentation and outcome. The clinical picture is dominated by upper and lower respiratory tract infections, leading to chronic lung disease, bronchiectasis, and eventually death.2-4 Moreover, granulomatous inflammation, gastrointestinal disorders, autoimmunity, and malignancies constitute complicating factors.2,3 The main treatment of primary antibody deficiencies is replacement immunoglobulin therapy administered by the intravenous or subcutaneous route. Restoration of physiologic levels of IgG in the patients' blood has been a profound therapeutic advance, preventing or alleviating the severity of infections.5-7
Several authors have demonstrated that B cells from IgA-deficient subjects and CVID patients undergo class-switch recombination (CSR) when stimulated through CD40 (TNFRSF5) together with interleukin-4 (IL-4) or interleukin-10 (IL-10), leading to reconstitution of IgG and IgA production ex vivo.8-12 However, these effects have not yet been established in vivo and it remains to be seen whether targeting of the CD40 molecule and stimulation with IL-4 and IL-10 are tolerated and therapeutically effective in humans.13,14 Immunomodulatory intervention with low doses of IL-2 has shown promise in vivo, despite insufficient evidence and proof of safety to support its routine use.15-18
Human interleukin-21 (IL-21) is a 4-helix-bundle cytokine of the γc family that is expressed exclusively by activated CD4+ T cells, including the recently discovered Th17 cells.19-21 IL-21 exerts variable and sometimes contrasting effects on natural killer, T, and B cells. IL-21 induces proliferation or apoptosis in B cells in a context-dependent manner, and promotes production of antigen-specific antibodies.22-24 IL-21 receptor (IL-21R)–deficient mice show no obvious developmental defects, but exhibit markedly diminished serum levels of IgG1, IgG2, and IgG3, whereas IgE is elevated.24,25 Data from experiments with human peripheral blood B cells also show that IL-21 can regulate immunoglobulin production both positively and negatively, depending on costimulation with IL-4 and anti-CD40 antibodies.26 Finally, IL-21R/IL-4 double-deficient mice exhibit a CVID phenotype, with remaining IgM production, pointing out a critical role for IL-21 in regulating immunoglobulin switching.24
In the present study we evaluated an IL-21–based approach to induce immunoglobulin production in B cells from patients with common variable immunodeficiency or selective IgA deficiency ex vivo.
Methods
Human subjects
Thirty-four patients with an established diagnosis of CVID, according to the European Society for Immunodeficiencies criteria, were included.27 The patients received monthly intravenous or weekly subcutaneous gammaglobulin replacement therapy. Clinical characteristics and the flow cytometric analysis of B-cell subpopulations of all included CVID patients are shown in supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). All enrolled CVID patients were tested previously or during the study to analyze known genetic causes of the CVID phenotype (ICOS, CD40L, CD19, and TACI). Only 1 included CVID patient (number 18) carried TACI mutations, being heterozygous for both C104R and A181E. Ten patients fulfilling the European Society for Immunodeficiencies diagnostic criteria for selective IgA deficiency (serum IgA < 0.07 g/L), and with a medical history of recurrent respiratory and/or gastrointestinal infections, were also included.27 In addition, 22 age- and sex-matched healthy blood donors or healthy volunteers with normal Ig levels were included as controls. The institutional review boards at both the Karolinska Institutet and the University of Leipzig approved this study. Consent was obtained in accordance with the Declaration of Helsinki.
Cell separation, antibodies, cytokines, and culture conditions
Heparinized peripheral venous blood (14 mL) was obtained twice from all included patients and healthy volunteers. Peripheral blood mononuclear cells (PBMCs) were isolated and cryopreserved as described by Kreher et al.28 Thawed PBMCs were cultured in Iscove modified Dulbecco medium with 1% l-alanyl-l-glutamine, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 1% penicillin-streptomycin (Invitrogen Corporation), and 10% heat-inactivated fetal bovine serum (PAA Laboratories GmbH) at 37°C in the presence of 5% CO2. IL-10 or IL-21 was added in a final concentration of 10 ng/mL and IL-2, -4, -6, -7, and -15, at 0.5 ng/mL (all from ImmunoTools). Anti–human CD40 monoclonal antibody (mAb; clone S2C6) or anti–human CD3 mAb (clone CD3-2) was added at 2 μg/mL (both from Mabtech AB). Tetanus and diphtheria toxoid was purchased from Statens Serum Institut.
Flow cytometry
Single-cell suspensions were prepared from PBMCs and immunophenotyping of lymphocytes was performed by 4-color cytometry on a FACSCalibur (BD Biosciences) using CellQuest software (BD Biosciences) as described previously.29 Three cocktails of monoclonal antibodies were used to determine B-cell and plasma cell populations: (1) anti–CD19-R-phycoerythrin-Cyanine 7 (PC7), anti-CD27–fluorescein isothiocyanate (FITC; both from DAKO), anti-CD5–allophycocyanin (APC; BD Biosciences), and anti-IgD–phycoerythrin (PE) (SouthernBiotech); (2) anti-CD19–PC7, anti-IgD–PE, anti-CD38–APC, and anti-CD77–FITC (both from BD Biosciences); (3) anti-CD19–PC7, anti-CD138–PE (Miltenyi Biotec), and anti-IgA-FITC and anti–IgG–APC (both from Jackson ImmunoResearch) or anti-CD38–APC. All antibodies were initially pretitrated to ensure uniform compensation characteristics, and isotype controls (mouse IgG1, rabbit F(ab′)2, goat F(ab′)2; all from Jackson ImmunoResearch) were embedded to detect unspecific staining. To analyze spontaneous apoptosis of purified CD19+ B cells, the following cocktail of monoclonal antibodies was used: anti-annexin V–FITC (BD Biosciences), anti-CD27–PC5 (Beckman Coulter), and anti-IgD–APC (Miltenyi Biotec). In addition, ethidium homodimer-1 (Invitrogen Corporation) was used to exclude dead cells. As an internal control, apoptosis was induced for 9 hours using an anti–human CD95 mAb (clone DX2; BD Biosciences).
Purification of CD19+ B cells and CD138+ plasma cells
CD19+ B cells or plasma cells expressing the CD138 (Syndecan-1) antigen were magnetically purified using either the EasySep Human CD19 or CD138 Positive Selection Kit (StemCell Technologies) according to the manufacturer's protocol. Flow cytometry (FCM) analysis of the positively selected CD19+ or CD138+ cells and the CD19− or CD138− fraction was carried out to verify purification efficacy.
ELISPOT protocol
MultiScreenHTS Filter Plates (Millipore Corp) were prewet with 30% ethanol, rinsed 3 times with sterile phosphate-buffered saline (sPBS), and coated overnight at 4°C with either polyclonal rabbit anti–human IgG or IgA capture antibody (DAKO) diluted in sPBS at 10 μg/mL. After washing, plates were blocked for 3 hours with sPBS containing 1% bovine serum albumin (Sigma-Aldrich). On day 5 of culture, PBMCs were washed with twice their culture volume and plated at 5 × 104 PBMCs/well and 105PBMCs/well in cell culture medium as described in “Cell separation, antibodies, cytokines, and culture conditions.” Enzyme-linked immunosorbent spot assay (ELISPOT) plates were incubated at 37°C for 20 hours in the presence of 5% CO2. Thereafter, the plates were washed 6 times using PBS containing 0.01% Tween20 (PBS-Tween; Sigma-Aldrich). Detection antibodies goat anti–human IgG-ALP (Mabtech AB) and goat anti–human IgA-ALP (SouthernBiotech) were diluted in PBS containing 0.5% bovine serum albumin and added at a final concentrations of 2 μg/mL. After overnight incubation at 4°C, the plates were washed 6 times with PBS-Tween. Spot development was carried out using the BCIP (5-bromo-4-chloro-3-indoyl phosphate/NBT (nitro blue tetrazolium) Liquid Substrate System (Sigma-Aldrich). ELISPOT plate analysis and subsequent enumeration of cell counts and immunoglobulin amount were performed on the AID EliSpot 04 HR Reader using appropriate AID reader software, release 4.0 (Autoimmun Diagnostika GmbH). The immunoglobulin amount is measured in a virtual unit that is equivalent to the surface in 0.01 mm2 multiplied by the intensity of a particular spot.
ELISA protocol
IgG antibodies to tetanus toxoid and diphtheria toxoid were quantified in culture supernatants at day 7 of culture from 2 × 106 PBMCs stimulated with a combination of IL-21, IL-4, and anti-CD40 mAb using VaccZyme Tetanus toxoid IgG or VaccZyme Diphtheria toxoid IgG assays (The Binding Site). Enzyme-linked immunosorbent assay (ELISA) protocols and calibrators were modified to allow detection of very low amounts of toxoid-specific IgG antibodies (> 0.004 IU/mL anti-tetanus toxoid IgG, > 0.001 IU/mL anti-diphtheria toxoid IgG). The absorbance of all ELISA plate samples was measured at 450 nm and analyzed with a SPECTRA Classic microplate reader and appropriate reader software (TECAN Trading).
Silencing of AID mRNA expression by RNA interference
For silencing of activation-induced cytidine deaminase (AID) mRNA expression, a siRNA reagent from Santa Cruz Biotechnology was used according to the manufacturer's instructions. Briefly, AID siRNA (sc-42729) was transfected into 3 × 105 PBMCs, stimulated previously with IL-21, IL-4, and mAb anti-CD40 for 24 hours. A scrambled siRNA (siRNA-A, sc-37007), not leading to degradation of any known cellular mRNA, was included as a control. RNA interference–mediated knockdown of AID mRNA expression was verified by reverse-transcription–polymerase chain reaction (RT-PCR) 72 hours after transfection. AID expression in control samples was considered as 100% expression level, whereas samples containing no RNA were treated as blank values (0% expression level).
RNA isolation and real-time quantitative RT-PCR
RNA was extracted from 106 PBMCs after 14 hours of culture with mAb anti-CD3 or 72 hours of culture with IL-21, IL-4, and mAb anti-CD40 using RNeasy Plus Mini Kits (QIAGEN). One-step cDNA reverse-transcription and real-time PCR was conducted using the SYBR Green I RNA Master Mix (Roche Applied Science) and run on a LightCycler 2.0 System (Roche Applied Science). Primer sequences are given in supplemental Table 2. Data were analyzed using the LightCycler Data Analysis software (Roche Applied Science). The results are given as the ratio of the calculated amount of candidate RNA in a given sample by the calculated amount of the housekeeping control β-actin gene in the same sample. β-Actin also served as an endogenous control and for intersample normalization.
Sequence analysis of the IL21 gene
Sequence-specific primers were used to amplify all exons of the IL21 gene as previously described by Salzer et al.30 The PCR products were run in a 1% agarose gel and specific bands were excised using a sterile scalpel. The PCR products from the agarose slices were purified using the QIAquick Gel Extraction Kit (QIAGEN) and sent for direct sequencing (Macrogen).
Results
IL-21 cooperates with IL-2 and IL-4 to potentiate immunoglobulin production in anti-CD40–activated human B cells
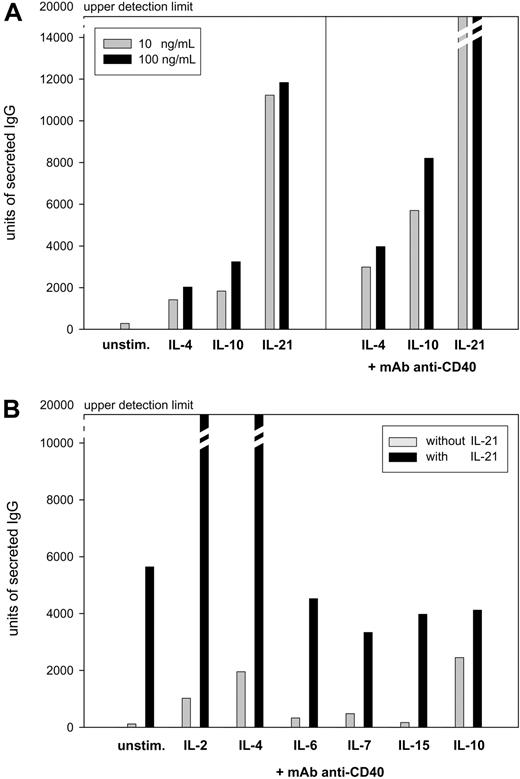
To compare the efficacy of IL-21 with known inducers of immunoglobulin production, freshly isolated PBMCs from healthy donors were stimulated with IL-4, IL-10, or IL-21 at 10 ng/mL alone or in combination with an anti-CD40 mAb at 2 μg/mL. IL-21 stimulation alone gave rise to higher amounts of IgG and IgA than cultures stimulated with IL-4 or IL-10 (Figure 1A; similar data for IgA production not shown). When B cells were activated by anti-CD40 stimulation, additional IL-21 stimulation resulted in much higher levels of IgG and IgA. In comparison with IL-4– or IL-10–induced Ig production of anti-CD40–stimulated PBMCs, the production of IgG and IgA induced by IL-21 was several-fold higher (Figure 1A; similar data for IgA production not shown). We furthermore investigated whether IL-21–induced immunoglobulin production could be potentiated by other members of the γc family of cytokines or IL-10 when added in a suboptimal concentration (0.5 ng/mL). In contrast to IL-6, IL-7, IL-15, and IL-10, both IL-2 and IL-4 strongly enhanced IL-21–driven IgG and IgA production (Figure 1B; data for IgA production not shown). Based on these data, all subsequent experiments were conducted using stimulation with a combination of IL-21, IL-4, and anti-CD40.
Induction of IgG production in PBMCs from 5 randomly chosen healthy donors presented as mean values. Units of secreted IgG were measured in a virtual unit that is equivalent to the surface in 0.01 mm2 multiplied by the intensity of a particular spot in ELISPOT analysis. (A) PBMCs (106) were stimulated for 5 days with either 10 or 100 ng/mL IL-4, IL-10, or IL-21 alone or in combination with 2 μg/mL anti-CD40 mAb. Subsequently, 105 PBMCs were subjected to ELISPOT assay for 20 hours of incubation. (B) PBMCs (106) were stimulated for 5 days with 2 μg/mL anti-CD40 mAb and 0.5 ng/mL of either IL-2, IL-4, IL-6, IL-7, IL-15, or IL-10 alone or in combination with 10 ng/mL IL-21. PBMCs (5 × 104) were subjected to ELISPOT assay for 20 hours of incubation.
Induction of IgG production in PBMCs from 5 randomly chosen healthy donors presented as mean values. Units of secreted IgG were measured in a virtual unit that is equivalent to the surface in 0.01 mm2 multiplied by the intensity of a particular spot in ELISPOT analysis. (A) PBMCs (106) were stimulated for 5 days with either 10 or 100 ng/mL IL-4, IL-10, or IL-21 alone or in combination with 2 μg/mL anti-CD40 mAb. Subsequently, 105 PBMCs were subjected to ELISPOT assay for 20 hours of incubation. (B) PBMCs (106) were stimulated for 5 days with 2 μg/mL anti-CD40 mAb and 0.5 ng/mL of either IL-2, IL-4, IL-6, IL-7, IL-15, or IL-10 alone or in combination with 10 ng/mL IL-21. PBMCs (5 × 104) were subjected to ELISPOT assay for 20 hours of incubation.
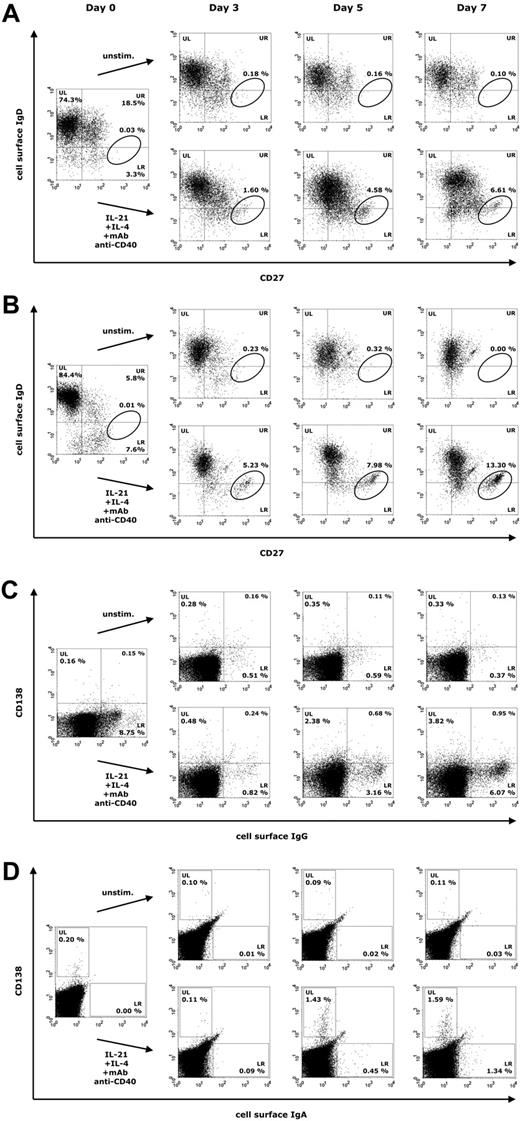
We next studied how a combination of IL-21, IL-4, and anti-CD40 mAb influences B-cell proliferation and differentiation in PBMCs at days 3, 5, and 7 of culture in 18 healthy blood donors. Already at day 3, both naive and memory B cells responded to this mixture, resulting in the formation of a CD27high IgD− (CD38low) population at day 7, representing activated B cells that are most likely committed to the plasma cell lineage (Figure 2A). In healthy donors, surface IgG+ (sIgG+) and surface IgA+ (sIgA+) B cells were present in substantial numbers, whereas activated CD138+ plasma cells were barely detectable in freshly isolated PBMCs (Figure 2B-C). During culture with IL-21, IL-4, and anti-CD40 mAb, 2 populations of Ig-secreting cells emerged, consisting of sIg+ CD138− B cells (Figure 2B-C, lower right [LR] quadrants) and CD138+ sIg− plasma cells (Figure 2B-C, upper left [UL] quadrants).
Representative expression of CD27, CD138 and surface IgD, IgG, and IgA on lymphogated cells in a healthy donor. Cell surface expression of these markers is presented on a 4-decade log scale as dot plots of correlated x-axis and y-axis fluorescence. FCM analysis was performed at days 0, 3, 5, and 7 with PBMCs cultured in the presence of IL-21 (10 ng/mL), IL-4 (0.5 ng/mL), and anti–human CD40 mAb (2 μg/mL). (A) Quadrant markers were positioned to include naive mature B cells (upper left [UL]), natural effector B cells (upper right [UR]), and IgD− memory B cells (lower right [LR]). The circle tags a population of CD27high IgD− B cells. (B) Regions were positioned to separate CD138+ plasma cells (UL) from sIgA+ B cells (LR). (C) Quadrant markers were positioned to separate CD138+ plasma cells (UL) from sIgG+ B cells (LR).
Representative expression of CD27, CD138 and surface IgD, IgG, and IgA on lymphogated cells in a healthy donor. Cell surface expression of these markers is presented on a 4-decade log scale as dot plots of correlated x-axis and y-axis fluorescence. FCM analysis was performed at days 0, 3, 5, and 7 with PBMCs cultured in the presence of IL-21 (10 ng/mL), IL-4 (0.5 ng/mL), and anti–human CD40 mAb (2 μg/mL). (A) Quadrant markers were positioned to include naive mature B cells (upper left [UL]), natural effector B cells (upper right [UR]), and IgD− memory B cells (lower right [LR]). The circle tags a population of CD27high IgD− B cells. (B) Regions were positioned to separate CD138+ plasma cells (UL) from sIgA+ B cells (LR). (C) Quadrant markers were positioned to separate CD138+ plasma cells (UL) from sIgG+ B cells (LR).
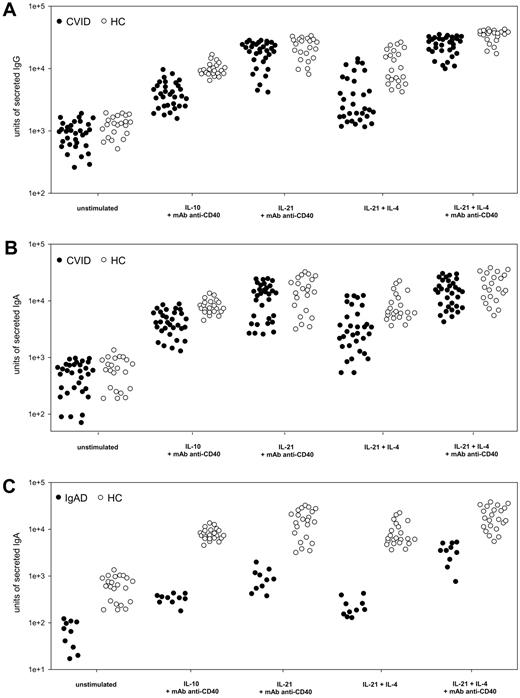
IL-21 induces immunoglobulin production in patients with CVID or IgAD
To investigate whether IL-21 stimulation is effective in patients with an established diagnosis of CVID (n = 34) or IgAD (n = 10), we studied Ig secretion in patients' PBMCs using an ELISPOT single-cell assay. Cells were cultivated with either IL-21 (10 ng/mL) alone or together with IL-4 (0.5 ng/mL) or with addition of anti-CD40 mAb (2 μg/mL). A B-cell differentiation protocol consisting of 10 ng/mL IL-10 plus 2 μg/mL anti-CD40 mAb was used for comparison, as described by Hummelshoj et al.11 Whereas IL-10 induced moderate Ig production, IL-21 stimulation resulted in several-fold higher amounts of secreted IgG and IgA (Figure 3A-C). Even IL-21 plus IL-4 without further CD40 stimulation was more effective than IL-10 plus mAb anti-CD40 in inducing Ig production in some patients. When Ig secretion in anti-CD40–activated PBMCs was compared between cells stimulated solely with IL-21 or IL-21 plus IL-4, the latter markedly increased the average IgG and IgA production almost to the levels observed in healthy donors (Figure 3A-C). In general, IgA production in CVID was not as effectively induced as IgG production. No correlation was found when IL-21–induced IgG or IgA production in patients with CVID was compared with patients' B-cell subsets (supplemental Table 1). To investigate the ability of IL-21 to induce antigen-specific immunoglobulin production ex vivo, PBMCs from 4 patients with CVID were stimulated for 7 days in the presence of IL-21, IL-4, anti-CD40 mAb, and either diphtheria or tetanus toxoid. Patients' files showed that previous immunization with diphtheria and tetanus toxoid vaccine within the last 4 years did not result in protective levels of toxoid-specific antibodies. In healthy controls, toxoid-specific IgG was detectable in samples stimulated by IL-21, IL-4, and anti-CD40 mAb, and was noticeably enhanced when diphtheria or tetanus toxoid was added to the cultures (supplemental Figure 1A). In CVID patients, toxoid-specific IgG was absent in samples stimulated by IL-21, IL-4, and anti-CD40 mAb alone. However, when diphtheria or tetanus toxoid was added during cell culture, a small amount of specific antibodies was detected (supplemental Figure 1A).
Immunoglobulin production in patients with CVID or IgAD. Effect of cytokine and anti-CD40 stimulation on IgG and IgA production in PBMCs from 32 patients with CVID (A-B) and 10 subjects with IgAD (C) in comparison with 22 healthy controls (HC). PBMCs (106) were stimulated for 5 days with cytokines (IL-10 and IL-21 at 10 ng/mL, IL-4 at 0.5 ng/mL) plus anti-CD40 mAb at 2 μg/mL, if stated. Subsequently, 5 × 104 and 1 × 105 PBMCs were subjected to ELISPOT assay for 20 hours of incubation. Units of secreted IgG (A) and IgA (B-C), presented on a logarithmic scale, were measured in a virtual unit that is equivalent to the surface in 0.01 mm2 multiplied by the intensity of a particular spot in ELISPOT analysis.
Immunoglobulin production in patients with CVID or IgAD. Effect of cytokine and anti-CD40 stimulation on IgG and IgA production in PBMCs from 32 patients with CVID (A-B) and 10 subjects with IgAD (C) in comparison with 22 healthy controls (HC). PBMCs (106) were stimulated for 5 days with cytokines (IL-10 and IL-21 at 10 ng/mL, IL-4 at 0.5 ng/mL) plus anti-CD40 mAb at 2 μg/mL, if stated. Subsequently, 5 × 104 and 1 × 105 PBMCs were subjected to ELISPOT assay for 20 hours of incubation. Units of secreted IgG (A) and IgA (B-C), presented on a logarithmic scale, were measured in a virtual unit that is equivalent to the surface in 0.01 mm2 multiplied by the intensity of a particular spot in ELISPOT analysis.
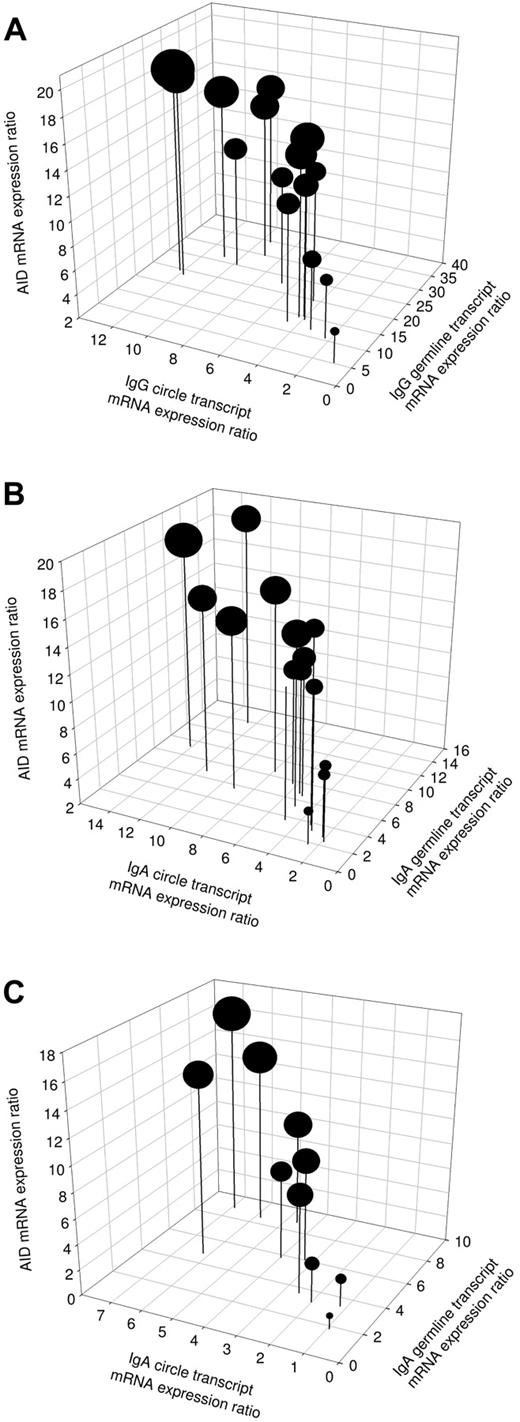
Involvement of active CSR in IL-21–reconstituted immunoglobulin production in patients with CVID or IgAD
To elucidate the molecular mechanism involved in immunoglobulin production induced by IL-21 plus IL-4 and mAb anti-CD40 stimulation, mRNA levels were analyzed for AID, Iγ-Cγ, or Iα-Cα germline transcripts (GLTs) and Iγ-Cμ or Iα-Cμ switch circle transcripts (CTs) in 15 patients with CVID and 10 subjects with IgAD. In unstimulated cells, the baseline mRNA expression of AID, GLT, and switch CT corresponded to expression ratios of 0 to 4 (data not shown). In patients' cells stimulated with IL-21, IL-4, and mAb anti-CD40, the expression of AID was up-regulated at day 3 of culture in all analyzed samples (Figure 4A-C). Furthermore, the production of Iγ-Cγ and Iα-Cα germline transcripts, representing early steps in immunoglobulin isotype switching, was up-regulated in all analyzed patient samples after stimulation with IL-21, IL-4, and mAb anti-CD40 (Figure 4A-C). In comparison with Iγ-Cγ GLT, there was a trend for a reduced expression of Iα-Cα GLT in all CVID patients. This reflects superior effects of IL-21 plus IL-4 and mAb anti-CD40 stimulation to induce IgG compared with IgA switching. Switch circle transcripts reflect active CSR events and provide a reliable parameter for detection of ongoing CSR. Therefore, the presence of CT was subsequently analyzed (Figure 4A-C). Expression of Iγ-Cμ switch CT was up-regulated in all CVID patients and Iα-Cμ switch CT was up-regulated in all CVID and IgAD patients after stimulation with IL-21, IL-4, and mAb anti-CD40. Patients with the lowest expression of CT also produced the least IgG and IgA (Figure 4A-B). No correlation was found between the patients' IgG or IgA serum levels and the expression ratios of Iγ-Cγ or Iα-Cα GLT. There were 3 CVID patients who showed a low AID expression, which was associated with a subsequent low expression of germline transcripts, switch circle transcripts, and Ig production (Figure 4A-B). This may suggest a lower level of CSR in response to the stimulation in this subset of patients, which may be explained by the heterogeneous etiology of CVID. Overall, these findings indicate that CSR is induced by a combination of IL-21, IL-4, and anti-CD40 stimulation in B cells from patients with CVID and IgAD.
Induction of active CSR in patients with CVID or IgAD. Expression of AID mRNA, rate of Iγ-Cγ or Iα-Cα germline transcription, and presence of Iγ-Cμ or Iα-Cμ switch circle transcripts in PBMCs from 15 patients with CVID (A-B) and 10 subjects with IgAD (C) at day 3 of cell culture with IL-21 (10 ng/mL), IL-4 (0.5 ng/mL), and anti–human CD40 mAb (2 μg/mL). The diameter of the dots correlates proportionally with units of secreted IgG (A) or IgA (B-C), detected in 20 hours of ELISPOT assay at day 5 of culture using 5 × 104 PBMCs.
Induction of active CSR in patients with CVID or IgAD. Expression of AID mRNA, rate of Iγ-Cγ or Iα-Cα germline transcription, and presence of Iγ-Cμ or Iα-Cμ switch circle transcripts in PBMCs from 15 patients with CVID (A-B) and 10 subjects with IgAD (C) at day 3 of cell culture with IL-21 (10 ng/mL), IL-4 (0.5 ng/mL), and anti–human CD40 mAb (2 μg/mL). The diameter of the dots correlates proportionally with units of secreted IgG (A) or IgA (B-C), detected in 20 hours of ELISPOT assay at day 5 of culture using 5 × 104 PBMCs.
We next investigated whether silencing of AID expression in PBMCs from 8 patients with CVID prevents production of isotype-switched immunoglobulins induced by a combination of IL-21, IL-4, and anti-CD40 stimulation. An AID-siRNA–based approach was used that reduced the expression of AID mRNA in patients' PBMCs by more than 84% at day 4 of culture compared with siRNA control samples (supplemental Figure 1B). Numbers of sIgG+ B cells within the CD19+ lymphogate were only marginally reduced by AID silencing (supplemental Figure 1B; FCM data for sIgA+ B cells not shown), and the production of IgG at day 5 of culture was reduced by approximately 58% (production of IgA reduced by ∼ 53%) in AID-silenced PBMCs (supplemental Figure 1B; data for IgA production not shown). Because of the remaining baseline AID expression in AID-silenced PBMCs, it is unclear whether these findings indicate AID-independent proliferation and differentiation of B cells by IL-21 in CVID.
Surface IgG+ and IgA+ B cells accumulate in cultures during IL-21 stimulation and account for early immunoglobulin production in patients with CVID or IgAD
B-cell proliferation induced by a combination of IL-21, IL-4, and anti-CD40 stimulation was subsequently analyzed by FCM in 26 patients with CVID and 9 subjects with IgAD to identify differentiation of Ig-secreting cells. Representative results are shown in Figure 5A-D. In the majority of analyzed CVID and IgAD patients, a CD27high IgD− (CD38low) population arises at day 5 of culture that is recruited mainly from naive B cells (Figure 5A for a CVID patient and Figure 5B for a patient with IgAD). In PBMCs stimulated with IL-21, IL-4, and anti-CD40 mAb, a substantial population of sIgG+ and sIgA+ B cells emerges in patients with CVID, accompanied by a population of CD138+ plasma cells, albeit smaller than that seen in healthy donors (Figure 5C; FCM data for sIgA not shown). CD138+ peripheral blood plasma cells were also reduced in patients with CVID at day 0 of culture, compared with age-matched control groups (supplemental Table 1). In patients with IgAD, no surface IgA+ B cells were detected in any patient at day 0 of culture (Figure 5D). At day 5 of culture with IL-21, IL-4, and anti-CD40 mAb, however, both a small-sized population of sIgA+ B cells and CD138+ plasma cells were detected.
Representative expression of CD27, CD138, and surface IgD, IgG, and IgA on lymphogated cells in patients with CVID or IgAD. Cell surface expression of these markers is presented in panels A and C for CVID patients and in panels B and D for subjects with IgAD on a 4-decade log scale as dot plots of correlated x-axis and y-axis fluorescence. FCM analysis was performed at days 0, 3, 5 (and 7), with PBMCs cultured in the presence of IL-21 (10 ng/mL), IL-4 (0.5 ng/mL), and anti–human CD40 mAb (2 μg/mL). (A-B) Quadrant markers were positioned to include naive mature B cells (UL), natural effector B cells (UR), and IgD− memory B cells (LR). The circle tags a population of CD27high IgD− B cells. (C) Quadrant markers were positioned to separate CD138+ plasma cells (UL) from sIgG+ B cells (LR). (D) Regions were positioned to separate CD138+ plasma cells (UL) from sIgA+ B cells (LR).
Representative expression of CD27, CD138, and surface IgD, IgG, and IgA on lymphogated cells in patients with CVID or IgAD. Cell surface expression of these markers is presented in panels A and C for CVID patients and in panels B and D for subjects with IgAD on a 4-decade log scale as dot plots of correlated x-axis and y-axis fluorescence. FCM analysis was performed at days 0, 3, 5 (and 7), with PBMCs cultured in the presence of IL-21 (10 ng/mL), IL-4 (0.5 ng/mL), and anti–human CD40 mAb (2 μg/mL). (A-B) Quadrant markers were positioned to include naive mature B cells (UL), natural effector B cells (UR), and IgD− memory B cells (LR). The circle tags a population of CD27high IgD− B cells. (C) Quadrant markers were positioned to separate CD138+ plasma cells (UL) from sIgG+ B cells (LR). (D) Regions were positioned to separate CD138+ plasma cells (UL) from sIgA+ B cells (LR).
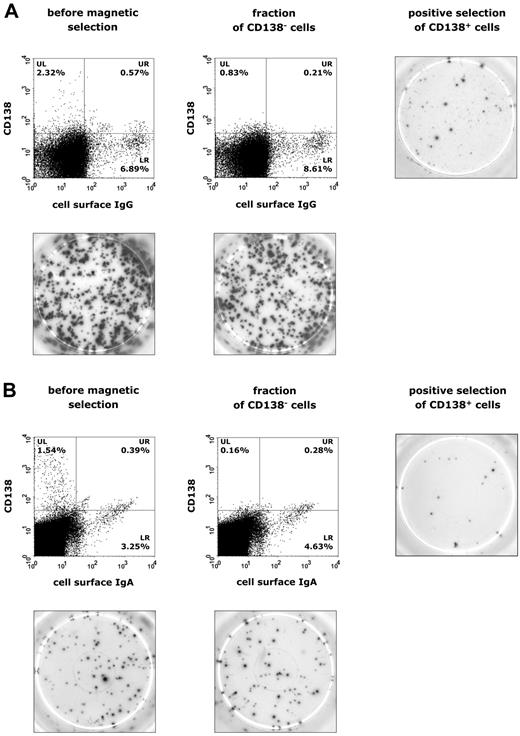
To relate IL-21–induced immunoglobulin production to potential Ig-secreting populations, PBMCs were separated by magnetic beads and purified into CD138+ plasma cells and CD138− cells, which were subsequently subjected to ELISPOT analysis of IgG and IgA secretion. Interestingly, in both CVID (n = 12) and IgAD (n = 5) patients, CD138+ plasma cells accounted for only a small fraction of overall IgG or IgA production (Figure 6A-B). FCM data showed that a weak population of sIgG+ and sIgA+ B cells is already present at day 3 of culture and, therefore, these B cells are likely to represent already isotype-committed cells that show surface Ig expression and Ig production in response to stimulation with IL-21, IL-4, and anti-CD40 (Figure 5C-D). The population of IL-21–induced CD138+ plasma cells may thus rather represent terminally differentiated, activated memory B cells, explaining their low-rate occurrence in CVID and IgAD patients.
Identification of IgG- and IgA-producing cell populations in patients with CVID or IgAD. Representative results are shown in panel A for CVID patients and in panel B for subjects with IgAD. Expression of CD138 and surface IgG or IgA on lymphogated cells was analyzed before and after magnetic separation of CD138+ plasma cells from PBMCs cultured in the presence of IL-21 (10 ng/mL), IL-4 (0.5 ng/mL), and anti–human CD40 mAb (2 μg/mL) for 5 days. Cell surface expression of these markers is presented on a 4-decade log scale as dot plots of correlated x-axis and y-axis fluorescence. Images of ELISPOT assay were taken after 20 hours of incubation of 5 × 104 PBMCs for IgG production (A) or 1 × 106 PBMCs for IgA production (B).
Identification of IgG- and IgA-producing cell populations in patients with CVID or IgAD. Representative results are shown in panel A for CVID patients and in panel B for subjects with IgAD. Expression of CD138 and surface IgG or IgA on lymphogated cells was analyzed before and after magnetic separation of CD138+ plasma cells from PBMCs cultured in the presence of IL-21 (10 ng/mL), IL-4 (0.5 ng/mL), and anti–human CD40 mAb (2 μg/mL) for 5 days. Cell surface expression of these markers is presented on a 4-decade log scale as dot plots of correlated x-axis and y-axis fluorescence. Images of ELISPOT assay were taken after 20 hours of incubation of 5 × 104 PBMCs for IgG production (A) or 1 × 106 PBMCs for IgA production (B).
IL-21 prevents increased spontaneous apoptosis of naive B cells from patients with CVID or IgAD
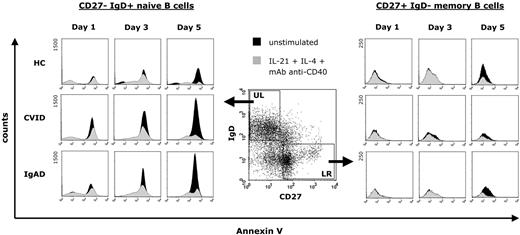
To investigate the influence of a stimulation with IL-21, IL-4, and anti-CD40 mAb on apoptosis of peripheral B cells, we cultured CD19+ purified B cells from 14 patients with CVID, 6 subjects with IgAD, and 12 healthy blood donors for 5 days. Representative results are shown in Figure 7. The rate of spontaneous apoptosis of CD27− IgD+ naive B cells from both CVID and IgAD patients was considerably higher already at day 1 of culture compared with naive B cells from healthy controls, and was continuously increasing during culture until day 5. In contrast, this was not observed in CD27+ IgD− memory B cells, suggesting that increased apoptosis is limited to peripheral blood B-cell subsets in patients with CVID or IgAD. When CD19+ purified B cells were cultured in the presence of IL-21, IL-4, and anti-CD40 mAb, the rate of apoptosis of both CD27− IgD+ naive B cells and CD27+ IgD− memory B cells was markedly reduced in all analyzed subjects and was comparable between B cells from patients and those from healthy controls.
Representative effect of a combination of IL-21, IL-4, and anti-CD40 stimulation on the spontaneous apoptosis of peripheral blood CD19+ purified B cells from patients with CVID or IgAD. After exclusion of dead cells, equal numbers of gated B cells were assayed for annexin V staining cells at days 1, 3, and 5 either without stimulation during culture or after stimulation with IL-21 (10 ng/mL), IL-4 (0.5 ng/mL), and anti–human CD40 mAb (2 μg/mL). Annexin V expression is presented on a 4-decade log scale as histogram plots with a linear scale of the number of events at the y-axis.
Representative effect of a combination of IL-21, IL-4, and anti-CD40 stimulation on the spontaneous apoptosis of peripheral blood CD19+ purified B cells from patients with CVID or IgAD. After exclusion of dead cells, equal numbers of gated B cells were assayed for annexin V staining cells at days 1, 3, and 5 either without stimulation during culture or after stimulation with IL-21 (10 ng/mL), IL-4 (0.5 ng/mL), and anti–human CD40 mAb (2 μg/mL). Annexin V expression is presented on a 4-decade log scale as histogram plots with a linear scale of the number of events at the y-axis.
Expression of IL-21 and IL-21R and sequencing of the IL-21 gene in patients with CVID
Given that T-cell abnormalities are common in CVID, we also studied the expression of IL-21 and IL-21R mRNA upon activation of T cells stimulated by an anti–human CD3 mAb. Thirty patients with CVID were compared with 22 healthy subjects, randomly selected from a pool of blood donors. In CVID patients, expression of IL-21 and IL-21R mRNA after 14 hours of stimulation was approximately 10% to 16% lower compared with the mean values of healthy controls (supplemental Figure 1C). The same trend was seen in unstimulated samples.
We subsequently screened 16 unrelated patients with an established diagnosis of CVID for mutations in the IL21 gene. These patients were all included in the experiments evaluating involvement of CSR induced by IL-21 (Figure 4A-B). We found no mutations in the coding regions of the IL21 gene in our patients. In exon 3 of the IL21 gene, a previously reported synonymous change (dbSNP: rs4833837) was found in all patients except 1, a frequency similar to that reported for healthy controls in public databases (PGA-EUROPEAN-PANEL). Furthermore, we found a heterozygous single nucleotide polymorphism (SNP; dbSNP: rs17879298) in the 3′ untranslated region in 5 of our patients that was linked to another heterozygous SNP (dbSNP: rs17886348), both being present insignificantly less frequently in healthy controls (PGA-EUROPEAN-PANEL).
Discussion
B-cell maturation defects and defective class-switch recombination, blocks in plasma cell differentiation, or abnormal apoptosis of B cells provide a basis for CVID and IgAD.10,31-35 Previous reports have demonstrated that blocks in B-cell differentiation, decreased CSR, and lowered Ig production in CVID and IgAD can be partly overcome ex vivo by anti-CD40 stimulation of B cells and simultaneous addition of IL-10, IL-4, IL-2, or transforming growth factor β.8-12 Although these findings suggest that normalization of Ig production in IgAD and CVID is achievable by CD40 triggering and that addition of cytokines is critical to induction of B-cell proliferation and CSR, little is known about the mechanisms allowing Ig production in IgAD and CVID.
This is the first report, to our knowledge, evaluating the efficacy of IL-21 to restore immunoglobulin production ex vivo in primary immunodeficiency diseases. Our results show that IL-21 in combination with CD40 costimulation is far more effective in inducing IgG or IgA production in patients with CVID or IgAD than known promoters of immunoglobulin production, such as IL-10 or IL-4, reflecting a major advantage of an IL-21–based approach. We observed that CD40 triggering enhances the IL-21–induced Ig production significantly. For this reason CD40 costimulation might be of critical importance for, and a limiting factor of, a therapeutic use of IL-21. Furthermore, we have shown that PBMCs from patients with CVID previously immunized with diphtheria and tetanus toxoid vaccine produce traces of toxoid-specific antibodies when cultured with a combination of IL-21, IL-4, and anti-CD40 mAb. However, future studies are needed to evaluate a potential use of therapies including IL-21 to improve the outcome of vaccinations in CVID.
Naive B cells represent the predominant B-cell subset in patients with CVID, whereas CD27+ IgD− isotype-switched memory B cells are generally reduced, with an association between their percentage and the frequency of infectious complications.32,36 This implies that a therapy expanding the proportion of switched B cells by favoring differentiation of naive B cells in vivo could be a major achievement in the treatment of CVID. Here, we have demonstrated that culturing of PBMCs with IL-21, IL-4, and anti-CD40 mAb ex vivo leads to the formation of a CD27high IgD− CD38low population recruited predominantly from naive B cells in patients with CVID, representing a transitional stage of plasma cell differentiation that is characterized by the ability to produce high levels of isotype-switched immunoglobulins.37,38 Consistent with the importance of IL-21 as a switch factor for the production of IgG by human B cells from healthy donors, we also show that stimulation with IL-21 together with IL-4 and anti-CD40 mAb induces active CSR in patients with CVID or IgAD.39,40 However, it is unclear whether AID-independent proliferation is also induced by IL-21 in B cells from patients with CVID, which could highlight the role of IL-21 as a powerful promoter of B-cell differentiation into Ig-secreting cells in addition to its effects on CSR.41-43 We confirm that the number of CD138+ circulating peripheral blood plasma cells is reduced in patients with CVID and might thus contribute to the lack of switched immunoglobulin isotypes. Although CD138+ plasma cells generated within 7 days of culture with IL-21 only marginally contributed to the overall immunoglobulin production, it is subject to future studies whether prolonged administration of IL-21 in vivo might further promote the differentiation of circulating plasma cells into long-lasting immunoglobulin producers.
Given that IL-21 is a crucial factor for the differentiation of normal mature human B cells into Ig-secreting cells, we hypothesize that alterations in the IL21 gene could be responsible for the lack of switched Ig production in CVID. However, screening of 16 unrelated patients with an established diagnosis of CVID did not reveal any mutations in the IL21 gene. This observation is consistent with a previous report by Salzer et al.30 Furthermore, functional data leave little doubt on the normal expression of both IL-21 and the IL-21 receptor upon anti-CD3 stimulation of PBMCs in CVID. These findings raise the question why additional IL-21 induces restoration of the immune function in CVID although the patients' cytokine expression and function are presumably unaltered in peripheral blood cells. Recent evidence confirms that IL-4 and IL-10 production and the frequencies of polymorphisms in these genes are comparable with healthy controls, suggesting a minor, if any, role in the pathogenesis of these diseases.30,44-47 Furthermore, CD40 ligand (CD40L/CD154), acting as an important component of the mechanism of Ig production, is normally expressed on stimulated T lymphocytes in a predominant proportion of CVID patients.45,46,48 Taken together, it appears that the present abnormalities of B-cell differentiation and failure to produce normal amounts of switched immunoglobulin in CVID and IgAD are not due to defect signaling through IL-4, IL-10, or IL-21 and CD40. Furthermore, the ability to undergo CSR and plasma cell differentiation when stimulated with IL-21, IL-4, and anti-CD40 mAb implies that B cells from patients with CVID or IgAD are not lacking the molecular machinery necessary for such processes. In addition, the population of surface IgG+ and IgA+ B cells, which are present at day 5 of culture after stimulation with IL-21, IL-4, and anti-CD40 mAb, suggests that CVID and IgAD B cells undergo differentiation when cultured under adequate stimulatory conditions.
Several reasons could account for the failure of stimulation during B-cell maturation and differentiation in CVID and IgAD. The first is that bone marrow progenitor cells in CVID show impaired growth and differentiation capacity, associated with a skewing of cytokine production toward a proapoptotic pattern.49 Moreover, increased apoptosis of CD20+ IgA+ B cells has previously been proposed to be involved in the etiology of IgAD.10,31 Our analyses also show that patients with IgAD lack surface IgA+ B cells, whereas a combination of IL-21, IL-4, and anti-CD40 mAb prevents apoptosis of B cells in IgAD and CVID patients and results in reconstitution of terminal B-cell differentiation. Based on these results, we suggest that an IL-21–based therapeutic approach also prevents abnormal apoptotic pathways in CVID and IgAD.10,22,31,50 A second possibility is that the impaired B-cell differentiation in CVID and IgAD might be due to inadequate production of cytokines or expression of costimulatory molecules at local sites of B-cell proliferation, including primary and secondary lymphoid tissues, or within cellular regulators of inflammation, such as Th17 cells.21 This could originate from abnormal epigenetic modifications stably induced in cells of lymphoid tissues, affecting not only cytokine production but also B-cell traffic and homing, resulting in insufficient B-cell stimulation and/or an insufficient period of time of B-cell differentiation.51-54 Abnormal cytokine release pattern or kinetics, the regulation of cellular signaling pathway intermediates, and the putative threshold effect in collaboration between cytokines such as IL-21 and IL-4 on B-cell activation could also account for the failure of in vivo B-cell differentiation in IgAD and CVID.43,55-57
In conclusion, our findings shed light on the potential immunotherapeutic perspective of IL-21 to revert the differentiation blockage of Ig-committed B cells and to expand the proportion of Ig-producing switched B cells in CVID and IgAD. However, there is limited information on aspects of tolerability and efficacy for clinically applicable treatment. Further research is needed to develop strategies by which the activity and availability of IL-21–based immunotherapy could be optimized to be beneficial in primary immunodeficiency diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.B. performed the research, analyzed results, made the figures, and wrote the paper; Q.P.-H. and C.L. performed experiments and analyzed results; U.S., M.B., U.W., and D.G. designed the research; and L.H. designed the research and wrote the paper.
Conflict-of-interest disclosure: S.B., L.H., and U.S. declare competing financial interests because of their patent application EP 09 150 038.9. The remaining authors declare no competing financial interests.
Correspondence: Stephan Borte, Department of Clinical Immunology and Transfusion Medicine, University of Leipzig, Johannis-allee 30, D-04103 Leipzig, Germany; e-mail: stephan.borte@medizin.uni-leipzig.de.

![Figure 2. Representative expression of CD27, CD138 and surface IgD, IgG, and IgA on lymphogated cells in a healthy donor. Cell surface expression of these markers is presented on a 4-decade log scale as dot plots of correlated x-axis and y-axis fluorescence. FCM analysis was performed at days 0, 3, 5, and 7 with PBMCs cultured in the presence of IL-21 (10 ng/mL), IL-4 (0.5 ng/mL), and anti–human CD40 mAb (2 μg/mL). (A) Quadrant markers were positioned to include naive mature B cells (upper left [UL]), natural effector B cells (upper right [UR]), and IgD− memory B cells (lower right [LR]). The circle tags a population of CD27high IgD− B cells. (B) Regions were positioned to separate CD138+ plasma cells (UL) from sIgA+ B cells (LR). (C) Quadrant markers were positioned to separate CD138+ plasma cells (UL) from sIgG+ B cells (LR).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/19/10.1182_blood-2009-02-207423/4/m_zh89990944230002.jpeg?Expires=1769097175&Signature=zTBzPUm~BYpxzKKBU10xBVZqHSFTMj3E52oIN~6MWtkInoh0EeCUTj704gP7IYH4bNWc813h-OK4KI83V1liptQAWd-y1Tymn0OlEy5wmfFnGYeB8Z7BTjD4ieBleo7iTQDuN0wPE1kgQHSB1h0fOnLe0TIG-oGbLQj7j2g7~Ydo87bkk-b56Wis3TeKa0q8slh9ZeQcwL78qxbAC3tCSI5peuWBP8A-2~IpGiJ6JUHe3XeIUwxsmkxcXrmT1Z~hwbVbdKR-xyskozhFhOBXc9tJ8zsYrDvuJECwyYX1XfcI-t8A17f0syZV46CwthpDIf410IvXsmN33GpmszORAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)