Abstract
UL16-binding proteins (ULBPs) belong to a family of ligands for NKG2D activating receptor of human natural killer (NK) cells. We previously reported that RAET1E2, a soluble isoform of the RAET1E (ULBP4), inhibits NKG2D-mediated NK cytotoxicity. In this study, we examined whether ULBP4 could be recognized by γδT cells via TCRγδ. Here we show that immobilized soluble ULBP4 (rULBP4) induces the proliferation of human ovarian epithelial carcinoma– or colonic carcinoma–derived Vδ2+ T cells in vitro. These Vδ2+ T cells secrete Th1 cytokines and display a strong cytolytic activity toward ULBP4-transfected targets. We also show that ULBP4 binds to a soluble chimeric protein containing TCRγ9/δ2 and activates TCR− Jurkat T cells transfected with TCRγ9/δ2. Moreover, both TCRγδ and NKG2D are involved in ULBP4-induced activation and cytotoxicity of γδT cells. We found that ULBP4 is expressed not only on human tumor cells, but also on Epstein-Barr virus (EBV)–infected peripheral blood cells. Taken together, our data suggest that ULBP4 functions as a ligand for both TCRγδ and NKG2D and may play a key role in immune surveillance of tumor development and clearance of viral infection.
Introduction
It is well established that γδ T cells function as sentinels in early host responses to microbial infections and malignancies. The human γδT cells are composed of 2 main subsets: Vδ1 T cells in epithelial tissues and epithelial tumors and Vδ2 T cells in peripheral blood.1,2 Vδ1 T cells recognize MHC class I–related proteins A and B (MICA/B)3-7 and other ligands,8-11 whereas Vδ2 T cells recognize phosphoantigens.12-14 Most human γδT cells express the activating natural killer (NK) receptor NKG2D,15 which plays an important role in γδT-cell recognition and activation.16-19 Although it was reported the NK receptor ligand MICA also was recognized by the Vδ1 T cells via γδT-cell receptor (TCRγδ),3,7 it remains to be determined how this simultaneous recognition of NK receptor ligand by both TCRγδ and NKG2D contributes to human γδT-cell function
UL16-binding proteins (ULBPs), along with MICA/B, are ligands for human NKG2D.20 ULBP gene family is composed of 10 members, 6 of which encode potentially functional glycoproteins.21 Among them, ULBP4 differs from other ULBPs (ULBP1-3). First, amino acid sequence analyses indicate that ULBP1, ULBP2, and ULBP3 are 55% to 60% identical, whereas ULBP4 is more divergent, with greater similarity to ULBP3 than ULBP1 or ULBP2. Second, ULBP4 molecule contains a predicted transmembrane and cytoplasmic domain.22 In contrast, ULBP1-3 molecules are anchored to membrane through GPI link.20 Third, ULBP1-3 mRNAs are expressed in a wide range of tissues and cell lines,20 whereas ULBP4 mRNA is largely restricted to skin and small intestine and up-regulated on transformed tumor22-25 or herpes simplex virus (HSV)–infected cells,23 suggesting that ULBP4 may play a role in antitumor or anti-infection immunity.
Functional analyses of ULBP4 demonstrate that ULBP4 can induce NK-cell activation and NK-mediated lysis.22-24 ULBP4-NKG2D interaction serves to enhance CD8+ T-cell activation.23 These results suggest that ULBP4 plays an important role in innate and adaptive immunity. Interestingly, we also found that tumor cells release soluble ULBP4 to down-modulate NKG2D and may serve as a novel mechanism to evade NKG2D-mediated immune surveillance.26 Despite the importance of ULBP4 in NK-mediated immune response, the role of ULBP4 in γδT-cell function has not been examined.
Here we report that recombinant ULBP4 induces cytotoxicity of human γδT cells. Our results suggest that ULBP4 is recognized by both TCRγδ and NKG2D on γδT cells and plays an important role in γδT-cell–mediated killing of tumor cells and virus-infected cells.
Methods
Cell lines and transfectants
Human embryonic kidney epithelial cell line HEK 293ET, a kind gift of Dr Brian Seed (Massachusetts General Hospital and Harvard Medical School) was cultured in 10% FCS DMEM medium (Gibco BRL). NK-92 cell line, provided by Prof Zhigang Tian (University of Science and Technology of China), was cultured in MEM-alpha medium (Gibco BRL) supplemented with 12.5% fetal bovine serum, 12.5% horse serum, and 200 UI/mL IL-2. Murine thymoma cell line EL4 was transfected by electroporation of 2 μg of the vector pcDNATM3.1-myc-His(A) containing a full-length ULBP4 open reading frame. Stable transfectant EL4-ULBP4 was selected in RPMI-1640 supplemented (Gibco BRL) with 1 mg/mL G418. The transfected cells were stained with anti-ULBP4 mAb 8C9 previously generated in our laboratory,27,28 followed by FITC-conjugated sheep anti–mouse IgG (Zhongshan) and sorted by flow cytometry. T-lymphoma cell line (J.RT3-T3.5), deficient in both TCR α and β chains (ATCC), was cotransfected with full-length human peripheral blood mononuclear cell (PBMC)–derived γ9 and δ2 chain (http://imgt.cines.fr/,29 sequence shown in Table 1) (CDR3δ region was OT3 nucleotide acid sequence).30,31 Stable transfectants J.RT3-T3.5-TCRγ9/δ2-OT3 were selected in RPMI-1640 supplemented with 1 mg/mL G418. Human gastric carcinoma cell line BGC823, human ovarian carcinoma cell line HO-8910, and human liver carcinoma cell line HepG2 were cultured in 10% FCS RPMI-1640 medium (Gibco BRL). Human lung adenocarcinoma cell line CaLu3 and human endometrium carcinoma cell line HEC-1B were cultured in 10% FCS MEM-NEAA medium (Gibco BRL). Human colorectal carcinoma cell line HCT-116 was cultured in 10% FCS IMDM medium (Gibco BRL). All of these tumor cell lines were in stock in our laboratory.
Information of sequences of TCRγ9/δ2 derived from IMGT repertoire
| Gene name . | Reference sequences . | Accession nos. . |
|---|---|---|
| TRGV9 | V9*A1 | X07205 |
| TRGJP1 | JP1 | X08084 |
| TRGC1*01 | Lambda-D19 (D-PLL) | M14996; M14997; M14998 |
| TRDV2 | V2*A1 | 5'UTR X15275; X15207 |
| TRDJ1 | J1 | M20289 |
| TRDC*01 | M22148; M22149; M22150 |
Cells and human tumor specimens
Fresh PBMCs separated from peripheral blood of healthy donors by density gradient centrifugation on Ficoll-Hypaque (Pharmacia) were grown in RPMI-1640 medium (Gibco BRL) with 10% FCS and IL-2 (200 UI/mL) in 24-well culture plates with immobilized anti–pan-TCRγδ mAb (Immunotech, Becton Dickinson). After 2 weeks of culture, the purity of γδT cells was more than 90% as assessed by flow cytometry analysis. Fresh PBMCs separated from peripheral blood of healthy donors were used for the establishment of an Epstein-Barr virus (EBV)–transformed lymphoblastoid cell line (LCL) by addition of exogenous EBV (B95.8 strain). The culture medium was 10% FCS RPMI-1640, 2 mM glutamine, initially supplemented with 0.05 mg/mL cyclosporin A (CsA; Novartis) and 0.5% (wt/vol) PHA. Primary B-cell lines were established by separating B cells from fresh PBMCs using human CD19 MicroBeads following the instructions of the manufacturer (Miltenyi Biotec).
Fresh tumor tissues obtained during surgical operations were from Peking Union Medical College (PUMC) Hospital. All the tumor specimens, including ovarian tumor carcinoma specimens and colonic tumor specimens, used in our study were diagnosed according to standard histopathological and immunohistochemical criteria and contained only malignant epithelium. Use of all human materials were carried out according to the Institutional Guidelines in PUMC and formally approved by the Institutional Review Board with informed consent obtained in accordance with the Declaration of Helsinki.
Construction and expression of soluble ULBP4
Soluble ULBP4 (rULBP4) was produced as a 8-histidine C-terminal tagged protein. In brief, a sequence encoding the extracellular domain of ULBP4 (aa 1-226) was amplified by polymerase chain reaction (PCR) using the primers U4f (5′-CGGAATTCATGCGAAGAATATCCCTGACTT-3′) and U4r (5′-GGCGGCCGCCTATTAGTGGTGGTGGTGGTGGTGGTGGTGTCTAT CTGGTAGACTAGAAGAA-3′) from a previously cloned vector pET22b(+)/ULBP426,27 and subcloned into the mammalian expression vector pEAK12, a kind gift from Dr Brian Seed (Massachusetts General Hospital and Harvard Medical School); the italicized portions of the sequences represent the restriction enzyme cutting site. Plasmid pEAK12/rULBP4 was stably transfected into HEK 293ET cells, and the fusion proteins were purified from culture supernatants using HisTrap kit (Amersham Pharmacia Biotech) following the instruction of the manufacturer. The molecular weight and purity of rULBP4 were confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) acrylamide gels. The samples were detected by anti-His mAb (Zhongshan) or anti-ULBP4 mAb 10F7.
IFN-γ secretion assay
A total of 5 × 105 NK-92 cells per well were seeded into 48-well plate with precoated 5 μg rULBP4 or other recombinant proteins expressed by 293ET cells per well and cocultured for 40 hours. Cell-free supernatants were collected and detected with the human IFN-γ Immunoassay Kit(R&D Systems).
Reverse-transcription PCR
RNA was prepared from EL4 or ULBP4-EL4 cells. The following PCR primers were used for cloning the genes: U4f/U4r stated in “Construction and expression of soluble ULBP4,” β-actin up (5′-ATGGATGATGATATCGCCGCGC-3′), and β-actin down (5′-CTAGAAGCATTTGCGGTGGACG-3′). The expected sizes of PCR products were 721 bp for ULBP4 and 1128 bp for β-actin. Thirty PCR cycles were applied.
Expansion of γδ TILs in vitro
The expansion of γδ tumor-infiltrating lymphocytes (TILs) by immobilized anti–pan-TCRγδ mAb or by immobilized rMICA in vitro has been described previously.6,7,32 The expansion of γδ TILs by rULBP4 was performed similarly. Purified rULBP4 protein (20 μg/well) diluted by RPMI-1640 was added to 24-well plates, incubated at 37°C for 2 hours, and then washed 3 times. The tumor tissues rinsed with RPMI-1640 supplemented with 100 UI/mL penicillin, 100 μg/mL streptomycin, and 50 μg/mL getamycin were minced thoroughly after discarding the necrosis and surrounding normal tissue and then transferred to the plates. Tissues cultured with immobilized anti–pan-TCRγδ mAb (Immunotech) served as a positive control and tissues cultured with IL-2 alone, tissues cultured with immobilized rULBP3 and leucine zipper (rULBP3/LZ) fusion protein expressed in CHO cell lines, or tissues cultured with immobilized rMICA expressed in Escherichia coli previously generated in our laboratory6 served as controls. After 3 weeks of expansion, the percentage of γδT cells in TILs was determined by fluorescence-activated cell sorting (FACS). When the proportion of γδT cells reached approximately 80%, the cells were used for functional assays.
Sequencing of TCR Vδ gene from rULBP4-expanded γδ TILs
RNA was prepared from γδ TILs expanded by immobilized rULBP4 protein. The cDNA of Vδ TCR was obtained by reverse-transcription (RT)–PCR. RT-PCR products were subcloned into T easy vector (Invitrogen) and sequenced.
Flow cytometry
T cells were analyzed by immunofluorescence assay.6,7,32 FITC-labeled anti-TCRγδ, and anti-TCR Vδ1/Vδ2/Vδ3, were from Immunotech. For binding assay of ULBP4 to TCRγ9/δ2-OT3, EL4 or EL4-ULBP4 cells pretreated with anti-ULBP4 mAb 8C9 were incubated with TCRγ9/δ2-OT3-Fc, chimeric protein containing the extracellular domains of the human γ9(TRγV9-JP1), and δ2 (TRδV2-J1, CDR3δ region was OT3 nucleotide acid sequence30,31 ) TCR chains fused to the hinge region CH2 and CH3 domains of human IgG1 heavy chain (produced by the Sino Biological Inc), or human recombinant NKG2D/Fc chimera (R&D Systems) for 1 hour. FITC-conjugated goat anti–human IgGFc(γ) (Zhongshan) was added to each tube and incubated for 30 minutes, followed by FACS analysis. Different cells including J.RT3-T3.5, J.RT3-T3.5-TCRγ9/δ2-OT3, or PBMC-derived Vδ2 T cells (90% purity) preincubated with anti-NKG2D (clone 1D11; eBioscience) were incubated with rULBP4 for an additional hour. FITC-conjugated anti-Histag mAb (Invitrogen) was added to each tube and incubated for 30 minutes, followed by FACS analysis.
For characterizing TCRγ9/δ2-OT3-Fc chimera, Daudi cells were stained with TCRγ9/δ2-OT3-Fc chimera for 1 hour. FITC-conjugated goat antihuman IgGFc(γ) (Zhongshan) was added and then incubated for 30 minutes, followed by FACS analysis.
For detecting ULBP4 expression, cells were stained with anti-ULBP4 mAb or control Ig followed by FITC-conjugated goat anti–mouse Ig (Zhongshan). After staining, all cells were analyzed with a FACSCalibur flow cytometer (Becton Dickinson).
Cytokines
Forty-eight–well plates were coated for 2 hours at 37°C with 1 μg/mL anti–pan-TCRγδ, 20 μg/mL rULBP4, or 20 μg/mL other recombinant human proteins expressed by 293ET cells. After thoroughly washing with PBS 3 times, 106 cells per well of PBMC-Vδ2 T cells or OEC-Vδ2 T cells were cultured in a total volume of 500 μL complete medium plus 50 UI/mL IL-2. After 40 hours, cytokine in cell-free supernatants was determined by enzyme-linked immunosorbent assay (ELISA; R&D Systems).
Cytotoxicity and monoclonal antibody inhibition
The colorimetric MTT (3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was used to evaluate the cytolytic activity of the γδT cells in vitro as reported previously.6,32 For the blocking assay, the effector cells were preincubated for 1 hour with 10 μg/mL antihuman TCRγδ mAb (clone B1.1; eBioscience), 20 μg/mL anti-NKG2D mAb (clone 1D11; eBioscience), or control mouse Ig. Target cells were incubated with anti-ULBP4 mAb 8C9 at 4°C for 1 hour and then used as for cytotoxicity assay.
Enzyme-linked immunosorbent binding assay
Binding activity of rULBP4 to TCRγ9/δ2-OT3-Fc was determined by ELISA. In brief, 1 μg rULBP4 was incubated for 2 hours at 37°C in 96-well plates in a total volume of 100 μL. After blocking with 15% FCS in PBS, the TCRγ9/δ2-OT3-Fc or control human IgG1 with gradient concentration was added to the wells. Bound mAb was detected using a HRP-labeled goat anti–human IgGFc(γ) (Zhongshan).
Stimulation of J.RT3-T3.5 transfectants
Parental or TCRγ9/δ2-transfected J.RT3-T3.5 cell lines were preincubated with 10 ng/mL PMA and then cultured in 24-well plates at 106 in the presence of 40 μg rULBP4 or control protein. After 24 hours, IL-2 in cell-free supernatants was detected by ELISA (R&D Systems).
Stimulation of PBMC-Vδ2 T cells and blocking assay
Ninety-six–well plates were coated with 10 μg/mL anti-NKG2D (clone 1D11; eBioscience), 1 μg/mL anti–pan-TCRγδ, 20 μg/mL rULBP4, or control mouse Ig. After washing, PBMC-Vδ2 T cells at 5 × 105 per well were cultured in a total volume of 100 μL complete medium plus 50 UI/mL IL-2. For the blocking assay, the effector cells were preincubated for 1 hour with 100 ng/mL of the immunosuppressive drug cyclosporin A (CsA; Novartis), 50 μM of the PI3-kinase inhibitor Ly294002 (Upstate Biotechnology), and/or 1 μM of the PI 3-kinase inhibitor wortmannin (Upstate Biotechnology). After 48 hours, cytokine in cell-free supernatants was determined by ELISA. For measurement of granule release, cells were stimulated for 5 hours at 37°C and supernatants were tested with a standard N-benzyloxycarbonyl lysine thiobenzyl ester (BLT3; Sigma-Aldrich) esterase assay.16,33 All assays were performed in triplicate wells, and results are presented as the mean from the given culture conditions.
Immunohistochemistry
ULBP4 expression was examined in paraffin-embedded sections of human tumor samples. The paraffin-embedded biopsies were sectioned to 4-mm thickness onto APES-coated glass slides. After deparaffinization and rehydration, the sections were subjected to target retrieval by microwave, rinsed in PBS, quenched with peroxide, treated with 5% goat serum in PBS, and incubated with anti-ULBP4 mAb 8C9 or 10F7. Mouse Ig was used as negative control. Then, HRP-goat anti–mouse antibody was used to locate the bound primary antibody (DAKO), and the complex was visualized using diaminobenzidine colorimetric reagent according to the manufacturer's protocol (DAKO).
Results
Generation of recombinant ULBP4 protein in HEK 293ET cells and ULBP4-expressing EL4 cells
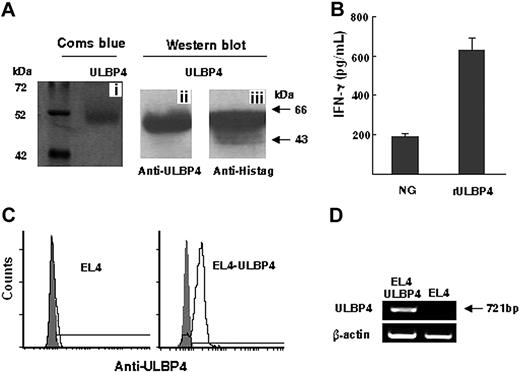
The recombinant ULBP4 generated from HEK 293ET cells was purified by affinity chromatography with Ni2+ ions. The rULBP4 exhibits a molecular mass of approximately 50 kDa most likely due to glycosylation of rULBP4 (Figure 1Ai). The protein identity was confirmed by Western blotting using anti-ULBP4 mAb (Figure 1Aii) or anti-Histag mAb (Figure 1Aiii). In contrast to control proteins, immobilized rULBP4 induced NK92 cells to secrete IFN-γ (Figure 1B). To examine the role of ULBP4 in γδT-cell–mediated cytotoxicity, we established ULBP4 stably transfected EL4 cell line. ULBP4 expression in these stable transfectants was confirmed by flow cytometry with anti-ULBP4 mAb (Figure 1C) and RT-PCR (Figure 1D).
Expression and identification of ULBP4. (A) Purified rULBP4 was analyzed by SDS-PAGE (i) and detected with anti-ULBP4 mAb (ii) or anti-Histag mAb (iii). (B) IFN-γ secretion of NK92 cells after stimulation with purified rULBP4 or control other human recombinant proteins (NG). Data represent means ± SD (error bars) of 3 independent experiments. (C) Flow cytometric staining of untransfected and ULBP4-transfected EL4 cells with anti-ULBP4 mAb 8C9 (black line histogram). Ig controls are shaded. (D) Expressions of ULBP4 transcripts in ULBP4-transfected and untransfected EL4 cells were detected by RT-PCR.
Expression and identification of ULBP4. (A) Purified rULBP4 was analyzed by SDS-PAGE (i) and detected with anti-ULBP4 mAb (ii) or anti-Histag mAb (iii). (B) IFN-γ secretion of NK92 cells after stimulation with purified rULBP4 or control other human recombinant proteins (NG). Data represent means ± SD (error bars) of 3 independent experiments. (C) Flow cytometric staining of untransfected and ULBP4-transfected EL4 cells with anti-ULBP4 mAb 8C9 (black line histogram). Ig controls are shaded. (D) Expressions of ULBP4 transcripts in ULBP4-transfected and untransfected EL4 cells were detected by RT-PCR.
Effect of ULBP4 on proliferation, cytokine production, and cytotoxicity of γδT cells
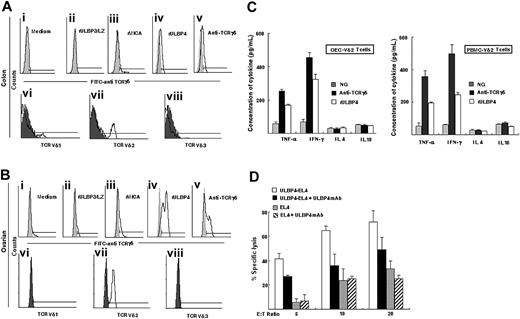
We previously found that the TILs can be expanded by anti–pan-TCRγδ mAb32 or by the NKG2D ligand, rMICA protein.6,7 In this study, a similar assay was conducted to determine whether γδ TILs from tumor specimens could be expanded by rULBP4 protein. We found that γδ TILs from colonic tumor specimens or ovarian tumor specimens were expanded by immobilized rULBP4 (Figure 2Aiv,Biv) or anti–pan-TCRγδ mAb (Figure 2Av,Bv) in vitro. In the absence of rULBP4 (Figure 2Ai,Bi) or in the presence of immobilized rULBP3/LZ (Figure 2Aii,Bii), no such expansion of γδT cells occurred. The majority of the rULBP4-expanded γδ TILs were identified as Vδ2 (Figure 2Avii,Bvii) rather than Vδ1 (Figure 2Avi,Bvi) or Vδ3 (Figure 2Aviii,Bviii) subset. We also detected weak expansion of rMICA-expanded γδ TILs from colonic tumor specimens (Figure 2Aiii) or ovarian tumor specimens (Figure 2Biii) compared with negative controls (Figure 2Ai,Bi).
Effect of immobilized rULBP4 on human γδT cells. (A-B) Expansion of human γδT cells from (A) colonic carcinoma specimen or (B) ovarian epithelial carcinoma specimen TILs after stimulation with immobilized rULBP4 in vitro. Flow cytometric analysis by FITC-labeled anti-TCRγδ mAb shows the proportions of γδT cells (A-B upper panel). The phenotype of rULBP4-expanded γδ TILs was analyzed by anti-Vδ1 FITC/anti-Vδ2 FITC mAb/anti-Vδ3 FITC mAb (A-B lower panel). Immobilized anti-pan-TCRγδ mAb, immobilized rULBP3/LZ, immobilized rMICA, or tissue culture with IL-2 alone was used to expand γδT cells. (C) Cytokines secreted by OEC-Vδ2 T cells or PBMC-Vγ9/δ2 T cells before and after stimulation with rULBP4. Forty-eight–well plates were coated with anti–pan-γδTCR mAb, rULBP4, or control other human recombinant proteins (NG). PBMC-Vδ2 T cells or OEC-Vδ2 T cells (106 cells/well) were cultured in a total volume of 500 μL complete medium plus 50 UI/mL IL-2. After 40 hours, cytokine in cell-free supernatants was determined by ELISA. Data represent means ± SD (error bars) of 3 independent experiments. (D) Anti-ULBP4 mAb blocks OEC-Vδ2 TIL cytotoxicity against the EL4-ULBP4 cells. In blocking assay, EL4-ULBP4 or EL4 cells were preincubated with anti-ULBP4 mAb 8C9.
Effect of immobilized rULBP4 on human γδT cells. (A-B) Expansion of human γδT cells from (A) colonic carcinoma specimen or (B) ovarian epithelial carcinoma specimen TILs after stimulation with immobilized rULBP4 in vitro. Flow cytometric analysis by FITC-labeled anti-TCRγδ mAb shows the proportions of γδT cells (A-B upper panel). The phenotype of rULBP4-expanded γδ TILs was analyzed by anti-Vδ1 FITC/anti-Vδ2 FITC mAb/anti-Vδ3 FITC mAb (A-B lower panel). Immobilized anti-pan-TCRγδ mAb, immobilized rULBP3/LZ, immobilized rMICA, or tissue culture with IL-2 alone was used to expand γδT cells. (C) Cytokines secreted by OEC-Vδ2 T cells or PBMC-Vγ9/δ2 T cells before and after stimulation with rULBP4. Forty-eight–well plates were coated with anti–pan-γδTCR mAb, rULBP4, or control other human recombinant proteins (NG). PBMC-Vδ2 T cells or OEC-Vδ2 T cells (106 cells/well) were cultured in a total volume of 500 μL complete medium plus 50 UI/mL IL-2. After 40 hours, cytokine in cell-free supernatants was determined by ELISA. Data represent means ± SD (error bars) of 3 independent experiments. (D) Anti-ULBP4 mAb blocks OEC-Vδ2 TIL cytotoxicity against the EL4-ULBP4 cells. In blocking assay, EL4-ULBP4 or EL4 cells were preincubated with anti-ULBP4 mAb 8C9.
Next, we determined the effect of rULBP4 stimulation on γδT-cell function. A strong enhancing effect on Th1 cytokine (IFN-γ and TNF-α), but not Th2 cytokine (IL-4 and IL-10), was observed in PBMC- or TIL-derived Vδ2 T cells stimulated with immobilized rULBP4 (Figure 2C).
Then, we examined whether ULBP4 expression on target cells affects γδT-cell–mediated cytotoxicity. OEC-Vδ2 T cells (∼ 80% pure) were assayed for lysis of EL4 or EL4-ULBP4 target cells. Vδ2 T cells exhibited a dramatically enhanced cytotoxicity against EL4-ULBP4 compared with EL4 target cells. Furthermore, lysis was inhibited by preincubating the EL4-ULBP4 target cells with anti-ULBP4 mAb but not with control Ig. In contrast, no specific cytolytic inhibition was observed in the antibody blocking assays in the killing of EL4 cells (Figure 2D). Taken together, these results demonstrate that immobilized or cell surface–expressed ULBP4 induces γδT cells to proliferate, produce cytokines, and exert cytotoxic activity.
Sequence of TCR Vδ gene from rULBP4-expanded γδ TILs
To determine the characteristics of amino acid sequences of CDR3δ in rULBP4-expanded γδT cells, we cloned and sequenced TCRVδ cDNA of rULBP4-expanded ovarian epithelial carcinoma–derived (Table 2) or colonic carcinoma–derived (data not shown) γδ TILs. The sequence analysis revealed that all the TCR Vδ transcripts were in frame. No obviously conserved motifs were observed except a few identical CDR3δ sequences in different individual clones. The lengths of CDR3δ of tested γδ TILs ranged from 14 to 24 amino acids.
Amino acid sequences of TCRVδ2-CDR3 regions derived from ULBP4-expanded γδ TILs of OEC
| Ratio . | CDR3 length, amino acid . | TCRVδ . | N–D–N . | Jδ . | Jδn . |
|---|---|---|---|---|---|
| 9/44 | 23 | CACD | FPSHTFHSTGGHT | TDKLIF | DJ1 |
| 5/44 | 18 | CACDT | VGSGEKY | TDKLIF | DJ1 |
| 3/44 | 16 | CACDT | MGDPPA | DKLIF | DJ1 |
| 3/44 | 16 | CACD | VLGVKY | TDKLIF | DJ1 |
| 2/44 | 16 | CACDT | LGDNPPRE | LIF | DJ1 |
| 2/44 | 23 | CACDT | VFAGGYTHGSEY | TDKLIF | DJ1 |
| 2/44 | 20 | CACD | ILGDSQHPTNA | DKLIF | DJ1 |
| 1/44 | 17 | CACD | VLGDMLD | DKLIF | DJ1 |
| 1/44 | 15 | CACDT | VGDKD | DKLIF | DJ1 |
| 1/44 | 18 | CACDT | VGGYAYN | TDKLIF | DJ1 |
| 1/44 | 24 | CACD | IVRAHTGGYAAPNH | TDKLIF | DJ1 |
| 1/44 | 16 | CACD | LVGEVG | TDKLIF | DJ1 |
| 1/44 | 14 | CACDT | DRN | TDKLIF | DJ1 |
| 1/44 | 23 | CACD | FPSHAFHSTGGHA | TDKLIF | DJ1 |
| 1/44 | 17 | CACDT | VLRRLGN | DKLIF | DJ1 |
| 1/44 | 19 | CACDT | VLRTGGLY | TDKLIF | DJ1 |
| 1/44 | 18 | CACDT | VGTLTPY | TDKLIF | DJ1 |
| 1/44 | 15 | CACDT | LDRN | TDKLIF | DJ1 |
| 1/44 | 19 | CACDT | HLGDPTNA | TDKLIF | DJ1 |
| 1/44 | 14 | CACD | NILAGSE | LIF | DJ1 |
| 1/44 | 19 | CACD | LVLGDPMVGFVQ | LIF | DJ1 |
| 1/44 | 18 | CACDT | VGSGEKY | TDKLIF | DJ1 |
| 1/44 | 14 | CACD | NILAGSE | LIF | DJ1 |
| 1/44 | 18 | CACDT | VGSGEKY | TDKLIF | DJ1 |
| 1/44 | 20 | CACDT | AYGGGYLR | DTRQMFF | DJ3 |
| 1/44 | 17 | CACDT | VVGA | WDTRQMFF | DJ3 |
| Ratio . | CDR3 length, amino acid . | TCRVδ . | N–D–N . | Jδ . | Jδn . |
|---|---|---|---|---|---|
| 9/44 | 23 | CACD | FPSHTFHSTGGHT | TDKLIF | DJ1 |
| 5/44 | 18 | CACDT | VGSGEKY | TDKLIF | DJ1 |
| 3/44 | 16 | CACDT | MGDPPA | DKLIF | DJ1 |
| 3/44 | 16 | CACD | VLGVKY | TDKLIF | DJ1 |
| 2/44 | 16 | CACDT | LGDNPPRE | LIF | DJ1 |
| 2/44 | 23 | CACDT | VFAGGYTHGSEY | TDKLIF | DJ1 |
| 2/44 | 20 | CACD | ILGDSQHPTNA | DKLIF | DJ1 |
| 1/44 | 17 | CACD | VLGDMLD | DKLIF | DJ1 |
| 1/44 | 15 | CACDT | VGDKD | DKLIF | DJ1 |
| 1/44 | 18 | CACDT | VGGYAYN | TDKLIF | DJ1 |
| 1/44 | 24 | CACD | IVRAHTGGYAAPNH | TDKLIF | DJ1 |
| 1/44 | 16 | CACD | LVGEVG | TDKLIF | DJ1 |
| 1/44 | 14 | CACDT | DRN | TDKLIF | DJ1 |
| 1/44 | 23 | CACD | FPSHAFHSTGGHA | TDKLIF | DJ1 |
| 1/44 | 17 | CACDT | VLRRLGN | DKLIF | DJ1 |
| 1/44 | 19 | CACDT | VLRTGGLY | TDKLIF | DJ1 |
| 1/44 | 18 | CACDT | VGTLTPY | TDKLIF | DJ1 |
| 1/44 | 15 | CACDT | LDRN | TDKLIF | DJ1 |
| 1/44 | 19 | CACDT | HLGDPTNA | TDKLIF | DJ1 |
| 1/44 | 14 | CACD | NILAGSE | LIF | DJ1 |
| 1/44 | 19 | CACD | LVLGDPMVGFVQ | LIF | DJ1 |
| 1/44 | 18 | CACDT | VGSGEKY | TDKLIF | DJ1 |
| 1/44 | 14 | CACD | NILAGSE | LIF | DJ1 |
| 1/44 | 18 | CACDT | VGSGEKY | TDKLIF | DJ1 |
| 1/44 | 20 | CACDT | AYGGGYLR | DTRQMFF | DJ3 |
| 1/44 | 17 | CACDT | VVGA | WDTRQMFF | DJ3 |
Specific binding of ULBP4 to Vγ9δ2 TCR
The fact that rULBP4 induces Vδ2 TILs to proliferate raises the possibility that ULBP4 may function as a TCRγδ ligand in addition to serving as a NKG2D ligand. To determine whether ULBP4 was able to bind to human Vδ2 TCR, we first determined the binding between ULBP4 and TCRγ9/δ2-OT3 by cell-surface staining.
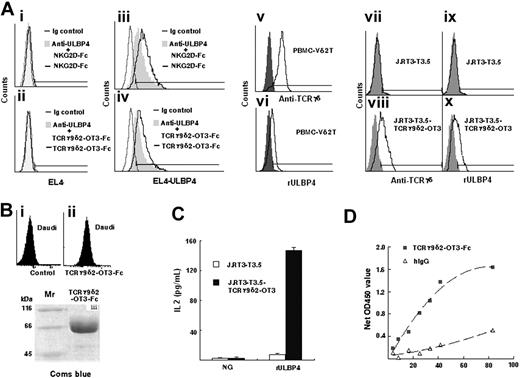
EL4-ULBP4 transfectants were stained positive by TCRγ9/δ2-OT3-Fc identified by immunofluorescence assay (Figure 3Bi-ii) and SDS-PAGE (Figure 3Biii) while NKG2D-Fc served as a positive control. The binding was inhibited by anti-ULBP4 mAb (Figure 3Aiii-iv). In contrast, untransfected EL4 cells were not reactive to NKG2D-Fc or the chimeric TCRγ9/δ2-OT3-Fc protein (Figure 3Ai-ii). In addition, PBMC-derived Vδ2 T cells (Figure 3Avi) and J.RT3-T3.5-TCRγ9/δ2-OT3 cells (Figure 3Ax) were stained positive by rULBP4, whereas J.RT3-T3.5 negative control cells exhibited no binding to rULBP4 (Figure 3Aix).
Specific binding of ULBP4 to TCRγ9/δ2-OT3-Fc chimeric protein or TCRγ9/δ2-OT3 expressed on transfected J.RT3-T3.5 cells. (A) EL4 cells (i-ii) or EL4-ULBP4 cells (iii-iv) pretreated with (gray filled histogram) or without (black line histogram) anti-ULBP4 mAb 8C9 were stained with human NKG2D-Fc or TCRγ9/δ2-OT3-Fc, followed by FITC-conjugated goat anti–human IgGFc(γ). Ig controls were shown as gray line histogram. PBMC-derived Vδ2 T cells (90% purity, v), pretreated with anti-NKG2D were incubated with rULBP4, followed by FITC-conjugated anti-Histag mAb (vi). TCR− J.RT3-T3.5 cells and TCR transfectants were stained with FITC-labeled anti-TCRγδ mAb (black line histogram) or with control Ig (vii-viii). TCR− J.RT3-T3.5 cells and TCR transfectants were stained with rULBP4 (black line histogram) or without rULBP4 (gray filled histograms), followed by FITC-conjugated anti-Histag mAb (ix-x). (B) Characterization of TCRγ9/δ2-OT3-Fc fusion protein. Daudi cells were stained with human TCRγ9/δ2-OT3-Fc, then followed by FITC-conjugated goat anti–human IgGFc(γ) (i-ii).TCRγ9/δ2-OT3-Fc fusion proteins were analyzed by reducing SDS-PAGE and visualized by Coomassie staining (iii). (C) IL-2 secretion by TCR− J.RT3-T3.5 cells and TCR transfectants, with or without the stimulation of immobilized rULBP4. J.RT3-T3.5 and J.RT3-T3.5-TCRγ9/δ2-OT3 were preactivated with PMA, then incubated with rULBP4 or control protein (NG). After 24 hours, IL-2 in the supernatants was detected by ELISA (R&D Systems). Data represent means ± SD (error bars) of 3 independent experiments. (D) ELISA assay of rULBP4 binding to TCRγ9/δ2-OT3-Fc. Increasing concentration of TCRγ9/δ2-OT3-Fc or control human IgG was incubated with coated rULBP4.
Specific binding of ULBP4 to TCRγ9/δ2-OT3-Fc chimeric protein or TCRγ9/δ2-OT3 expressed on transfected J.RT3-T3.5 cells. (A) EL4 cells (i-ii) or EL4-ULBP4 cells (iii-iv) pretreated with (gray filled histogram) or without (black line histogram) anti-ULBP4 mAb 8C9 were stained with human NKG2D-Fc or TCRγ9/δ2-OT3-Fc, followed by FITC-conjugated goat anti–human IgGFc(γ). Ig controls were shown as gray line histogram. PBMC-derived Vδ2 T cells (90% purity, v), pretreated with anti-NKG2D were incubated with rULBP4, followed by FITC-conjugated anti-Histag mAb (vi). TCR− J.RT3-T3.5 cells and TCR transfectants were stained with FITC-labeled anti-TCRγδ mAb (black line histogram) or with control Ig (vii-viii). TCR− J.RT3-T3.5 cells and TCR transfectants were stained with rULBP4 (black line histogram) or without rULBP4 (gray filled histograms), followed by FITC-conjugated anti-Histag mAb (ix-x). (B) Characterization of TCRγ9/δ2-OT3-Fc fusion protein. Daudi cells were stained with human TCRγ9/δ2-OT3-Fc, then followed by FITC-conjugated goat anti–human IgGFc(γ) (i-ii).TCRγ9/δ2-OT3-Fc fusion proteins were analyzed by reducing SDS-PAGE and visualized by Coomassie staining (iii). (C) IL-2 secretion by TCR− J.RT3-T3.5 cells and TCR transfectants, with or without the stimulation of immobilized rULBP4. J.RT3-T3.5 and J.RT3-T3.5-TCRγ9/δ2-OT3 were preactivated with PMA, then incubated with rULBP4 or control protein (NG). After 24 hours, IL-2 in the supernatants was detected by ELISA (R&D Systems). Data represent means ± SD (error bars) of 3 independent experiments. (D) ELISA assay of rULBP4 binding to TCRγ9/δ2-OT3-Fc. Increasing concentration of TCRγ9/δ2-OT3-Fc or control human IgG was incubated with coated rULBP4.
To further confirm the interaction between ULBP4 and TCRγδ, we tested the effect of ULBP4 stimulation on Jurkat cells with or without TCRγδ expression. J.RT3-T3.5 transfected with TCRγ9/δ2 but not the parental cells produced a large amount of IL-2 in response to rULBP4 stimulation (Figure 3C). These data provided further support that ULBP4 was recognized by TCRγδ.
To directly measure the interaction between rULBP4 and TCRγδ, we performed enzyme-linked immunosorbent binding assay using rULBP4 and TCRγ9/δ2-OT3-Fc. As shown in Figure 3D, TCRγ9/δ2-OT3-Fc specifically bound to coated rULBP4 in a concentration-dependent manner, whereas control human IgG exhibited little binding to coated rULBP4.
Both TCR and NKG2D mediate ULBP4-induced activation of γδT cells
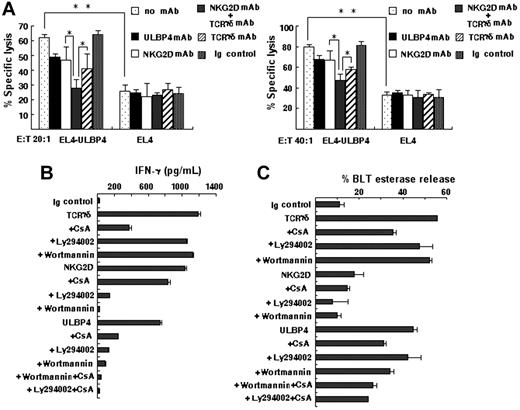
As γδT cells also express NKG2D, we examined the relative contribution of TCRγδ and NKG2D in ULBP4-induced activation. PBMC-derived Vγ9/δ2 T cells were used as effectors in a cytotoxicity assay against EL4 or EL4-ULBP4 target cells. We tested the effect of blocking mAbs to NKG2D or TCRγδ on their cytotoxicity. At an E/T ratio of 20 or 40, Vγ9/δ2 T effectors displayed a more than 2-fold enhanced cytotoxicity against the EL4-ULBP4 cells compared with against EL4 cells (P < .01). The cytotoxicity was significantly inhibited by mAbs to ULBP4, NKG2D, or TCRγδ. Importantly, combined use of anti-NKG2D and anti-TCRγδ further decreased the cytotoxicity of Vγ9/δ2 T effectors compared with use of anti-NKG2D or anti-TCRγδ alone (P < .05) (Figure 4A).
Involvement of both TCR and NKG2D during ULBP4-induced γδT-cell activation. (A) MTT assay evaluating inhibition of cytotoxicity by PBMC-Vδ2T cells against EL4-ULBP4 cells. Anti-NKG2D (20 μg/mL), anti-TCRγδ (10 μg/mL), a combination of both, and control mAb. Data represent means ± SD of 3 independent experiments (*P < .05, **P < .01). (B-C) Effect of TCR and NKG2D signal on IFN-γ secretion and granule release. Immobilized rULBP4, anti-NKG2D mAb, anti–pan-TCRγδ mAb, and Ig control were precoated on microtiter plates. PBMC-Vγ9/δ2T cells were incubated. In blocking assay, CsA (100ng/mL), Ly294002 (50 μM), or wortmannin (1 μM) was added to some cultures. Supernatants collected after 48 hours (B) or after 5 hours (C) were measured for IFN-γ secretion (B) or BLT esterase release (C) by ELISA. Each error bar represents means ± SD of triplicate samples.
Involvement of both TCR and NKG2D during ULBP4-induced γδT-cell activation. (A) MTT assay evaluating inhibition of cytotoxicity by PBMC-Vδ2T cells against EL4-ULBP4 cells. Anti-NKG2D (20 μg/mL), anti-TCRγδ (10 μg/mL), a combination of both, and control mAb. Data represent means ± SD of 3 independent experiments (*P < .05, **P < .01). (B-C) Effect of TCR and NKG2D signal on IFN-γ secretion and granule release. Immobilized rULBP4, anti-NKG2D mAb, anti–pan-TCRγδ mAb, and Ig control were precoated on microtiter plates. PBMC-Vγ9/δ2T cells were incubated. In blocking assay, CsA (100ng/mL), Ly294002 (50 μM), or wortmannin (1 μM) was added to some cultures. Supernatants collected after 48 hours (B) or after 5 hours (C) were measured for IFN-γ secretion (B) or BLT esterase release (C) by ELISA. Each error bar represents means ± SD of triplicate samples.
We next examined IFN-γ production by PBMC-derived Vγ9/δ2 T cells stimulated with immobilized rULBP4. After 48 hours of culture in the presence of 50 UI/mL IL-2, the concentration of IFN-γ in the supernatants after stimulation with immobilized rULBP4 was 743 pg/mL. To determine the signaling pathways involved in ULBP4-induced activation of γδT cells, we treated ULBP4-stimulated Vγ9/δ2 T cells with PI3-kinase inhibitors wortmannin or Ly294002. Previous reports showed that DAP10 adapter protein recruits the p85 subunit of PI3-kinase, which is activated during NKG2D engagement by ULBPs.34-36 We found that use of Ly294002 (50 μM, final concentration) caused an 80% reduction in IFN-γ production in ULBP4-stimulated Vγ9/δ2 T cells. Wortmannin also had a similar effect (80%–90% inhibition) on ULBP4-induced cytokine production. Importantly, the immunosuppressive drug CsA that blocks TCR signal also caused a large reduction (60%–70% inhibition) in IFN-γ production in ULBP4-stimulated Vγ9/δ2 cells. Combined use of CsA plus Ly294002 or CsA plus wortmannin further inhibited IFN-γ production in these cells. These data suggest that both TCRγδ and NKG2D are involved in ULBP4-induced IFN-γ in γδT cells (Figure 4B).
We further tested cytolytic granule release in PBMC-derived Vγ9/δ2 T cells induced by ULBP4. The extent of granule release from effectors reached 44% after stimulation with rULBP4. The inhibition values of CsA, Ly294002, or wortmannin were 30%, 5%, or 23%, respectively. Use of CsA in combination with Ly294002 or wortmannin further inhibited the granule release (Figure 4C). Together, these results further support that TCRγδ and NKG2D are involved in ULBP4-induced γδT-cell activation.
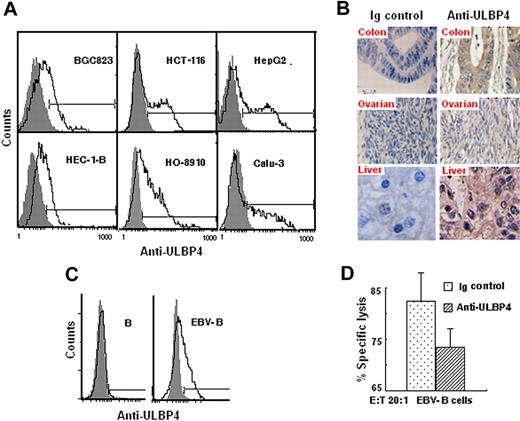
Expression of ULBP4 on tumor cells/tissues and infected peripheral blood cells
ULBP4 mRNA is not only expressed in some tissues such as skin,20 small intestine, brain, breast, colon, and spleen23 but also can be induced in other cells by a stressful stimulus. To determine whether ULBP4 is expressed on the cell surface of tumor cells, we screened tumor cells or tumor specimens of different origins using anti-ULBP4 mAb by flow cytometry (Figure 5A) and immunohistochemistry (Figure 5B). All these tumor cell lines expressed ULBP4 on their surface (Figure 5A). Immunohistochemistry assay revealed that all tumor cells were ULBP4 positive. In contrast, connective tissue cells were negative for ULBP4 staining (Figure 5B right panel). As expected, negative controls showed no staining (Figure 5B left panel). These results were consistent with previous reports showing that ULBP4 mRNA was expressed in tumors from ovary23,25 and colon.23
ULBP4 expression on tumor- and EBV-infected B cells. (A) Cell-surface expression of ULBP4 on tumor cells. The indicated tumor cells were stained with unconjugated anti-ULBP4 mAb 8C9 (black line histograms) or control Ig (gray filled histogram) followed by FITC-conjugated goat anti–mouse IgG. Analyses were measured on a FACSCalibur flow cytometer. (B) Immunohistochemistry staining of colonic carcinoma specimen (40×/0.65 NA oil objective lens), ovarian epithelial carcinoma specimens (20×/0.4 NA oil objective lens), and liver carcinoma specimens (40×/0.65 NA oil objective lens). Slides were viewed with a Leica DM3000 microscope. Images were acquired using a Leica DFC420 camera, and were processed with Leica QWin plus version 3.5.0 software. Staining was visualized using diaminobenzidine as the substrate. (C) Surface expression of ULBP4 in B lymphocytes before or after EBV infection. The indicated cells were stained with the anti-ULBP4 mAb 8C9 (black line histograms) or control Ig (gray filled histogram) followed by FITC-conjugated goat anti–mouse IgG. (D) Cytotoxicity of PBMC-Vδ2T cells against EBV-B cells with or without anti-ULBP4 mAb 8C9. Data represent means ± SD (error bars) of 3 independent experiments.
ULBP4 expression on tumor- and EBV-infected B cells. (A) Cell-surface expression of ULBP4 on tumor cells. The indicated tumor cells were stained with unconjugated anti-ULBP4 mAb 8C9 (black line histograms) or control Ig (gray filled histogram) followed by FITC-conjugated goat anti–mouse IgG. Analyses were measured on a FACSCalibur flow cytometer. (B) Immunohistochemistry staining of colonic carcinoma specimen (40×/0.65 NA oil objective lens), ovarian epithelial carcinoma specimens (20×/0.4 NA oil objective lens), and liver carcinoma specimens (40×/0.65 NA oil objective lens). Slides were viewed with a Leica DM3000 microscope. Images were acquired using a Leica DFC420 camera, and were processed with Leica QWin plus version 3.5.0 software. Staining was visualized using diaminobenzidine as the substrate. (C) Surface expression of ULBP4 in B lymphocytes before or after EBV infection. The indicated cells were stained with the anti-ULBP4 mAb 8C9 (black line histograms) or control Ig (gray filled histogram) followed by FITC-conjugated goat anti–mouse IgG. (D) Cytotoxicity of PBMC-Vδ2T cells against EBV-B cells with or without anti-ULBP4 mAb 8C9. Data represent means ± SD (error bars) of 3 independent experiments.
To determine whether virus infection induces cell-surface expression of ULBP4, we infected peripheral blood cells from healthy donor with EBV and analyzed the expression of surface ULBP4. Uninfected B cells expressed little surface ULBP4. In contrast, virus-infected B cells up-regulated cell-surface ULBP4 expression (Figure 5C).
Then, we tested effect of EBV infection on the cytotoxicity of PBMC-γδT cells. At 20 E/T, PBMC-γδT cells readily lysed EBV-infected targets and the specific lysis can be inhibited by anti-ULBP4 mAb (Figure 5D). This result suggests that ULBP4 is involved in the specific lysis of EBV-infected cells.
Discussion
In the present study, our results support that NKG2D ligand ULBP4 also serves as a ligand for TCRγδ. Several lines of evidence have demonstrated the interaction between ULBP4 and TCRγδ. First, recombinant ULBP4 not only induces the expansion of human γδT cells from TILs, but importantly, directly binds to soluble TCRγδ and stimulates the activation of TCRγδ transfectants. Second, mAb blocking assay also indicates that both NKG2D and TCRγδ are engaged during ULBP4-induced γδT-cell activation.
We initially tested whether ULBP4 activates Vδ1 T cells because other MHC class I–related molecules, MICA or ULBP3, activate this population. However, ULBP4 induced marked expansion of Vδ2 TILs in vitro. Furthermore, specific binding of ULBP4 to TCRγ9/δ2-OT3-Fc chimeric protein or TCRγ9/δ2-transfected cells provides strong evidence that ULBP4 functions as a ligand for TCRγ9/δ2. Studies have so far demonstrated that γδT cells recognize endogenous molecules induced by stressful stimuli. Recent data show that Vγ9/δ2 T cells in PBMCs react not only to unique nonpeptide antigen,12-14 but also to protein antigen ATPase.37 However, the mechanism of nonpeptide phosphoantigen recognition by Vγ9/δ2 T cells remains to be established. This recognition might be due to the existence of a surface molecule that either presents phosphoantigens to γδT cells acting as a professional antigen-presenting molecule or is modified by phosphoantigens, which in turn are recognized by γδT cells.38,39 Based on the special role of phosphate and the presence of ecto-phosphorylation of cell-surface proteins such as heparin-binding fibroblast growth factor receptor tyrosine kinase and collagen XVII,40-42 we predict that the phosphorylation sites in ULBP4 sequence may be recognized by Vδ2 T cells. There are in total 15 predicted phosphorylation sites in ULBP4 sequence. Further study of the recognition sites on ULBP4 may provide insights to the mechanism of γδT-cell recognition.
In addition to Vδ2 TCR, Vδ1 TCR may mediate the recognition of ULBP4. Poggi et al9 and Catellani et al10 reported that Vδ1 T lymphocytes from B-cell chronic lymphocytic leukemia patients recognized ULBP3.9,10 In line with this finding, we also detected that Vδ1 phenotype of human colonic carcinoma specimen–derived γδ TILs was induced to expand after ULBP4 stimulation (Figure 2A lower panel). Moreover, ULBP4 gene has greater similarity to ULBP3 than to ULBP1 or ULBP2.22 Thus, ULBP4 may serve as a ligand for multiple γδTCRs.
Hayday predicted that γδT-cell activation may require other stimulating signals besides the signal provided by TCR.1 NKG2D becomes one of such candidates for γδT-cell costimulators. We observed additive inhibitory effect by inhibitor of TCRγδ signal plus NKG2D signal in blocking γδT-cell–mediated killing of ULBP4 expression target cells, IFN-γ secretion, and granule release. These results suggest a role of both signaling pathways in ULBP4-mediated activation of γδT cells.
Human γδT cells recognize ULBP4 stressfully expressed in tumors or pathogen-infected tissue cell via TCR and NKG2D. Afterward, they are activated, and display strong cytotoxicity toward tumors and EBV-infected B cells. Because EBV infection of B cells leads to lymphomas, it suggests that ULBP4 may be a target for γδT cells to monitor EBV infection of B cells and the spreading of solid tumors in bloodstream when neoplasm metastasis happens.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants (2001CB510009, 2007CB512405, and 2004CB518706) from the National Program for Key Basic Research Project, Ministry of Science and Technology, People's Republic of China (W.H.) and projects funded by National Natural Science Foundation of China (30490244 [W.H.] and 30400391 [C.M.]).
Authorship
Contribution: Y.K. performed research, analyzed data, and wrote the paper; W.C. and X.X. performed research and contributed vital new reagents; C.M. and L.C. analyzed data and wrote the paper; W.H. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wei He, Department of Immunology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences and School of Basic Medicine, Peking Union Medical College, National Key Laboratory of Medical Molecular Biology, 5 Dong Dan San Tiao, Beijing 100005, China; e-mail: heweiimu@public.bta.net.cn.