Abstract
Blood group antigen immunogenicity is a crucial factor in red blood cell alloimmunization. Previous calculated estimates of immunogenicity suffered from several key shortcomings. To address these issues we have (1) introduced a correction factor for antibody persistence rates into traditional immunogenicity calculations, (2) calculated immunogenicities only in men to eliminate pregnancy-related antibodies, and (3) excluded antibodies reactive only at room temperature to minimize the contribution of naturally occurring antibodies. With these corrections, we have calculated the immunogenicities of common blood group antigens using data collected on clinically significant alloantibodies (n = 452) in a male patient population. We observed a 3- to 5-fold increase in immunogenicity for some antigens (ie, Jka, Cw, Lua) and smaller changes in others compared with traditionally calculated estimates. In addition, we have calculated the transfusion-related immunogenicities of antigens traditionally associated with naturally occurring antibodies (eg, anti-Lea, -Leb, -M, and -P1).
Introduction
The immunogenicity of a blood group antigen is an important factor in determining whether a person transfused with red blood cells expressing that antigen will develop the corresponding alloantibody.1 The “Giblett equation” is a common method used in estimating the immunogenicity of blood group antigens.2 This calculation involves dividing the total number of antibodies of a given specificity by the probability that an antigen-negative person will be transfused with antigen-positive red blood cells. This value is then normalized to the immunogenicity of the K antigen by dividing by the corresponding calculation for K. To date, the immunogenicities of antigens other than D and K have been based largely on this calculated approach.2-7
These calculated estimates are subject to several potential inaccuracies. For example, any factors that reduce antibody detection will cause an erroneous reduction in the calculated immunogenicity of the corresponding antigen. Antibody evanescence, that is, a decrease in antibody titers to below the limits of detection, is one such factor. Evanescence is a particular problem because alloantibody evanescence rates differ depending on the antigenic specificity of the antibody.8-11 No prior study has attempted to correct for this potential artifact.
Errors in the estimation of transfusion-related immunogenicity may also result from the inclusion of naturally occurring and pregnancy-related antibodies. Only transfusion-induced antibodies should be counted for the calculation, otherwise immunogenicities will be artifactually inflated. Moreover, because the circumstances leading to the development of pregnancy-related antibodies are different from those leading to transfusion-related antibodies, we believe that they should be studied separately. However, to our knowledge no prior study has excluded pregnancy-related antibodies from immunogenicity calculations. To eliminate naturally occurring antibodies, most previous immunogenicity estimates used a “shotgun” approach of excluding all anti-Lea, -Leb, -M, -N, and -P1 antibodies, because they are often naturally occurring.2-4 Because of this shotgun exclusion, the transfusion-related immunogenicities of antigens such as Lea, Leb, M, N, and P1 are not known, although these antigens can induce alloantibodies after transfusion.6 No prior study has used the selective approach of excluding antibodies reactive at room temperature, which would eliminate naturally occurring antibodies while permitting the calculation of any antigen's transfusion-related immunogenicity.
The goals of our study, therefore, were to develop an approach to reduce these sources of error. To accomplish this we (1) corrected traditional immunogenicity calculations for antibody persistence, (2) eliminated pregnancy-related alloantibodies by studying an exclusively male patient population, and (3) excluded antibodies reactive only at room temperature to minimize the contribution of naturally occurring antibodies. Our additional goal was to calculate transfusion-related immunogenicities of antigens traditionally associated with naturally occurring antibodies (eg, anti-Lea, -Leb, -M, -N, and -P1).
Methods
The number, specificities, and persistence rates of blood group alloantibodies documented in the paper transfusion records of 18 750 military veterans between 1961 and 2006 at the Veterans Affairs Connecticut Healthcare System (VACHCS) were described previously.8,12 We examined antibodies in this dataset detected in male patients and that could be found on typical commercial antibody screening panels.13 For immunogenicity calculations, we used previously published antigen frequency data for white Europeans because 80% of alloimmunized patients self-reported their race as white.12,14 Naturally occurring antibodies were defined as those that reacted only at room temperature. Alloantibodies detected in female patients and autoantibodies were excluded. Immunogenicity calculations for the D antigen were omitted because transfusions were matched for D at the VACHCS, causing the number of anti-D to be artifactually underrepresented compared with all other antibodies.
Fractional persistence rates for alloantibodies represent the ratio of persistently detected antibodies of an indicated specificity to the total antibodies of that specificity; rates were obtained from a previous study of this patient population.8 We calculated antibody persistence rates only for antibodies whose initial induction was documented at the study center to obtain more accurate persistence rates.8 The persistence rates of antibodies already present at the time of initial testing before transfusion at the study center can never truly be known, because we cannot determine the total number of antibodies induced before that initial testing.
Results and discussion
Of 540 total antibodies formed by male patients, 85.6% (462/540) were directed against clinically significant antigens included in a typical commercial panel (Table 1's “Total antibodies”). Antibodies likely to be naturally occurring, that is, those reactive only at room temperature, were subtracted from the total of each corresponding specificity to approximate those that were transfusion induced (Table 1's “Antibodies reacting at 37°C and/or antiglobulin phase”). With this definition, transfusion-induced antibodies constituted 97.8% (452/462) of antibodies made by men.
Comparison of the immunogenicities of blood group antigens obtained with corrected and traditional calculations, showing key data used in the calculation
| Antigen . | Total antibodies* . | Antibodies reacting at 37°C and/or antiglobulin phase* . | Traditional antigen potency† . | Fractional persistence rate‡ . | Antigen potency corrected for antibody persistence§ . | Fold change‖ . |
|---|---|---|---|---|---|---|
| Jka | 20 | 20 | 0.077 | 0.11 | 0.37 | 4.81 |
| Lua | 11 | 10 | 0.094 | 0.13 | 0.40 | 4.26 |
| Cw | 8 | 8 | 0.19 | 0.14 | 0.70 | 3.68 |
| P1 | 21 | 18 | 0.074 | 0.33 | 0.12 | 1.62 |
| Leb | 19 | 18 | 0.062 | 0.37 | 0.089 | 1.44 |
| M | 18 | 18 | 0.073 | 0.43 | 0.090 | 1.23 |
| Lea | 40 | 37 | 0.15 | 0.50 | 0.16 | 1.07 |
| K | 118 | 118 | 1.00 | 0.53 | 1.00 | 1.00 |
| E | 105 | 105 | 0.35 | 0.52 | 0.35 | 1.00 |
| c | 26 | 26 | 0.11 | 0.62 | 0.097 | 0.88 |
| Fya | 29 | 29 | 0.090 | 0.75 | 0.064 | 0.71 |
| C | 25 | 25 | 0.080 | 0.77 | 0.055 | 0.69 |
| S | 10 | 9 | 0.025 | 1.00 | 0.013 | 0.52 |
| V | 3 | 3 | 0.21 | |||
| e | 2 | 2 | 0.071 | |||
| s | 2 | 2 | 0.014 | |||
| N | 3 | 2 | 0.007 | |||
| Fyb | 1 | 1 | 0.005 | |||
| Jkb | 1 | 1 | 0.004 |
| Antigen . | Total antibodies* . | Antibodies reacting at 37°C and/or antiglobulin phase* . | Traditional antigen potency† . | Fractional persistence rate‡ . | Antigen potency corrected for antibody persistence§ . | Fold change‖ . |
|---|---|---|---|---|---|---|
| Jka | 20 | 20 | 0.077 | 0.11 | 0.37 | 4.81 |
| Lua | 11 | 10 | 0.094 | 0.13 | 0.40 | 4.26 |
| Cw | 8 | 8 | 0.19 | 0.14 | 0.70 | 3.68 |
| P1 | 21 | 18 | 0.074 | 0.33 | 0.12 | 1.62 |
| Leb | 19 | 18 | 0.062 | 0.37 | 0.089 | 1.44 |
| M | 18 | 18 | 0.073 | 0.43 | 0.090 | 1.23 |
| Lea | 40 | 37 | 0.15 | 0.50 | 0.16 | 1.07 |
| K | 118 | 118 | 1.00 | 0.53 | 1.00 | 1.00 |
| E | 105 | 105 | 0.35 | 0.52 | 0.35 | 1.00 |
| c | 26 | 26 | 0.11 | 0.62 | 0.097 | 0.88 |
| Fya | 29 | 29 | 0.090 | 0.75 | 0.064 | 0.71 |
| C | 25 | 25 | 0.080 | 0.77 | 0.055 | 0.69 |
| S | 10 | 9 | 0.025 | 1.00 | 0.013 | 0.52 |
| V | 3 | 3 | 0.21 | |||
| e | 2 | 2 | 0.071 | |||
| s | 2 | 2 | 0.014 | |||
| N | 3 | 2 | 0.007 | |||
| Fyb | 1 | 1 | 0.005 | |||
| Jkb | 1 | 1 | 0.004 |
Values were obtained from a previous study of this patient population.12
Calculations were based on the traditional equation of Giblett.2 The potency of K was set at 1.00 and all other potencies were expressed relative to K.
Fractional persistence rates represent the ratio of persistently detected antibodies of the indicated specificity to total antibodies of that specificity, as calculated in a previous study of this same patient population.8
Values were obtained according to the modified immunogenicity calculation presented in Figure 1B and described in ″Results and discussion.″ Blood group specificities associated with 3 or less total antibodies were excluded from the modified immunogenicity calculation due to potential errors associated with small sample size.
Fold change was calculated as follows: ″Antigen potency corrected for antibody persistence″ divided by ″Traditional antigen potency.″
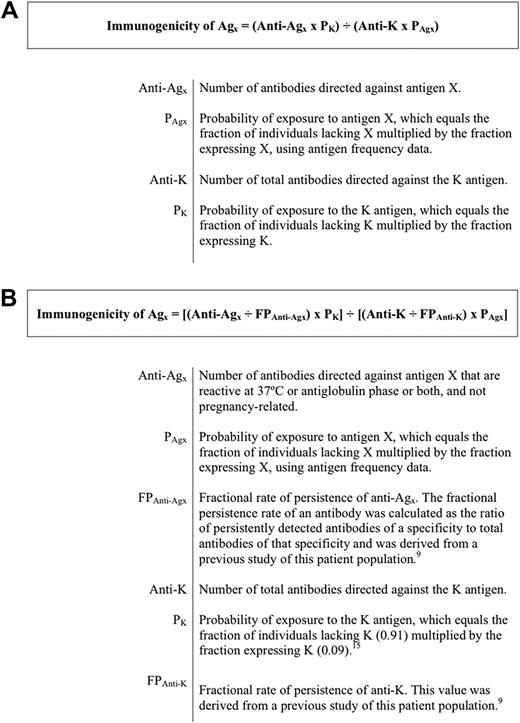
The traditional immunogenicity calculation of Giblett assumes that the total number of antibodies of a given specificity is related to the immunogenicity of the corresponding antigen (Figure 1A).2 However, the number of antibodies of a given specificity is also related to the persistence of that antibody over time. As such, we introduced a correction factor into the Giblett equation for antibody persistence to account for antibodies that were induced, but disappeared, before they could have been detected. This modification consisted of dividing the number of antibodies detected for a given specificity by the fractional persistence rate for that antibody (Figure 1B). This ratio should yield the total number of antibodies that would have been detected if persistence rates were 100%.
Equations for calculating blood group antigen immunogenicity. (A) The traditional Giblett immunogenicity calculation.2 (B) The modified immunogenicity calculation.
Equations for calculating blood group antigen immunogenicity. (A) The traditional Giblett immunogenicity calculation.2 (B) The modified immunogenicity calculation.
Antibody data from our study population were used to calculate immunogenicities by both modified and traditional equations (Table 1's “Traditional antigen potency” and “Antigen potency corrected for antibody persistence”). The largest correction was observed for Jka, whose immunogenicity was approximately 5-fold higher than that obtained with the original Giblett equation. As a result, the relative potency of Jka rose from the tenth most potent antigen in the uncorrected ranking to fourth. This corrected estimate for Jka ranged between 2- and 26-fold higher than that calculated in previous publications using the original Giblett equation.2-5 Lua and Cw also demonstrated relatively large increases in immunogenicity of approximately 4-fold. The potencies of C, c, Fya, and S appeared to decrease slightly, whereas those of M, Leb, and P1 appeared to increase slightly. The potencies of E and Lea were essentially unchanged.
Given that most antibodies traditionally considered to be naturally occurring (eg, anti-Lea, -Leb, -M, -N, and -P1) appeared to be predominantly transfusion induced in men,12 we were able to calculate transfusion-related immunogenicities for these antigens. Based on the modified equation, Lea, Leb, P1, and M were among the 10 most potent antigens overall with a potency rank order of Lea > P1 > M > Leb. To our knowledge, the only other study to calculate relative immunogenicities for Lewis, P1, and M antigens was that of Winters et al.5 However, most antibodies with those specificities in that study were naturally occurring.5 As such, the relevance of their calculations to transfusion-associated immunogenicity is questionable. Several other studies have addressed this issue by excluding all anti-M, -P1, -Lea, and -Leb antibodies.2-4 However, that approach prevented the calculation of the immunogenicities of their corresponding antigens, and did not exclude antibodies of other specificities that may have been naturally occurring. Our approach of calculating antigen immunogenicities only for antibodies reactive at 37°C and/or antiglobulin phase avoided these earlier pitfalls.
Because our patient population included only alloimmunized men, the immunogenicity calculations excluded pregnancy-related antibodies. Although many pregnancy-induced antibodies are clinically significant, the circumstances of their induction are sufficiently different from those of transfusion-induced antibodies to merit their separate study. For example, the route of exposure, the volume of antigen-positive red blood cells (antigenic load), and perhaps host immune status may differ in the settings of pregnancy and transfusion. However, it should be noted that by studying only men, we may have introduced a sex-related bias to immunogenicity estimates.
Despite our efforts to reduce errors associated with immunogenicity estimates, the possibility for inaccuracy remains. For example, even though we attempted to exclude naturally occurring antibodies, some may have been inadvertently included if they reacted at 37°C and/or antiglobulin phase. Warm-reactive, naturally occurring anti-E, -Lua, -Cw, -M, -N, and -P1 have been reported.6,14,15 In theory, our approach would also exclude transfusion-induced antibodies reactive only at room temperature, such as newly formed antibodies before isotype switching from immunoglobulin M to immunoglobulin G.6 Rare examples of antibodies typically considered clinically significant, but that react only at room temperature, such as anti-D, -E, and -Fya, would also be excluded.6,14-16 Because only 2% of the antibodies in our study reacted at room temperature alone, and most of these have specificities known to be naturally occurring,6,14,15 the number of such antibodies would be very low.
Inaccurate antibody persistence rates are another potential source of error. Unfortunately, antibody numbers for many specificities were likely too small to yield accurate results. Moreover, accurate persistence rates require regular antibody testing at defined intervals, which was not possible with a retrospective study.8 In addition, antibody evanescence is potentially subject to variation over time, because serologic detection techniques and reagents changed over the course of the data collection period. Even with these shortcomings, our modified approach should provide an improved theoretical framework for future calculations.
In summary, we have modified the calculation of antigen immunogenicity to correct for several potential errors. Specifically, we have corrected the Giblett equation for antibody persistence and have excluded pregnancy-related and naturally occurring antibodies from the calculation. The effect of these corrections was most evident for Jka, whose immunogenicity was previously underestimated because of the high evanescence rate of anti-Jka. We have also reported the transfusion-related immunogenicities of antigens traditionally associated with naturally occurring antibodies, several of which ranked among the top 10 most immunogenic antigens.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the VACHCS.
Authorship
Contribution: C.A.T. and G.S. designed the project, collected and reviewed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christopher A. Tormey, Department of Laboratory Medicine, Yale University School of Medicine, 333 Cedar St, PO Box 208035, New Haven, CT 06520; e-mail: christopher.tormey@yale.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal