Abstract
MicroRNAs (miRNAs, miRs) modulate a multitude of cellular events. Here, we identify functional miRNA-protein networks that regulate human monocyte-derived dendritic cell (MDDC) differentiation. miRNA profiling revealed stage-specific differential expression of 20 miRNAs during days 1, 3, and 5 of MDDC differentiation. To identify and prioritize miRNA-protein networks for functional validation, we developed a target ranking algorithm that incorporates many features of miRNA regulatory networks. This system prioritized miR-21, miR-34a, and their cognate targets WNT1 and JAG1 for functional validation. Inhibition of both miR-21 and miR-34a stalled MDDC differentiation, as quantified by DC-SIGN/CD14 expression ratios, showing cooperative involvement of these miRNAs in MDDC differentiation. We confirmed that the 3′ untranslated regions of WNT1 and JAG1 were functional targets of these miRNAs and provide evidence that these targets were translationally suppressed. Significantly, exogenously added Wnt-1 and Jagged-1 also stalled MDDC differentiation, suggesting that miRNA-mediated inhibition of endogenous WNT1 and JAG1 expression was important for proper MDDC differentiation. Finally, inhibition of miR-21 and miR-34a, or addition of Wnt-1 and Jagged-1, led to a decrease in endocytic capacity, a key function of immature DCs. Thus, our novel approach identified and validated some miRNA-protein networks involved in phenotypic and functional MDDC differentiation.
Introduction
MicroRNAs (miRNAs, miRs) are small (∼ 21-mer) regulatory RNA molecules encoded in plant and animal genomes. miRNAs regulate the expression of target genes by binding to the 3′-untranslated regions (3′-UTR) of specific mRNAs and triggering mRNA degradation or translational repression.1 There are hundreds of miRNAs in humans and each is predicted to regulate multiple genes, making the potential regulatory connections controlled by miRNAs enormous.2 miRNAs act as key regulators of diverse developmental and cellular differentiation processes.1,3-7
miRNAs fine-tune gene expression by effecting more subtle and rapid changes than global transcriptional control mechanisms.6 These effects may be most significant in systems in which relative expression levels of genes in a common pathway define the functional outcome, as is thought to occur during hematopoietic development.6,8 To this end, comparisons of miRNA expression profiles in hematopoietic cell populations during differentiation show stage-specific expression, strengthening the idea that miRNAs play a crucial role in the maintenance and progression of specific stages during hematopoietic development.9,10
In the current study, we investigate the role of miRNAs in stage-specific human monocyte-derived dendritic cell (MDDC) differentiation using miRNA microarrays and a stepwise target ranking system. Dendritic cells (DCs) serve a crucial function in initiating and regulating immunity,11 and they can develop directly from myeloid progenitors in the bone marrow as well as circulating blood monocytes.12 Although the expression profile of miRNAs in MDDCs has been reported,13 the identification and functional evaluation of the miRNAs and their corresponding target genes in MDDC differentiation have not been investigated.
Although miRNAs have been implicated in diverse biologic processes, the target genes of many of these miRNAs remain unresolved. Only a small subset of predicted human miRNA targets have been directly characterized thus far.14 Narrowing down actual target genes has become increasingly difficult because of the abundance of prediction algorithms, high rate of false positives, and hundreds of possible targets generated by each algorithm.15 Furthermore, when multiple miRNAs are known to coordinately regulate a particular process, the high number of predicted targets makes it technically challenging to evaluate the importance of each target gene or to implicate the relevant miRNA-protein regulatory network. Thus, for functional miRNA studies, it would be beneficial to have a restricted pool of predicted targets that can be experimentally verified.
Here, we identify and investigate the role of stage-specific miRNAs in regulating MDDC differentiation. We used computational and experimental approaches that first culled and then prioritized the target genes for functional validation. Our analysis identified miR-21 and miR-34a as well as their corresponding target genes, WNT1 and JAG1, for further investigation as to their roles in MDDC differentiation.
Methods
Flow cytometric analysis of MDDC surface phenotype
Human MDDCs were differentiated with granulocyte-macrophage colony-stimulating factor (GM-CSF)/interleukin-4 (IL-4) as described.16 At the indicated time points, cells were processed for standard flow cytometry using fluorescein isothiocyanate (FITC)–conjugated anti-CD14 (Invitrogen) and/or allophycocyanin-conjugated anti-CD209 (DC-SIGN; R&D Systems). Samples were analyzed using a FACSCalibur flow cytometer (BD Biosciences) and FCSExpress3 software (De Novo Software).
RNA preparation, miRNA microarray hybridization
Total RNA was independently isolated for each donor at the indicated time point using the mirVana miRNA isolation kit (Ambion). For miRNA microarray, RNA samples were processed and hybridized to the Ambion Bioarray v1566, containing 389 miRNA probes, by Asuragen Services according to standard protocol.17 Data were analyzed using analysis of variance with a multiple comparison corrected P value less than .005 and a false discovery rate set to 0.05 by Asuragen Services. The microarray data have been deposited in the GEO public database under accession number GSE15644.
miRNA and mRNA real-time quantitative RT-PCR
miRNA and mRNA expression was independently quantified using the TaqMan MicroRNA and TaqMan gene expression assays, respectively (Applied Biosystems) according to the manufacturer's protocols. miRNA expression was normalized to RNU43 small nuclear RNA endogenous controls. For mRNA, transcripts were quantified by real-time quantitative polymerase chain reaction (RT-PCR) and normalized to the amount of β-actin mRNA expressed, as described previously.16
Hierarchical clustering and principle component analysis
Hierarchical clustering was carried out using Euclidian distance as the distance metric and average linkage between clusters to perform the clustering. Principal component analysis (PCA) was performed using covariance for the dispersion matrix and normalized scaling (Asuragen).
Target gene prediction and functional analysis
Target gene prediction strategy is provided with detailed rationale in the text accompanying supplemental Figure 9 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Functional analysis of these datasets was performed using GOstat18 with a P value less than .05.
Immunoblot analysis of Wnt-1 and Jagged-1
MDDCs were lysed at indicated time points after culture with GM-CSF and IL-4 (as described16 ). Equal concentrations of protein were separated on a denaturing sodium dodecyl sulfate–10% polyacrylamide gel and then transferred to nitrocellulose by electroblotting. Proteins were detected with a 1:125 dilution of rabbit anti–Wnt-1 clone ZMD.326 (Invitrogen) and a 1:40 000 dilution of horseradish peroxidase (HRP)–conjugated donkey anti–rabbit antibodies (Pierce Chemical). For Jagged-1 immunoprecipitation, equivalent amounts of protein were precleared with protein G agarose beads (Pierce Chemical) coated with a 1:100 dilution of rabbit IgG (Pierce Chemical). Cleared lysates were subjected to standard immunoprecipitation using a 1:200 dilution of anti–Jagged-1 (clone J59, a kind gift from Gerry Weinmaster at University of California–Los Angeles, Los Angeles, CA). Beads were washed 2 times with wash buffer (phosphate-buffered saline [PBS]/2 mM ethylenediaminetetraacetic acid/0.2% NP-40). Immunoprecipitated proteins were analyzed by Western blotting using a 1:1000 dilution of mouse anti–Jagged-1 clone 1E12 (Novus Biologicals) and 1:30 000 of goat anti–mouse-HRP (Pierce Chemical). HRP was detected with ECL Plus (GE Healthcare).
Reporter vectors and DNA constructs
The 3′-UTRs of WNT1 (1082 bp) and JAG1 (1812 bp) were PCR amplified from human monocyte genomic DNA and cloned downstream of CMV-driven firefly luciferase cassette in pMIR-REPORT vector (Ambion). As positive controls, reporter vectors with directly matched miRNA-binding site oligos (∼ 51 bp) for either miR-34a or miR-21 were generated. For miRNA target validation, approximately 105 293T cells per well in a 24-well plate were transiently transfected with 25 to 50 ng of each firefly luciferase reporter construct, 150 to 175 ng pcDNA3 empty vector, 200-ng Renilla luciferase vector cloned in pcDNA3, and 30 pmol of pre-miR-21, pre-miR-34a, pre-let-7e, or pre-miR-neg (Ambion). Renilla luciferase vector was used to normalize transfection efficiency. At 24 hours after transfection, both firefly and Renilla luciferase activity was assayed (Promega). Normalized relative light units represent firefly luciferase activity/Renilla luciferase activity.
Conditioned media assays
The 293T cells were plated at a density of 5 × 106 cells/10-cm-diameter dish and transfected with 15 μg pcDNA3 empty vector, WNT1 expression vector, or JAG1 expression vector in OptiMEM I media. Media was changed 4 hours after transfection to equal volumes of Dulbecco modified Eagle medium and RPMI plus 10% fetal bovine serum. Twenty-four hours later, conditioned media was harvested, cleared to remove cell debris, and either used immediately or stored at −80°C. For flow cytometry, 5 × 105 monocytes/well in a 12-well plate were cultured in differing conditioned media plus GM-CSF and IL-4 and then stained for cell surface markers at the indicated time points.
Recombinant Jagged-1-Fc assays
Recombinant Jagged-1-Fc (R&D Systems) or ephrinB2-Fc (R&D Systems) was preclustered with goat anti–human-Fc (Jackson ImmunoResearch Laboratories) for 1 hour at room temperature in RPMI media with 10% fetal bovine serum. Media and protein were then used to culture MDDCs in the presence of GM-CSF and IL-4 as described.16
Transfection of anti-miR inhibitors
Cy3-labeled nontargeting negative anti-miR oligonucleotides (Ambion) were mixed with 100 nM targeting anti-miRNAs (miR-21, miR-34a, let-7e, miR-99b, miR-125a, or miR-342; Ambion) at a ratio of 1:5 in OptiMEM I media. In the negative anti-miR only transfection, 100 nM total of Cy3-labeled nontargeting negative anti-miR was used. The mixture was reverse transfected into 105 monocytes using lipofectin reagent (Invitrogen) according to the manufacturer's instructions. Media and cytokines were resupplemented 24 hours after transfection. MDDC cell surface phenotype was then assayed by flow cytometry as described in “Flow cytometry analysis of MDDC surface phenotype.”
FITC-dextran uptake assay
MDDCs were differentiated for 5 days under the indicated conditions, harvested, and washed with ice-cold PBS. MDDCs (5 × 105) were incubated with 1 mg/mL FITC-dextran (Sigma-Aldrich) in 1-mL culture medium for 30 minutes at 37°C or 4°C (background). Cells were washed 3 times with ice-cold PBS and then fixed with 2% paraformaldehyde and analyzed with flow cytometry as described in “Flow cytometric analysis of MDDC surface phenotype.”
Results
miRNA microarrays show unique miRNA expression profiles at distinct MDDC differentiation stages
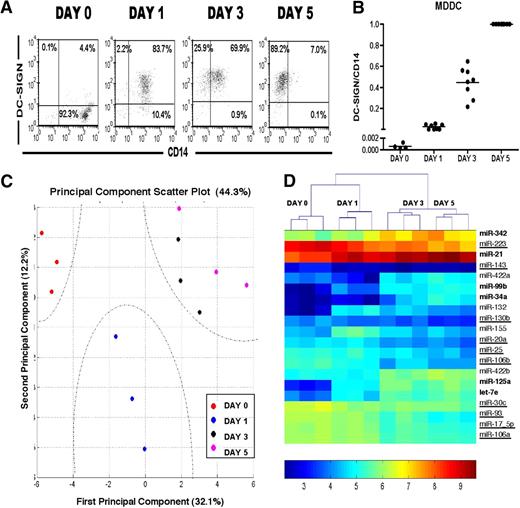
Human monocytes cultured with GM-CSF and IL-4 acquire the characteristics of immature DCs after approximately 5 to 7 days.12,19,20 However, Figure 1A shows that monocyte to MDDC differentiation progresses through intermediate stages reflected by specific up-regulation of DC-SIGN and down-regulation of CD14 expression from day 0 through days 1, 3, and 5. Two metrics can be used to examine and quantify progression of MDDC differentiation. The first metric (Figure 1A) uses the percentage positive cells in each quadrant as a qualitative reflection of MDDC differentiation. For example, day 0 monocytes were essentially all DC-SIGN− CD14+. At day 1, cells began to increase DC-SIGN expression while still maintaining CD14 expression. By day 3, most cells have down-regulated CD14 at the apparent expense of DC-SIGN up-regulation. By day 5, most cells are fully differentiated DC-SIGN++CD14− MDDCs. The second metric (Figure 1B) uses the relative mean fluorescence intensity (MFI) of DC-SIGN and CD14 expression on differentiation, and the relative increase in DC-SIGN/CD14 MFI ratios from day 1 to day 5 allows for a more quantitative measure of MDDC differentiation. Indeed, this ratio steadily increased during MDDC differentiation and showed clustering of the numerical values at each stage of differentiation, and thus, is probably a reliable indicator of differentiation progression. Although microarray and proteomic studies have identified many genes involved in MDDC differentiation,21-23 miRNA regulation of MDDC differentiation has not been examined.4,5,10 Thus, we sought to assess the role of miRNAs in regulating MDDC differentiation.
MDDCs exhibit unique stage-specific patterns in miRNA expression. (A) Flow cytometric analysis showing cell surface phenotype during MDDC differentiation. Monocytes and DCs can be distinguished by their CD14 and DC-SIGN expression profiles. Data from one representative donor (of 3) are shown. (B) Quantification of DC-SIGN/CD14 expression ratios in 5 independent donors from 7 independent experiments. Ratios at day 0 were measured in 4 of 7 independent experiments. Ratios at days 1, 3, and 5 were measured in 7 of 7 independent experiments. Ratios were calculated using MFI of DC-SIGN/ MFI of CD14 and normalized to the day 5 ratio of each donor, which was set at 1.0. (C) PCA of all 389 miRNA probes shows distinct clustering of expression profiles from day 0, 1, 3, and 5 differentiated monocytes. First and second principal components, represented on the x-axis and y-axis, respectively, account for approximately 32% and 12% of the variability between the various samples. Different time points (in 3 different donors; each represented by one circle) are represented by the indicated colors. (D) Hierarchical clustering of statistically significant differential miRNAs (human miRNAs) with analysis of variance (P < .005). Boldface type represents upregulated miRNAs. Downregulated miRNAs are underlined. Remaining miRNAs have sinusoidal expression. Clustering on the top represents the relationships between the various time points, and each column in a given day represents an independent donor. Color legend indicates relative log scale intensity of expression.
MDDCs exhibit unique stage-specific patterns in miRNA expression. (A) Flow cytometric analysis showing cell surface phenotype during MDDC differentiation. Monocytes and DCs can be distinguished by their CD14 and DC-SIGN expression profiles. Data from one representative donor (of 3) are shown. (B) Quantification of DC-SIGN/CD14 expression ratios in 5 independent donors from 7 independent experiments. Ratios at day 0 were measured in 4 of 7 independent experiments. Ratios at days 1, 3, and 5 were measured in 7 of 7 independent experiments. Ratios were calculated using MFI of DC-SIGN/ MFI of CD14 and normalized to the day 5 ratio of each donor, which was set at 1.0. (C) PCA of all 389 miRNA probes shows distinct clustering of expression profiles from day 0, 1, 3, and 5 differentiated monocytes. First and second principal components, represented on the x-axis and y-axis, respectively, account for approximately 32% and 12% of the variability between the various samples. Different time points (in 3 different donors; each represented by one circle) are represented by the indicated colors. (D) Hierarchical clustering of statistically significant differential miRNAs (human miRNAs) with analysis of variance (P < .005). Boldface type represents upregulated miRNAs. Downregulated miRNAs are underlined. Remaining miRNAs have sinusoidal expression. Clustering on the top represents the relationships between the various time points, and each column in a given day represents an independent donor. Color legend indicates relative log scale intensity of expression.
We first performed miRNA microarrays on miRNA isolated from human MDDCs at differentiation days 0, 1, 3, and 5 from 3 separate donors. To capture a universal picture of the expression profile of all miRNAs in each sample, PCA was used to reduce the large dimensionality of expression profiles to 2 dimensions, which can be mapped out for comparison. PCA comparison shows that stage-specific unique miRNA profiles exist on differentiation (Figure 1C), as evidenced by distinct clustering of samples at each stage. Each temporal group (eg, day 0, day 3) clustered separately from one another, implying that the miRNA expression profile from each differentiation time point was unique. In concordance with our cell surface phenotype analysis (Figure 1A), miRNA expression profiles from days 0 and 1 clustered quite distinctly from each other and from days 3 and 5 profiles, which were closest to each other (Figure 1C).
miRNA microarrays identify 20 unique miRNAs involved in MDDC differentiation
Analysis of variance (using a corrected P value < .005) identified 20 miRNAs that are differentially regulated between the various stages of MDDC differentiation (Figure 1D). Hierarchical clustering of these statistically significant miRNAs further confirmed the distinct clustering of miRNA expression profiles according to the day of differentiation, in agreement with the PCA comparison (Figure 1C-D). Indeed, Figure 1D shows that day 0, 1, 3, and 5 MDDCs can be identified via a specific pattern of miRNA expression. This dendogram also shows a wide range of expression changes, such as the low level of miR-34a in day 0 and its more than 10-fold increase in subsequent days (Figure 1D). Table 1 details the fold change and P value of the differentially regulated miRNAs, which include multiple miRNAs (miR-20a, miR-17_5p, miR-106a) previously implicated in myeloid differentiation.24 In all, approximately 10 miRNAs were significantly decreased during MDDC differentiation, whereas 6 were significantly increased. The remaining 4 miRNAs had sinusoidal patterns of increases or decreases as differentiation proceeded. Table S3 contains a complete list of miRNA expression and statistics. Quantitative RT-PCR of 12 miRNA candidates in 3 independent donors confirmed the microarray expression results and their myeloid specificity because these miRNAs had little to no expression in CD4+ T cells (supplemental Figure 8).
Fold change and P values of miRNAs with P < .005
| Expression trend/miRNA . | Fold change relative to day 0 . | P (ANOVA) . | ||
|---|---|---|---|---|
| Day 1 . | Day 3 . | Day 5 . | ||
| Increasing | ||||
| hsa-miR-125a | 4.9 | 11.4 | 12.0 | 2.18 × 10−7 |
| hsa-miR-99b | 4.0 | 10.5 | 13.5 | 4.65 × 10−6 |
| hsa-let-7e | 2.6 | 5.1 | 5.3 | 3.66 × 10−5 |
| hsa-miR-34a | 1.4 | 6.1 | 13.4 | 4.79 × 10−5 |
| hsa-miR-21 | 2.3 | 2.7 | 3.4 | 2.60 × 10−4 |
| hsa-miR-342 | 1.3 | 3.3 | 3.4 | 4.51 × 10−3 |
| Decreasing | ||||
| hsa-miR-106a | 0.9 | 0.5 | 0.5 | 1.57 × 10−5 |
| hsa-miR-17–5p | 0.9 | 0.5 | 0.4 | 6.90 × 10−5 |
| hsa-miR-106b | 0.7 | 0.4 | 0.3 | 4.18 × 10−4 |
| hsa-miR-20a | 1.0 | 0.6 | 0.5 | 1.62 × 10−3 |
| hsa-miR-223 | 0.6 | 0.4 | 0.2 | 3.13 × 10−4 |
| hsa-miR-30c | 0.7 | 0.6 | 0.5 | 2.15 × 10−3 |
| hsa-miR-93 | 0.8 | 0.5 | 0.3 | 7.81 × 10−5 |
| hsa-miR-130b | 0.8 | 0.7 | 0.5 | 2.82 × 10−3 |
| hsa-miR-25 | 0.9 | 0.6 | 0.4 | 2.52 × 10−3 |
| hsa-miR-143 | 0.6 | 0.5 | 0.5 | 1.64 × 10−3 |
| Sinusoidal | ||||
| hsa-miR-155 | 3.3 | 1.2 | 1.1 | 8.85 × 10−4 |
| hsa-miR-132 | 11.1 | 7.7 | 4.7 | 2.55 × 10−6 |
| hsa-miR-422a | 0.4 | 2.2 | 2.9 | 2.03 × 10−4 |
| hsa-miR-422b | 0.9 | 2.0 | 2.6 | 5.23 × 10−5 |
| Expression trend/miRNA . | Fold change relative to day 0 . | P (ANOVA) . | ||
|---|---|---|---|---|
| Day 1 . | Day 3 . | Day 5 . | ||
| Increasing | ||||
| hsa-miR-125a | 4.9 | 11.4 | 12.0 | 2.18 × 10−7 |
| hsa-miR-99b | 4.0 | 10.5 | 13.5 | 4.65 × 10−6 |
| hsa-let-7e | 2.6 | 5.1 | 5.3 | 3.66 × 10−5 |
| hsa-miR-34a | 1.4 | 6.1 | 13.4 | 4.79 × 10−5 |
| hsa-miR-21 | 2.3 | 2.7 | 3.4 | 2.60 × 10−4 |
| hsa-miR-342 | 1.3 | 3.3 | 3.4 | 4.51 × 10−3 |
| Decreasing | ||||
| hsa-miR-106a | 0.9 | 0.5 | 0.5 | 1.57 × 10−5 |
| hsa-miR-17–5p | 0.9 | 0.5 | 0.4 | 6.90 × 10−5 |
| hsa-miR-106b | 0.7 | 0.4 | 0.3 | 4.18 × 10−4 |
| hsa-miR-20a | 1.0 | 0.6 | 0.5 | 1.62 × 10−3 |
| hsa-miR-223 | 0.6 | 0.4 | 0.2 | 3.13 × 10−4 |
| hsa-miR-30c | 0.7 | 0.6 | 0.5 | 2.15 × 10−3 |
| hsa-miR-93 | 0.8 | 0.5 | 0.3 | 7.81 × 10−5 |
| hsa-miR-130b | 0.8 | 0.7 | 0.5 | 2.82 × 10−3 |
| hsa-miR-25 | 0.9 | 0.6 | 0.4 | 2.52 × 10−3 |
| hsa-miR-143 | 0.6 | 0.5 | 0.5 | 1.64 × 10−3 |
| Sinusoidal | ||||
| hsa-miR-155 | 3.3 | 1.2 | 1.1 | 8.85 × 10−4 |
| hsa-miR-132 | 11.1 | 7.7 | 4.7 | 2.55 × 10−6 |
| hsa-miR-422a | 0.4 | 2.2 | 2.9 | 2.03 × 10−4 |
| hsa-miR-422b | 0.9 | 2.0 | 2.6 | 5.23 × 10−5 |
Inhibition of up-regulated miRNAs alters normal MDDC differentiation
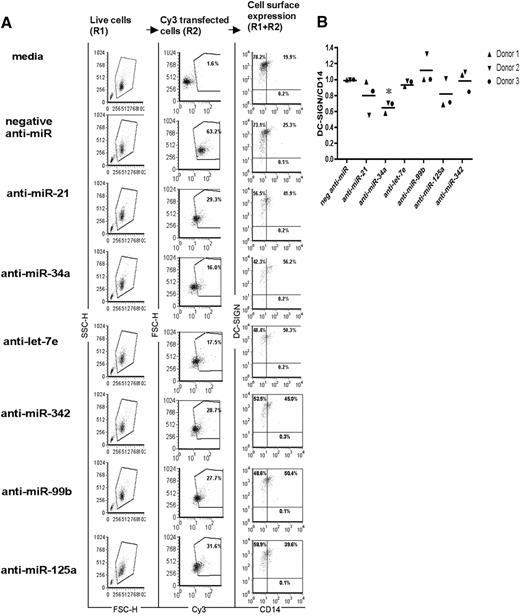
To test the functional significance of the up-regulated miRNAs identified from our statistical analysis, we used miRNA inhibitors to block endogenous miRNA expression in differentiating MDDCs. Monocytes were transfected with anti-miR oligonucleotide inhibitors25 and then differentiated and analyzed for DC-SIGN and CD14 expression. Figure 2A shows our gating strategy for such an analysis: the first panel shows the live cell gate (R1), the second panel shows our gating for anti-miR transfected (Cy3+) transfected cells (R2), and the third panel shows DC-SIGN and CD14 expression in the R1 + R2 gate. Each miRNA had an apparent stalling effect on the progression of MDDC differentiation as indicated by the 20% to 30% increase of dual-positive (DC-SIGN+/CD14+) cells at day 5 compared with the media or negative anti-miR controls (Figure 2A panel 3). Anti-miR34a had the most prominent effect as indicated by the largest percentage of dual-positive cells. Indeed, when MDDC differentiation was more rigorously quantified using DC-SIGN/CD14 MFI expression ratios as defined in Figure 1B, only inhibition of miR-34a seemed to have a reproducible and statistically significant effect on stalling progression (Figure 2B).
miRNAs regulate MDDC differentiation. Monocytes were untransfected, transfected with 100 nM Cy3-labeled nontargeting negative anti-miR, or transfected with a 1:5 ratio of Cy3-labeled nontargeting negative anti-miR and anti–miR-21, anti–miR-34a, anti–let-7e, anti–miR-99b, anti–miR-125a, or anti-miR-342 (100 nM). (A) FACS analysis showing (from left to right): live cell gating (R1), percentage of Cy3 positive cells (R2) (positive cells gated based on live cell gates), and cell surface phenotype (R1 + R2) during MDDC differentiation at day 5 with the indicated miRNA inhibitors. Numbers in each quadrant represent percentage of positive cells. Data from 1 representative donor (of 3) are shown. (B) DC-SIGN/CD14 expression was normalized to MFI ratios of negative anti-miR transfected MDDCs at day 5. Ratios were calculated using MFI of DC-SIGN/MFI of CD14. P values were calculated using a paired t test comparison with the negative anti-miR transfected MDDCs: *P < .05; **P < .01; ***P < .001. Scatter plots show data from 3 independent donors. Monocytes were differentiated in the presence of GM-CSF and IL-4. Cells were stained and analyzed at day 5 of MDDC differentiation for DC-SIGN and CD14 cell surface expression. Live cell populations were further gated on the positively transfected (Cy3+) cells for analysis.
miRNAs regulate MDDC differentiation. Monocytes were untransfected, transfected with 100 nM Cy3-labeled nontargeting negative anti-miR, or transfected with a 1:5 ratio of Cy3-labeled nontargeting negative anti-miR and anti–miR-21, anti–miR-34a, anti–let-7e, anti–miR-99b, anti–miR-125a, or anti-miR-342 (100 nM). (A) FACS analysis showing (from left to right): live cell gating (R1), percentage of Cy3 positive cells (R2) (positive cells gated based on live cell gates), and cell surface phenotype (R1 + R2) during MDDC differentiation at day 5 with the indicated miRNA inhibitors. Numbers in each quadrant represent percentage of positive cells. Data from 1 representative donor (of 3) are shown. (B) DC-SIGN/CD14 expression was normalized to MFI ratios of negative anti-miR transfected MDDCs at day 5. Ratios were calculated using MFI of DC-SIGN/MFI of CD14. P values were calculated using a paired t test comparison with the negative anti-miR transfected MDDCs: *P < .05; **P < .01; ***P < .001. Scatter plots show data from 3 independent donors. Monocytes were differentiated in the presence of GM-CSF and IL-4. Cells were stained and analyzed at day 5 of MDDC differentiation for DC-SIGN and CD14 cell surface expression. Live cell populations were further gated on the positively transfected (Cy3+) cells for analysis.
Bioinformatic target ranking system uncovers functional groups and target genes coordinately regulated by miRNAs during MDDC differentiation
Because several miRNAs are coordinately up-regulated during MDDC differentiation, it is doubtful that inhibition of a single miRNA would be capable of completely blocking MDDC differentiation. Thus, we sought to identify miRNAs that may have a cooperative effect and the target genes that may be regulated by these miRNAs. Target prediction programs can predict hundreds of targets for any given miRNA15 ; hence, the task of identifying the cognate targets that are actually regulated by the multiple miRNAs in question becomes nontrivial. Thus, to identify functionally relevant targets, we developed a target ranking system that uses multiple target prediction programs, incorporates the functional ontologies of the predicted target genes, and integrates the prospect of coordinated miRNA regulation.
First, we used multiple target prediction programs to generate a reduced target list for each miRNA (supplemental Table 4) by only keeping targets that are predicted by more than one program. Because each target prediction program uses a different basis for target prediction, encompassing all these methods will probably produce a more reliable set of predictions.15 This target prediction methodology is based on the hypothesis that miRNAs that are up-regulated or down-regulated in a given system may actually work in coordination to regulate a specific function in that system.26 Next, we prioritized genes that are targeted by multiple miRNAs1,27,28 and are overrepresented in a common functional ontology group.
A reduced target gene list was generated for each miRNA shown in Figure 1D. Increasing and decreasing miRNAs were grouped separately. Within each group, the reduced target gene lists for each of these miRNAs were cross-referenced with each other, and only unique target genes that were targets of 2 or more miRNAs in the group were kept (supplemental Figure 9 shows an explanatory diagram and logic flowchart of our target ranking procedure). This procedure reduced the total number of predicted target genes from 1148 to 112 for the group of increasing miRNAs, and from 2784 to 514 for the group of decreasing miRNAs (supplemental Tables 4–5).
Next, to concentrate on unique functions and targets that may be regulated by increasing or decreasing miRNAs, we analyzed these 2 reduced target gene lists using GOstat functional analysis (supplemental Tables 6–7) and eliminated all overlapping functional ontologies and target genes between these 2 sets. GOstat assigns significant P values to overrepresented functional groups within a set of genes.18 Our final culling resulted in 10 target genes (15 functional groups) regulated by increasing miRNAs during MDDC differentiation (Table 2). Supplemental Table 8 shows the corresponding list for decreasing miRNAs (402 target genes). For validation purposes, we first chose to focus our attention on the more manageable number of genes putatively targeted by increasing miRNAs (Table 2).
GOstat functional analysis of miRNA targets for miRNAs that are increased upon MDDC differentiation
| GO ID . | Functional group . | Count . | Targets . | miRNAs predicted to target these genes . | Total . | P . |
|---|---|---|---|---|---|---|
| Biologic process | ||||||
| Development | ||||||
| Organ development | ||||||
| GO:0048637 | Skeletal muscle development | 2 | JAG1; MBNL1 | miR-21, 34a, 99b | 21 | .025 |
| GO:0048747 | Muscle fiber development | 2 | JAG1; MBNL1 | miR-21, 34a, 99b | 21 | .025 |
| GO:0048741 | Skeletal muscle fiber development | 2 | JAG1; MBNL1 | miR-21, 34a, 99b | 21 | .025 |
| GO:0009790 | Embryonic development | 3 | MBNL1; KIF1B; FUT 8 | miR-21, 99b, 125a, 7e, 34a, 342 | 92 | .007 |
| GO:0009792 | Embryonic development (sensu Metazoa) | 2 | MBNL1; FUT 8 | miR-21, 99b, 34a, 342 | 27 | .035 |
| GO:0043009 | Embryonic development (sensu Vertebrata) | 2 | MBNL1; FUT 8 | miR-21, 99b, 34a, 342 | 12 | .011 |
| GO:0001701 | Embryonic development (sensu Mammalia) | 2 | MBNL1; FUT 8 | miR-21, 99b, 34a, 342 | 11 | .010 |
| Cellular process | ||||||
| GO:0051865 | Protein autoubiquitination; cell differentiation | 1 | UHRF2 | miR-34a, 7e | 1 | .031 |
| GO:0045445 | Myoblast differentiation | 2 | JAG1; MBNL1 | miR-21, 34a, 99b | 16 | .018 |
| GO:0045165 | Cell fate commitment | 2 | WNT 1; JAG1 | miR-21, 34a, 7e | 20 | .025 |
| Molecular function | ||||||
| Enzyme regulator activity | ||||||
| Enzyme activator activity | ||||||
| GO:0005099 | Ras GTPase activator activity; catalytic activity | 1 | RASAL2 | miR-342, 125a | 12 | .011 |
| GO:0046921 | α(1,6)-fucosyltransferase activity | 1 | FUT 8 | miR-34a, 342 | 1 | .031 |
| GO:0008424 | Glycoprotein 6-alpha-L-fucosyltransferase activity | 1 | FUT 8 | miR-34a, 342 | 1 | .031 |
| Binding | ||||||
| GO:0051018 | Protein kinase A binding | 1 | AKAP6 | miR-34a, 7e | 11 | .010 |
| GO:0051020 | GTPase binding | 2 | DOCK3; MYRIP | miR-125a, 7e, 34a | 92 | .035 |
| GO ID . | Functional group . | Count . | Targets . | miRNAs predicted to target these genes . | Total . | P . |
|---|---|---|---|---|---|---|
| Biologic process | ||||||
| Development | ||||||
| Organ development | ||||||
| GO:0048637 | Skeletal muscle development | 2 | JAG1; MBNL1 | miR-21, 34a, 99b | 21 | .025 |
| GO:0048747 | Muscle fiber development | 2 | JAG1; MBNL1 | miR-21, 34a, 99b | 21 | .025 |
| GO:0048741 | Skeletal muscle fiber development | 2 | JAG1; MBNL1 | miR-21, 34a, 99b | 21 | .025 |
| GO:0009790 | Embryonic development | 3 | MBNL1; KIF1B; FUT 8 | miR-21, 99b, 125a, 7e, 34a, 342 | 92 | .007 |
| GO:0009792 | Embryonic development (sensu Metazoa) | 2 | MBNL1; FUT 8 | miR-21, 99b, 34a, 342 | 27 | .035 |
| GO:0043009 | Embryonic development (sensu Vertebrata) | 2 | MBNL1; FUT 8 | miR-21, 99b, 34a, 342 | 12 | .011 |
| GO:0001701 | Embryonic development (sensu Mammalia) | 2 | MBNL1; FUT 8 | miR-21, 99b, 34a, 342 | 11 | .010 |
| Cellular process | ||||||
| GO:0051865 | Protein autoubiquitination; cell differentiation | 1 | UHRF2 | miR-34a, 7e | 1 | .031 |
| GO:0045445 | Myoblast differentiation | 2 | JAG1; MBNL1 | miR-21, 34a, 99b | 16 | .018 |
| GO:0045165 | Cell fate commitment | 2 | WNT 1; JAG1 | miR-21, 34a, 7e | 20 | .025 |
| Molecular function | ||||||
| Enzyme regulator activity | ||||||
| Enzyme activator activity | ||||||
| GO:0005099 | Ras GTPase activator activity; catalytic activity | 1 | RASAL2 | miR-342, 125a | 12 | .011 |
| GO:0046921 | α(1,6)-fucosyltransferase activity | 1 | FUT 8 | miR-34a, 342 | 1 | .031 |
| GO:0008424 | Glycoprotein 6-alpha-L-fucosyltransferase activity | 1 | FUT 8 | miR-34a, 342 | 1 | .031 |
| Binding | ||||||
| GO:0051018 | Protein kinase A binding | 1 | AKAP6 | miR-34a, 7e | 11 | .010 |
| GO:0051020 | GTPase binding | 2 | DOCK3; MYRIP | miR-125a, 7e, 34a | 92 | .035 |
miR-21 and miR-34a together further regulate MDDC differentiation
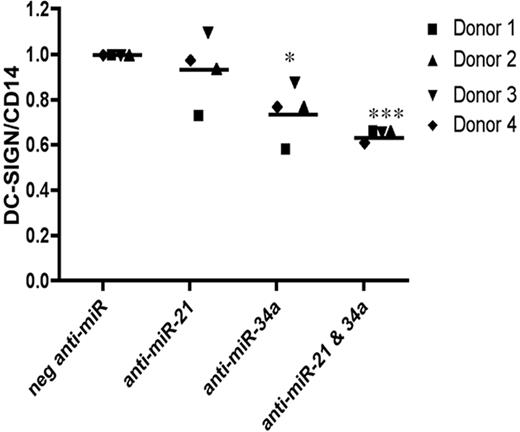
Table 2 shows that miR-21 and miR-34a are the most highly represented miRNAs among the group of increasing miRNAs, respectively, targeting 9 and 14 of the 15 unique functional ontology groups. Because every functional ontology appears to be targeted by miR-21 and/or miR-34a, and neither miRNA alone was able to completely block MDDC differentiation (Figure 2A-B), we sought to assess the role of miR-21 and 34a together in regulating MDDC differentiation, using anti-miR inhibitors as described in “Methods.” Although the transfections of anti-miR-34a or anti–miR-21 alone resulted in some decrease in DC-SIGN/CD14 ratios, respectively (Figure 3), cotransfection of both anti–miR-21 and anti–miR-34a more significantly decreased DC-SIGN/CD14 expression ratios (Figure 3), underscoring the importance of considering coordinated miRNA expression when evaluating miRNA regulatory function. We later confirmed that both anti-miRs together were also more potent in stalling the functional differentiation of MDDCs (Figure 7).
miRNAs 21 and 34a together further regulate MDDC differentiation. Monocytes were either untransfected or transfected with Cy3-labeled nontargeting negative anti-miR and anti–miR-21, anti–miR-34a, or both. DC-SIGN/CD14 expression was normalized to MFI ratios of untransfected MDDCs at day 5. Ratios were calculated using MFI of DC-SIGN/ MFI of CD14. P values were calculated using a paired t test comparison with the untransfected MDDCs: *P < .05; **P < .01; ***P < .001. Scatter plots show data from 4 independent donors. Monocytes were differentiated in the presence of GM-CSF and IL-4. Cells were stained and analyzed at day 5 of MDDC differentiation for DC-SIGN and CD14 cell surface expression. Live cell populations were further gated on the top 30% of positively transfected (Cy3+) cells for analysis.
miRNAs 21 and 34a together further regulate MDDC differentiation. Monocytes were either untransfected or transfected with Cy3-labeled nontargeting negative anti-miR and anti–miR-21, anti–miR-34a, or both. DC-SIGN/CD14 expression was normalized to MFI ratios of untransfected MDDCs at day 5. Ratios were calculated using MFI of DC-SIGN/ MFI of CD14. P values were calculated using a paired t test comparison with the untransfected MDDCs: *P < .05; **P < .01; ***P < .001. Scatter plots show data from 4 independent donors. Monocytes were differentiated in the presence of GM-CSF and IL-4. Cells were stained and analyzed at day 5 of MDDC differentiation for DC-SIGN and CD14 cell surface expression. Live cell populations were further gated on the top 30% of positively transfected (Cy3+) cells for analysis.
WNT1 and JAG1 are targets of miRNAs that are increased on MDDC differentiation
Because we have bioinformatically and functionally implicated miR-21 and miR-34a in MDDC differentiation, we now sought to validate genes and functional groups that are targeted by both miR-21 and miR-34a. Interestingly, the only gene (JAG1) that is the predicted target of both miR-21 and miR-34a is also in the “cell fate commitment” functional group, the functional ontology that is most evidently relevant to MDDC differentiation. The other target gene in this group is WNT1, predicted to be a target of both let-7e and miR-34a. Both JAG1 and WNT1 signaling pathways are involved in diverse hematopoietic differentiation and developmental processes, although they have not been classically associated with human MDDC differentiation.29-32
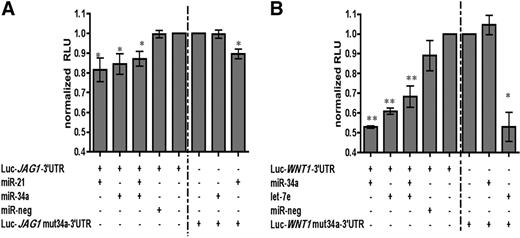
To confirm that these genes are true miRNA targets, JAG1 and WNT1 full-length 3′-UTRs were cloned into a luciferase reporter vector. We used the full-length 3′-UTRs of the predicted target genes to better reflect miRNA regulation in an endogenous context.26 JAG1 3′-UTR and WNT1 3′-UTR luciferase reporter vectors were cotransfected into 293T cells along with the indicated precursor miRNAs or cognate negative nontargeting controls (Figure 4). We verified that precursor miRNAs were processed into their mature form via quantitative RT-PCR (data not shown). The lysate was then assayed for luciferase expression. In the JAG1 3′-UTR vector, both miR-21 and miR-34a, alone or in combination, suppressed luciferase activity by a modest but significant amount compared with the negative nontargeting miRNA (Figure 4A, ∼ 20% inhibition, P < .05). In the case of the WNT1 3′-UTR vector, miR-34a and let-7e, individually or in combination, but not the negative nontargeting miRNA, suppressed the luciferase activity by approximately 30% to 50% (Figure 4B).
WNT1 and JAG1 are target genes of miR-21, miR-34a, and let-7e. Luciferase reporter gene assays of vector constructs with (A) JAG1-3′-UTR (left of dotted line) and JAG1mut 34a-3′-UTR (right of dotted line) or (B) WNT1-3′-UTR (left side) and WNT1mut34a-3′-UTR (right side) in the absence or presence of the indicated miRNAs. The luciferase expression of each vector used was set to 1 and used for normalization of all the respective assays. The negative miRNA control is nontargeting, having no sequence homology to the human genome. Error bars represent SEM from 3 independent experiments. P values (above each bar) were calculated using a paired t test comparison of the condition with the no miRNA condition: *P < .05; **P < .01; ***P < .001.
WNT1 and JAG1 are target genes of miR-21, miR-34a, and let-7e. Luciferase reporter gene assays of vector constructs with (A) JAG1-3′-UTR (left of dotted line) and JAG1mut 34a-3′-UTR (right of dotted line) or (B) WNT1-3′-UTR (left side) and WNT1mut34a-3′-UTR (right side) in the absence or presence of the indicated miRNAs. The luciferase expression of each vector used was set to 1 and used for normalization of all the respective assays. The negative miRNA control is nontargeting, having no sequence homology to the human genome. Error bars represent SEM from 3 independent experiments. P values (above each bar) were calculated using a paired t test comparison of the condition with the no miRNA condition: *P < .05; **P < .01; ***P < .001.
To further verify the specificity of the miRNA-mediated repression, the miR-34a binding sites were mutated in each of the 3′-UTRs. When the second miR-34a binding site in the JAG1 3′-UTR was mutated, miR-34a was no longer able to suppress luciferase expression (Figure 4A). However, miR-21 was still able to modestly inhibit luciferase expression. Concordantly, when the miR-34a binding site was mutated in the WNT1 3′-UTR, luciferase expression was no longer inhibited by miR-34a (Figure 4B). Importantly, let-7e was still able to suppress luciferase activity similar to wild-type WNT1 3′-UTR (∼ 50%; Figure 4B). The modest, yet specific, miRNA-mediated suppression seen in our luciferase reporter assays may not reflect the biologic significance of these miRNA-protein regulatory networks in their endogenous setting. For example, 293T cells may already have endogenous miR-21/miR-34a, which may affect the degree of suppression that can be seen in these reporter assays. Thus, we sought to explore the putative regulation of JAG1 and WNT1 mRNA/protein levels during MDDC differentiation.
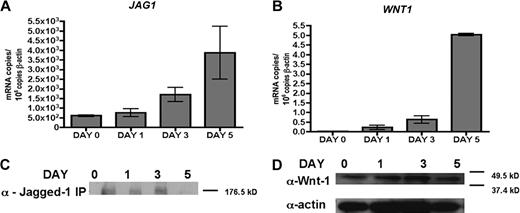
Discordance between target mRNA and protein expression levels suggests that JAG1 and WNT1 are translationally suppressed
To determine whether the miRNA may be acting via target mRNA degradation or translational suppression,1 we evaluated the target mRNA and protein levels during MDDC differentiation. Surprisingly, quantitative RT-PCR showed a dramatic increase of JAG1 (6-fold) and WNT1 (> 200-fold) mRNA as differentiation progressed (Figure 5A-B). However, Western blots of Jagged-1 immunoprecipitated from MDDCs indicated either constant (day 0, 1, and 3) or decreased (day 5) Jagged-1 protein expression through differentiation (Figure 5C). Similarly, Wnt-1 protein was detected at relatively constant levels during differentiation, contrary to the more than 200-fold increase in mRNA expression from day 0 to day 5 (compare Figure 5D with 5B). Such a discordance between mRNA and protein expression values suggests that miR-21 and/or miR-34a function by translational suppression of WNT1 and JAG1. In addition, the relatively dramatic suppression of Jagged-1 and Wnt-1 protein expression in MDDCs compared with the modest suppressive effect of miR-21 and/or miR34a on the WNT1 and JAG1 3′-UTR in the luciferase reporter assay (Figure 4) suggests that additional miRNAs may be involved in regulating JAG1 and WNT1 expression in MDDCs.
Discordance between JAG1 and WNT1 mRNA and protein expression levels. Quantitative RT-PCR of (A) JAG1 and (B) WNT1 mRNA in day 0, 1, 3, and 5 differentiated MDDCs. One representative donor from 3 is shown. Error bars represent SD from 3 replicates. (C) Immunoprecipitation-Western blot of Jagged-1 protein (∼ 180 kDa) in lysate (350 μg protein per sample). (D) Top: Western blot detection of Wnt-1 protein (∼ 41 kDa) in lysate (33 μg protein per lane) from day 0, 1, 3, and 5 differentiated MDDCs. Bottom: Western blot detection of actin loading control.
Discordance between JAG1 and WNT1 mRNA and protein expression levels. Quantitative RT-PCR of (A) JAG1 and (B) WNT1 mRNA in day 0, 1, 3, and 5 differentiated MDDCs. One representative donor from 3 is shown. Error bars represent SD from 3 replicates. (C) Immunoprecipitation-Western blot of Jagged-1 protein (∼ 180 kDa) in lysate (350 μg protein per sample). (D) Top: Western blot detection of Wnt-1 protein (∼ 41 kDa) in lysate (33 μg protein per lane) from day 0, 1, 3, and 5 differentiated MDDCs. Bottom: Western blot detection of actin loading control.
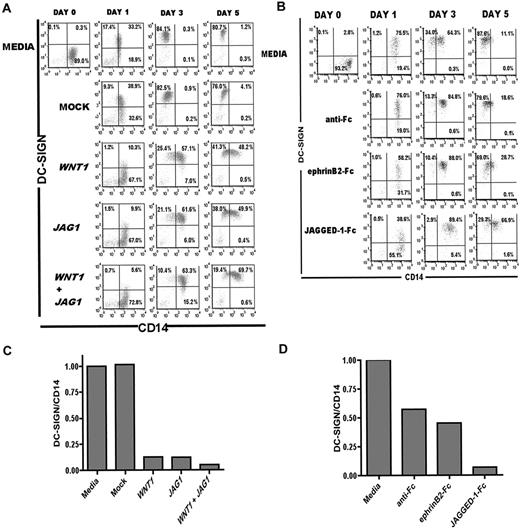
Conditioned media from 293T cells expressing WNT1 and/or JAG1 inhibit complete MDDC differentiation
Finally, to functionally implicate WNT1 and JAG1 as bona fide target genes, we differentiated MDDCs in the presence of conditioned media (CM) from WNT1 and/or JAG1-transfected 293T cells. We reasoned that, if miR-21 and miR-34a-mediated repression of WNT1 and JAG1 activity was required for proper MDDC differentiation, then addition of exogenous Wnt-1 and/or Jagged-1 proteins would antagonize the function of miR-21 and miR-34a. Wnt-1 is a secreted signaling protein and transmembrane Jagged-1 can be shed off as a soluble protein by intramembrane proteases, exerting its function in trans, even when expressed on 293T cells.33 Thus, to test the effects of these proteins on MDDC differentiation, the indicated CM was added and cells were analyzed for DC-SIGN and CD14 cell surface expression at the designated stages of differentiation. Both media control and mock CM-treated monocytes resembled normal differentiating MDDCs with respect to their DC-SIGN and CD14 expression profiles (compare Figure 6A with Figure 1A). However, monocytes differentiated in the presence of WNT1, JAG1, or both WNT1 and JAG1 CM showed limited or blocked differentiation (Figure 6A) illustrated by a decreased gain in DC-SIGN expression on day 1 and the persistence of CD14 expression on days 3 and 5 (Figure 6A). Quantification of DC-SIGN/CD14 expression ratios showed that DC-SIGN/CD14 expression ratios were significantly reduced in the monocytes differentiated in the presence of WNT1, JAG1, or WNT1 and JAG1 CM compared with media or mock-treated controls (Figure 6B). Interestingly, the monocytes differentiated in the presence of both WNT1 and JAG1 CM had the biggest effect on DC-SIGN/CD14 ratios at day 5.
Secreted Wnt-1 and Jagged-1 block complete MDDC differentiation. Flow cytometric analysis showing DC-SIGN and CD14 expression of MDDCs differentiated with IL-4 and GM-CSF plus (A) CM from cells transfected with empty pcDNA3 vector (Mock), WNT1, JAG1, or a combination of WNT1 and JAG1 (WNT1 + JAG1) expression vectors. Top row of dot plots: MDDCs differentiated with IL-4 and GM-CSF plus CM from untransfected cells (Media control). Quadrants were set based on isotype controls for each time point. (B) Flow cytometric data were quantified for comparison of DC-SIGN/CD14 expression ratios at day 3 in differentiating MDDCs. Ratios were calculated using MFI of DC-SIGN/ MFI of CD14 and normalized to media control. (C) Flow cytometry analysis showing DC-SIGN and CD14 expression of MDDCs differentiated with IL-4 and GM-CSF plus secondary anti-Fc antibodies only, preclustered ephrinB2-Fc, or preclustered Jagged-1-Fc. Quadrants were set based on isotype controls for each time point. (D) DC-SIGN/CD14 expression ratios at day 3 were calculated using MFI of DC-SIGN/ MFI of CD14 and normalized to media control.
Secreted Wnt-1 and Jagged-1 block complete MDDC differentiation. Flow cytometric analysis showing DC-SIGN and CD14 expression of MDDCs differentiated with IL-4 and GM-CSF plus (A) CM from cells transfected with empty pcDNA3 vector (Mock), WNT1, JAG1, or a combination of WNT1 and JAG1 (WNT1 + JAG1) expression vectors. Top row of dot plots: MDDCs differentiated with IL-4 and GM-CSF plus CM from untransfected cells (Media control). Quadrants were set based on isotype controls for each time point. (B) Flow cytometric data were quantified for comparison of DC-SIGN/CD14 expression ratios at day 3 in differentiating MDDCs. Ratios were calculated using MFI of DC-SIGN/ MFI of CD14 and normalized to media control. (C) Flow cytometry analysis showing DC-SIGN and CD14 expression of MDDCs differentiated with IL-4 and GM-CSF plus secondary anti-Fc antibodies only, preclustered ephrinB2-Fc, or preclustered Jagged-1-Fc. Quadrants were set based on isotype controls for each time point. (D) DC-SIGN/CD14 expression ratios at day 3 were calculated using MFI of DC-SIGN/ MFI of CD14 and normalized to media control.
To further confirm the specificity of our observations, we evaluated the role of exogenously added purified recombinant Jagged-1-Fc on MDDC differentiation. Previous studies have shown that monomeric and dimeric soluble Notch ligands are inactive in signaling, whereas preclustered soluble ligands are active, suggesting that preclustering more closely resembles the physical state of the ligands.34-36 Thus, recombinant Jagged-1-Fc preclustered with secondary anti-Fc antibodies were added to monocytes differentiated in the presence of GM-CSF and IL-4, and cells were analyzed for DC-SIGN/CD14 expression at indicated time points. Because binding to Fc-receptors can affect MDDC differentiation37 and secondary antibodies alone had a moderate effect on MDDC differentiation (Figure 6C-D), we used an irrelevant chimeric protein, ephrinB2-Fc, with no known roles in MDDC differentiation as a negative control. Preclustered recombinant Jagged-1-Fc, but not ephrinB2-Fc, had similar effects on stalling MDDC differentiation as did JAG1 CM (Figure 6C), indicating that the effect of the CM was probably the result of Jagged-1 itself and not some downstream proteins. Again, quantification of DC-SIGN/CD14 expression ratios shows that DC-SIGN/CD14 ratios were significantly decreased in the MDDCs differentiated in the presence of recombinant Jagged-1-Fc compared with secondary anti-Fc alone or ephrinB2-Fc (Figure 6D).
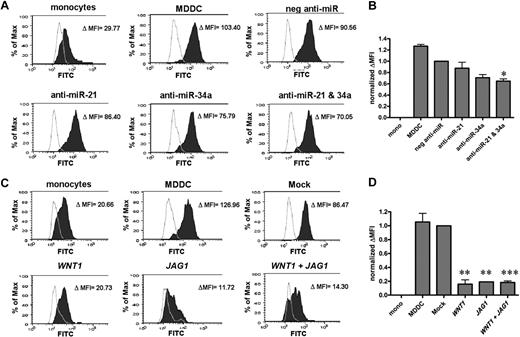
Inhibition of miR-21/miR34a or addition of Wnt-1/Jagged-1 can stall MDDC differentiation in a functional manner
Immature DCs are very efficient at antigen uptake to enable sampling of the environment; thus, increased endocytic capacity is a key functional characteristic of monocyte to DC differentiation. As shown in Figure 7A and B, when both miR-21 and miR-34a were inhibited, MDDCs showed a decreased endocytic uptake of FITC-dextran compared with untransfected MDDCs and negative anti-miR transfected MDDCs. In addition, MDDCs differentiated in the presence of exogenous Wnt-1, Jagged-1, or both had a much more dramatic reduction in FITC-dextran uptake compared with untreated or mock-treated MDDCs. Therefore, the efficacy of anti-MiR inhibitors or exogenous Wnt-1/Jagged-1 in inhibiting functional MDDC differentiation mirrors their ability in stalling phenotypic differentiation.
Inhibition of miR-21 and miR-34a or addition of WNT1 and JAG1 functionally stalls MDDC differentiation. MDDCs were treated with anti–miR-21 and anti–miR-34a (A-B) or secreted products of WNT1 and JAG1 (C-D) during differentiation. Endocytic activity (FITC-dextran uptake) was measured by flow cytometry on day 5 of MDDC differentiation. (A,C) Representative histograms of FITC-dextran uptake at both 37°C (filled histogram) and 4°C (background control, unfilled histogram) are shown. The raw Δ MFI value (37°C MFI to 4°C MFI) for each condition is shown. The relative endocytic activity is quantified in panels B and D, as normalized ΔMFI = ΔMFI (condition) − ΔMFI of monoctyes (background)/ΔMFI of control. The controls for panels B and D are negative anti-miR or mock-treated MDDCs, respectively. Error bars represent SEM for 3 independent experiments. *P < .05; **P < .005; ***P < .0005.
Inhibition of miR-21 and miR-34a or addition of WNT1 and JAG1 functionally stalls MDDC differentiation. MDDCs were treated with anti–miR-21 and anti–miR-34a (A-B) or secreted products of WNT1 and JAG1 (C-D) during differentiation. Endocytic activity (FITC-dextran uptake) was measured by flow cytometry on day 5 of MDDC differentiation. (A,C) Representative histograms of FITC-dextran uptake at both 37°C (filled histogram) and 4°C (background control, unfilled histogram) are shown. The raw Δ MFI value (37°C MFI to 4°C MFI) for each condition is shown. The relative endocytic activity is quantified in panels B and D, as normalized ΔMFI = ΔMFI (condition) − ΔMFI of monoctyes (background)/ΔMFI of control. The controls for panels B and D are negative anti-miR or mock-treated MDDCs, respectively. Error bars represent SEM for 3 independent experiments. *P < .05; **P < .005; ***P < .0005.
Discussion
miRNAs function as an endogenous mode of fine gene regulation and have been implicated in multiple differentiation and developmental processes. However, the specific functional miRNA-protein networks that modulate these many processes have not been established. Here, we characterize the specific miRNA regulation of MDDC differentiation and develop a target ranking system to identify and prioritize relevant miRNAs and target genes for functional validation. Using this system, we identified the roles of several miRNAs (eg, miR-21, miR-34a, let-7e) and their cognate gene targets (WNT1 and JAG1) in regulating MDDC differentiation.
We first show that monocyte to DC differentiation progresses through specific stages exhibiting distinct DC-SIGN/CD14 and miRNA expression profiles (Figure 1). miRNA expression patterns and individual miRNAs have been implicated in a myriad of differentiation processes; however, we wanted to identify the functional miRNA-protein regulatory network that underlies MDDC differentiation. Our work presents a novel ranking system that combines a variety of previously reported components along with innovative parameters to prioritize a list of biologically relevant miRNA-targeted genes for functional validation. We first reduced the target gene list by bioinformatic parsing and then grouped the parsed genes into functional ontologies. Next, to better focus on genes with unique functions regulated by each group of miRNAs, we eliminated overlapping functional ontologies and decreased our final target gene lists 115-fold (> 1100 to 10 genes) and 7-fold (> 2700 to 402), respectively, for the group of increasing versus decreasing miRNAs. Our primary goal was to prioritize a manageable number of target genes for functional verification. Thus, reducing false positives at the expense of increasing false negatives was an acceptable trade-off for our current purpose.
Our target gene ranking system may compliment other methods for functional miRNA studies, and it makes no assumptions regarding the mechanism of miRNA-mediated suppression. Other studies incorporating mRNA profiling into their analysis have successfully predicted target genes38-40 ; however, this method probably misses target genes regulated by translational mechanisms and relies on numerous microarrays. Our system makes no prior assumptions on the method of gene silencing but relies on stringent bioinformatic parsing to identify biologically relevant targets. Of note, the mRNA expression of our validated target genes (WNT1 and JAG1) was highly discordant with the level of protein expression (Figure 5). This suggests that these genes were translationally suppressed and may not have been identified for further analysis had we relied on mRNA microarray profiling in our analysis.
The number of functionally characterized miRNAs and miRNA targets remains limited.7 Our study provides evidence that miR-21, miR-34a, let-7e, miR-99b, miR-125a, and miR-342 were coordinately up-regulated during MDDC differentiation, and we validated 2 genes targeted by 3 of these miRNAs as being involved in MDDC differentiation. Transfection of anti–miR-21 and miR-34a inhibitors together stalled phenotypic and functional MDDC differentiation more than either inhibitor alone (Figures 3,7). More dramatically, exogenous addition of Wnt-1 and Jagged-1 can also block phenotypic and functional MDDC differentiation and overcome endogenous miRNA repression of WNT1 and JAG1-mediated signaling (Figures 6–7). Thus, antagonizing the differential expression of miR-21 and miR-34a by either transfection of miRNA inhibitors or by exogenous addition of Wnt-1 and Jagged-1 results in the parallel effect of stalling MDDC differentiation, suggesting that this regulatory pathway is necessary for MDDC differentiation.
Our study also made the surprising finding that WNT1 and JAG1 mRNA expression was paradoxically increased on MDDC differentiation; thus, miR-21, miR-34a, and let-7e appear to regulate WNT1 and JAG1 at the functional level by controlling the amount of protein expressed (Figure 5). Why produce so much mRNA for proteins that are detrimental to the process at hand? Perhaps transcription factors that promote JAG1 and WNT1 mRNA expression are also required for other genes whose increased expression are themselves required for MDDC differentiation; thus, miRNA posttranscriptional control may be a way to achieve functional balance of JAG1 and WNT1 expression while keeping the expression of other needed genes. This is a testable hypothesis, which may reveal an additional level of genetic regulation that has evolved to meet the complex and competing needs during cellular differentiation.
Many pathways have been implicated in the terminal differentiation of DCs. In the current study, we report the involvement of WNT1 and JAG1 in MDDC differentiation and the probable posttranscriptional regulation of these proteins via specific miRNAs. Wnt proteins compose a group of secreted signaling proteins with pleiotropic effects on hematopoietic cell differentiation,29,41 although WNT1 has not been previously implicated in human MDDC differentiation. Interestingly, in mice, JAG1 on bone marrow stroma prevented terminal DC differentiation,42 consistent with the inhibitory effects of JAG1 on human MDDC differentiation (Figure 6).
Differentiation of MDDCs and other cells of the hematopoietic lineage are generally viewed in terms of regulation by cytokines and transcription factors. Here, we have shown that 6 miRNAs (miR-21, miR-34a, let-7e, miR-99b, miR-125a, and miR-342) are significantly up-regulated during MDDC differentiation. We show that 2 of these, miR-21 and miR-34a, play a role in regulating phenotypic and functional MDDC differentiation via modulating expression of their target genes (WNT1 and JAG1). It is possible that other miRNAs discovered since this study was initiated may also contribute to the regulation of these target genes. It is not unreasonable to speculate that altering the expression of a combination of other coregulated miRNAs and miRNA targets may further lead to altered MDDC differentiation and disrupt immune balance. Our algorithm provides one way to parse target gene lists for future functional miRNA studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank H. Nassanian, H. C. Aguilar, and Y. Wang for technical assistance, Gerry Weinmaster for providing Jagged-1 antibodies, and Paul Boutz and Linda Baum for helpful comments.
S.T.H. was supported by National Science Foundation Integrative Graduate Education and Research Traineeship Award (DGE-9987641). J.A.F. was supported by the National Institutes of Health (training grants GM08042, Medical Scientist Training Program; and AI07126-30, Clinical and Fundamental Immunology). L.G. was supported by the National Institutes of Health (training grant GM55052, Initiative for Maximizing Student Diversity). This work was also supported by the UCLA AIDS Institute Virology Core and the UCLA Flow Cytometry Core (UCLA Center for AIDS Research CA-16042).
National Institutes of Health
Authorship
Contribution: S.T.H. and B.L. designed research; S.T.H., J.A.F., M.H.C., L.G., and S.W. performed research; S.T.H. developed analytic tools; and S.T.H., J.A.F. and B.L. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benhur Lee, Department of Microbiology, Immunology, and Molecular Genetics, University of California–Los Angeles, p257 BSRB, 615 Charles E. Young Dr East, Los Angeles, CA 90095; e-mail: bleebhl@ucla.edu.
References
Author notes
*S.T.H. and J.A.F. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal