Abstract
During surface-initiated blood coagulation in vitro, activated factor XII (fXIIa) converts factor XI (fXI) to fXIa. Whereas fXI deficiency is associated with a hemorrhagic disorder, factor XII deficiency is not, suggesting that fXI can be activated by other mechanisms in vivo. Thrombin activates fXI, and several studies suggest that fXI promotes coagulation independent of fXII. However, a recent study failed to find evidence for fXII-independent activation of fXI in plasma. Using plasma in which fXII is either inhibited or absent, we show that fXI contributes to plasma thrombin generation when coagulation is initiated with low concentrations of tissue factor, factor Xa, or α-thrombin. The results could not be accounted for by fXIa contamination of the plasma systems. Replacing fXI with recombinant fXI that activates factor IX poorly, or fXI that is activated poorly by thrombin, reduced thrombin generation. An antibody that blocks fXIa activation of factor IX reduced thrombin generation; however, an antibody that specifically interferes with fXI activation by fXIIa did not. The results support a model in which fXI is activated by thrombin or another protease generated early in coagulation, with the resulting fXIa contributing to sustained thrombin generation through activation of factor IX.
Introduction
The plasmas of placental and marsupial mammals contain factor XI (fXI),1 the zymogen of a protease (fXIa) that contributes to fibrin formation and stability through activation of factor IX (fIX).2-4 Congenital fXI deficiency is associated with a variable trauma-induced bleeding disorder in humans and other species.5-8 The mechanism by which fXI is converted to fXIa during blood coagulation has been a topic of recent debate.9,10 When blood is exposed to a surface in vitro, the process of contact activation converts factor XII (fXII) to the protease fXIIa, which then activates fXI.3,4 Substances, such as RNA,11 polyphosphates,12 and collagen,13 induce pathologic coagulation in mice in a fXII-dependent manner13,14 and may represent physiologic surfaces for fXII activation. However, the contribution of fXIIa-mediated fXI activation to normal hemostasis is unclear, as fXII deficiency, unlike fXI deficiency, is not associated with abnormal bleeding in any species in which it has been identified.4 This key observation supports hypotheses proposing that fXI is either activated during hemostasis by a protease other than fXIIa or that auxiliary mechanisms for fXI activation compensate in the absence of fXII.3,15-17
Candidates for fXI-activating proteases include α-thrombin,15,16 meizothrombin,18 and fXIa (autoactivation).15,16 Thrombin has received much attention in this regard. Several laboratories have presented evidence suggesting that a protease generated early in coagulation, such as thrombin, converts fXI to fXIa.19-23 This hypothesis has been challenged by a recent study that did not find evidence for fXI activation in thrombin or tissue factor (TF) stimulated plasma in the absence of fXII.9 This work also showed that the process of preparing plasma can generate fXIa, giving the false impression in subsequent assays that fXIIa-independent fXI activation has occurred. These observations have been presented in support of a hypothesis,9,10 also proposed by other investigators,24,25 that normal hemostasis in fXII deficiency reflects loss of fXIIa-initiated processes, such as fibrinolysis, that negate the propensity to bleed from simultaneous loss of fXI activation. Here, we present results of studies on the contribution of fXII-independent fXI activation to thrombin generation in plasma, using systems designed to limit fXIa contamination and its effects. The results show that fXI can be activated by a fXII-independent mechanisms in a plasma environment.
Methods
Reagents
fXII-deficient plasma was from George King Bio-Medical. fXI, fXIa, fXa, α-thrombin, and fXI-deficient plasma were from Hematologic Technologies. Recombi-plastin TF was from Instrument Laboratories. fXIIa and corn-trypsin-inhibitor (CTI) were from Enzyme Research Laboratories. Lepirudin was from Bayer. S-2366 (L-pyro-glutamyl-L-prolyl-L-arginine-p-nitroanilide) was from DiaPharma. Z-Gly-Gly-Arg-AMC was from Bachem. Dioleoylphosphatidylcholine:dioleoylphosphatidylserine (7:3 wt/wt) was from Avanti Polar Lipids. STA PTT Automate 5 reagent was from Diagnostic Stago. Bovine serum albumin (BSA), rabbit brain cephalin, and diisopropylfluorophosphate (DFP) were from Sigma-Aldrich.
Expression and purification of recombinant fXI
Recombinant human fXI was expressed in HEK 293 cells as described.26 cDNAs expressed were for (1) wild-type fXI (fXIWT), (2) fXI with Lys83 and Gln84 replaced with alanine (fXI-Ala83-84), (3) fXI with Ser195, Asn196, and Ile197 replaced with alanine (fXI-Ala195-197), and (4) fXI with Ser557 replaced with alanine (fXI-Ala557). fXI was purified from conditioned media (Cellgro Complete) by chromatography using anti–human fXI-IgG 1G5.12.26 Protein was eluted with 2 M sodium thiocyanate in 50 mM Tris-HCl, pH 7.5, 100 mM NaCl (Tris/NaCl), concentrated by ultrafiltration, dialyzed against Tris/NaCl, and stored at −80°C. fXI (∼ 200 μg/mL) was converted to fXIa by incubation with 2 μg/mL fXIIa at 37°C. fXIa was separated from fXIIa by reapplying it to the 1G5.12 affinity column.
Characterization of recombinant fXI
Serial 1:2 dilutions of fXI (65 μL), starting at 5 μg/mL, in 50 mM Tris-HCl pH 7.4, 100 mM NaCl, 0.1% BSA (TBSA) were mixed with equal volumes of fXI-deficient plasma and STA PTT Automate 5 reagent, and incubated for 5 minutes at 37°C. After incubation, 65 μL of 25 mM CaCl2 was added, and time to clot formation determined on a Dataclot II fibrometer (Helena Laboratories). Results for 5 μg/mL fXIWT was designated 100% activity. The specific activity of fXIWT was similar to plasma fXI (∼ 200 units/mg, with one unit representing the fXI activity in 1 mL of normal plasma). Activities of fXIa in plasma were compared by adding 65 μL of serial dilutions of protease to equal volumes of fXI-deficient plasma and rabbit brain cephalin. After 30 seconds, 65 μL of 25 mM CaCl2 was added and the time to clot formation determined. Activities were determined relative to 5 μg/mL fXIWT (assigned a value of 100%).
fXIWT and fXI83-84 (25 nM) were incubated with 5 nM fXIIa or 15 nM α-thrombin at 37°C. At intervals, 50-μL aliquots were removed and supplemented with 750 μM CTI (for fXIIa) or 150 μM lepirudin (for α-thrombin). Samples were mixed with equal volumes of TBSA containing 1 mM S-2366, and changes in OD 405 nm were followed on a SpectraMAX 340 microtiter plate reader (Molecular Devices). In some reactions, monoclonal antibodies to fXI were included.
Characterization of murine anti-fXI monoclonal antibodies O1A6 and 14E11
Murine IgG O1A6 was raised against human fXI.27 The antibody prolongs the clotting time of human plasma (IC99 ∼ 10 nM) in a partial thromboplastin time (PTT) assay. IgG 14E11 was raised against murine fXI in a fXI-deficient mouse,28 and prolongs the PTT of mouse and human plasma. Recombinant human fXI, prekallikrein (PK), and fXI/PK chimeras have been described.29 Western blots of these recombinant proteins size-fractionated on 10% polyacrylamide–sodium dodecyl sulfate gels were performed using O1A6 or 14E11 as the primary antibody and chemiluminescence for detection. The effect of O1A6 and 14E11 on fXI activation was tested using the chromogenic assay described in “Characterization of recombinant fXI.”
Preparation of human platelets
Blood was drawn from healthy volunteers into a one-tenth volume of acid citrate dextrose anticoagulant, followed by sedimentation at 200g for 20 minutes at room temperature. These experiments were approved by the Vanderbilt University Institutional Review Board, and informed consent was obtained in accordance with the Declaration of Helsinki. Platelet-rich plasma was removed from the pellet. Platelets were pelleted in the presence of 1 U/mL VII grade Apyrase (Sigma-Aldrich) at 800g for 20 minutes, resuspended in Tyrode buffer (15 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 6.5, 125 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 0.4 mM NaH2PO4, 5.6 mM dextrose, 0.35% BSA), and passed over a Sepharose 4B (Sigma-Aldrich) size exclusion column.30 After pelleting at 800g for 20 minutes, platelets were resuspended in Tyrode buffer, pH 7.4, and counted on a Hemavet HV950FS multispecies hematology instrument (Drew Scientific).
Thrombin generation assay
Thrombin generation in plasma was measured by following cleavage of the fluorogenic substrate Z-Gly-Gly-Arg-AMC at 37°C on a Thrombinoscope (Thrombinoscope BV), with thrombin-α2-macroglobulin calibrators supplied by the manufacturer.31,32 Studies were performed in 96-well plates (Immulon 2HB, Thermo Fisher Scientific) coated with PEG 20000. Before use, all plasma and recombinant fXI preparations (0.7-1.9 μM in Tris/NaCl) were treated with a 1000-fold molar excess of DFP for 30 minutes at room temperature, followed by dialysis against Tris/NaCl. To avoid variation resulting from differences between donor plasmas, all experiments were performed on single lots of fXI- or fXII-deficient plasma containing 50 μg/mL CTI and 415 μM Z-Gly-Gly-Arg-AMC. fXI-deficient plasma was supplemented with fXI (30 nM) or vehicle. fXII-deficient plasma was supplemented with O1A6 or 14E11 IgG (300 nM) or vehicle 30 minutes before use. Addition of supplements diluted the plasma by less than 10%.
Supplemented plasma (80 μL) was mixed with 20 μL Tyrode buffer, pH 7.4, containing phosphatidylcholine/phosphatidylserine vesicles (30 μM) or gel-filtered platelets (600000/mm3), and TF (0.96-9.6 pM), α-thrombin (30-300 nM), fXa (36-180 pM), or fXIIa (0.6-6 nM). Final concentrations are 5 μM phosphatidylcholine/phosphatidylserine vesicles, 100000/mm3 platelets, 0.16 to 1.6 pM TF, 5 to 50 nM α-thrombin, 6 to 30 pM fXa, or 0.1 to 1 nM fXIIa. For controls, 80-μL supplemented plasma was mixed with 20 μL calibrator. Reactions were initiated by adding 20 μL of 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, 100 mM CaCl2, 6% BSA, and fluorescence was monitored (excitation λ 390 nM, emission λ 460 nM). Each set of conditions was run at least 2 times in triplicate. Thrombin generation was determined using Thrombinoscope Analysis Software, version 3.0. The area under the thrombin generation curves is referred to as the endogenous thrombin potential (ETP).31
Results
fXI-dependent thrombin generation in fXI-deficient plasma supplemented with plasma fXI
The contribution of fXI to coagulation was assessed by measuring thrombin-mediated cleavage of a fluorogenic substrate, as described by Hemker et al.31,32 Initial studies were performed in fXI-deficient plasma supplemented with CTI, a trypsin inhibitor that selectively binds and inhibits fXII and fXIIa in plasma.33 Different sources of fXI-deficient plasma were tested, including plasma from a patient homozygous for a null mutation in the fXI gene. All plasmas tested gave similar results, and subsequent studies used a single source of plasma. fXI was added immediately before addition of calcium and an initiator of coagulation (TF, fXa, or α-thrombin). In the absence of CTI, thrombin generation was detected in some (but not all) reactions in the absence of an initiator. This is consistent with fXI activation by fXIIa. In addition, some fXI preparations promoted thrombin generation in the absence of an initiator, even with CTI present, consistent with fXIa contamination of fXI. To address this, fXI preparations were treated with DFP, which irreversibly inhibits fXIa. When DFP-treated fXI was added to fXI-deficient plasma with CTI, thrombin generation was not observed after recalcification in the absence of an initiator over a 2-hour period. Based on these results, CTI was included in all reactions, and all preparations of fXI were treated with DFP before use.
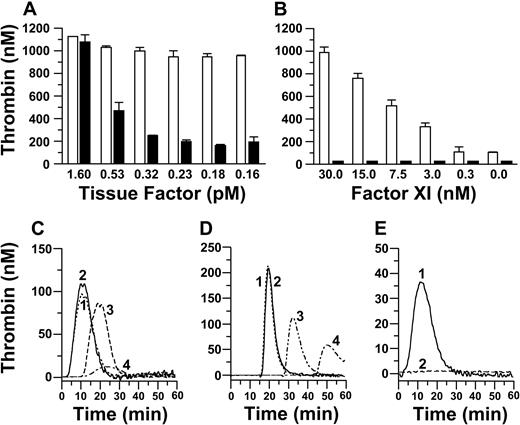
In published studies, thrombin generation did not require fXI when coagulation was initiated by more than or equal to 5 pM TF.18,20,21 In our system, the contribution of fXI to thrombin generation was observed with TF less than 1.6 pM (Figure 1A-B). With 0.23 pM TF, thrombin generation was significantly greater in the presence of fXI than in its absence (Figure 1C, ETP 810 ± 174 and 180 ± 20 nM, respectively), whereas results were similar with 1.6 pM TF (ETP 1160 ± 79 and 1131 ± 78 nM). Similarly, thrombin generation occurred earlier, and was greater, in the presence of fXI when reactions were initiated with 6 pM, but not 30 pM, fXa (Figure 1D). These findings suggest that fXI is activated by a protease generated after addition of TF to plasma. This protease may be thrombin, as 5 nM α-thrombin promoted fXI-dependent thrombin generation (Figure 1E). At this concentration, α-thrombin does not cleave the fluorogenic substrate appreciably, and the observed signal is the result of activation of endogenous prothrombin.
Effect of fXI on thrombin generation in fXI-deficient plasma. Thrombin generation in plasma is shown as areas under the curve for panels A and B or thrombin generation over time for panels C through E. Coagulation was initiated with (A) TF and Ca2+ in the presence (□) or absence (■) of fXI; (B) TF (0.23 pM) and Ca2+ (□) or Ca2+ alone (■) at various fXI concentrations; (C) Ca2+ and 1.6 pM (1 and 2) or 0.23 pM (3 and 4) TF in the presence (1 and 3) or absence (2 and 4) of fXI; (D) Ca2+ and 30 pM (1 and 2) or 6 pM (3 and 4) fXa, in the presence (1 and 3) or absence (2 and 4) of fXI; and (E) 5 nM α-thrombin in the presence (1) or absence (2) of fXI. Error bars for panels A and B represent one SD.
Effect of fXI on thrombin generation in fXI-deficient plasma. Thrombin generation in plasma is shown as areas under the curve for panels A and B or thrombin generation over time for panels C through E. Coagulation was initiated with (A) TF and Ca2+ in the presence (□) or absence (■) of fXI; (B) TF (0.23 pM) and Ca2+ (□) or Ca2+ alone (■) at various fXI concentrations; (C) Ca2+ and 1.6 pM (1 and 2) or 0.23 pM (3 and 4) TF in the presence (1 and 3) or absence (2 and 4) of fXI; (D) Ca2+ and 30 pM (1 and 2) or 6 pM (3 and 4) fXa, in the presence (1 and 3) or absence (2 and 4) of fXI; and (E) 5 nM α-thrombin in the presence (1) or absence (2) of fXI. Error bars for panels A and B represent one SD.
Sensitivity of the thrombin generation assay to fXIa
Experiments were conducted to determine the sensitivity of the thrombin generation assay to fXIa, and to specifically address the possibility that small residual amounts of fXIa in the fXI preparations were influencing the results. In fXI-deficient plasma, robust thrombin generation occurred after addition of fXIa to a final concentration of 30 pM (0.1% of plasma fXI concentration; Figure 2A). Late thrombin generation was detectable in some, but not all, experiments with 3 pM fXIa, and was not observed with 0.3 pM fXIa (Figure 2A). If we accept the premise that fXIa contamination of fXI explains the apparent fXII-independent contribution of fXI reported in the literature, then our fXI preparations would need to be contaminated with sufficient fXIa to produce a final plasma concentration in excess of 3 pM. This seems unlikely, as no preparation of DFP-treated fXI promoted thrombin generation after plasma recalcification over a 2-hour period.
Effect of fXIa on thrombin generation in fXI-deficient plasma. Thrombin generation in plasma supplemented with (A) vehicle or (B) 30 nM fXI. Coagulation was initiated with (1) 300, (2) 30, (3) 3.0, (4) 0.3, or (5) 0.0 pM fXIa. Note that curves 4 and 5 in panel A, and curve 5 in panel B, are essentially flat lines (ie, no thrombin generated).
Effect of fXIa on thrombin generation in fXI-deficient plasma. Thrombin generation in plasma supplemented with (A) vehicle or (B) 30 nM fXI. Coagulation was initiated with (1) 300, (2) 30, (3) 3.0, (4) 0.3, or (5) 0.0 pM fXIa. Note that curves 4 and 5 in panel A, and curve 5 in panel B, are essentially flat lines (ie, no thrombin generated).
Next we tested the sensitivity of the assay to fXIa in the presence of a normal plasma concentration of fXI. In contrast to the results in the absence of fXI, 0.3 pM fXIa reproducibly initiated thrombin generation (Figure 2B, ETP 441 nM), comparable with results with initiation by 5 nM α-thrombin (Figure 1E, ETP 549 nM). Given the results in Figure 2A, there is insufficient fXIa contamination from the fXI preparation in this system to account for the result. That thrombin generation occurs with fXIa as an initiator at a concentration (0.3 pM) more than 10-fold below that needed to trigger thrombin generation (> 3 pM) strongly indicates that fXIa is generated after addition of the initiator. Again, note that no thrombin generation was detected in the absence of the 0.3 pM fXIa initiator, despite the presence of a physiologic fXI concentration in the plasma. The time to peak thrombin generation was longer with 0.3 pM fXIa as an initiator than with TF or α-thrombin (Figures 1C,E, 2B). This is consistent with the fXIa initiator generating a small amount of thrombin through fIX activation, with thrombin subsequently converting fXI to fXIa, promoting a subsequent larger burst of thrombin formation.
Recombinant wild-type fXI and platelets in the thrombin generation assay
Previously, we showed that recombinant wild-type fXI (fXIWT) and plasma fXI have similar activities in a variety of assays,26,29 including a PTT assay (specific activity ∼ 200 units/mg). Peak thrombin generation induced by TF was consistently higher in the presence of fXIWT than plasma fXI (Figures 1C, 3A), and total thrombin formed was moderately greater (ETP 1267 ± 20 vs 810 ± 174 nM, respectively). fXIWT is activated faster than plasma fXI in purified systems (M.-f.S. and D.G., unpublished data, December 2008), perhaps resulting from differences in glycosylation. This may explain the differences in the shapes of the thrombin generation curves. Whereas platelets have been reported to enhance fXII-independent fXI activation,22,23 in our system thrombin generation was similar in the presence of phospholipid or human platelets (Figure 3A,B, ETP 764 and 699 nM, respectively). fXI binds to platelet glycoprotein 1bα (GP1bα).34 An anti-GP1b antibody did not affect thrombin generation (data not shown), again indicating that platelets are not required for fXI activation or for fIX activation by fXIa in this system.
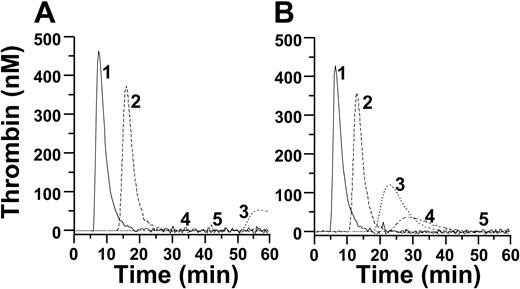
Effect of recombinant fXI on thrombin generation in fXI-deficient plasma. Thrombin generation with (A-B) recombinant (1) fXIWT, (2) vehicle, (3) fXI-Ala557, or (4) fXI-Ala195-197; or (D) (1) fXIWT, (2) vehicle, or (3) fXI-Ala.83-84 Assays contain phospholipid (A,D) or gel-filtered platelets (B). (C) Activation of 25 nM fXIWT (□, ○) or fXI-Ala83-84 (■, ●) by 5 nM fXIIa (□, ■) or 15 nM α-thrombin (○, ●). fXIa generation was followed by cleavage of S2366, as described in “Characterization of recombinant fXI.”
Effect of recombinant fXI on thrombin generation in fXI-deficient plasma. Thrombin generation with (A-B) recombinant (1) fXIWT, (2) vehicle, (3) fXI-Ala557, or (4) fXI-Ala195-197; or (D) (1) fXIWT, (2) vehicle, or (3) fXI-Ala.83-84 Assays contain phospholipid (A,D) or gel-filtered platelets (B). (C) Activation of 25 nM fXIWT (□, ○) or fXI-Ala83-84 (■, ●) by 5 nM fXIIa (□, ■) or 15 nM α-thrombin (○, ●). fXIa generation was followed by cleavage of S2366, as described in “Characterization of recombinant fXI.”
Recombinant fXI variants in the thrombin generation assay
The contribution of fXIa to hemostasis is mediated largely, if not exclusively, through fIX activation. To confirm the importance of fIX activation in our system, fXI-deficient plasma was supplemented with recombinant fXI, followed by addition of TF. Two fXI variants that activate fIX poorly were compared with fXIWT. fXI195-197 is activated normally but has a low affinity for fIX resulting from mutations in a putative fIX-binding site.26 Activated fXI-Ala557 has normal affinity for fIX but lacks catalytic activity because of replacement of the active site serine.35 Both variants have low specific activity (< 5% of fXIWT) in the zymogen (fXI) and activated (fXIa) forms in plasma clotting assays, and both supported thrombin generation poorly (ETP < 100 nM; Figure 3A).
The results so far indicate that thrombin generation in this system requires fXI to be activated in a fXII-independent manner, with fXIa subsequently activating fIX. Previously, an α-thrombin-binding site was identified in the fXI A1 domain.36 Using a saturation mutagenesis approach, we determined that replacing fXI Lys83 and Gln84 with alanine reduces affinity for α-thrombin 100-fold (data not shown). In a PTT assay, fXI-Ala83-84 preparations exhibited 100% to 150% of the specific activity (200-300 units/mg) of fXIWT (200 units/mg), whereas fXIa-Ala83-84 has 70% to 100% of the activity of fXIaWT. These studies show that fXI-Ala83-84 is activated by fXIIa and subsequently activates fIX in plasma. In a purified system, α-thrombin activates fXI-Ala83-84 at approximately 10% of the rate of fXIWT, whereas fXIIa activates fXI-Ala83-84 at approximately 65% of the rate of fXIWT (Figure 3C). In thrombin generation assays initiated with 5 nM α-thrombin, fXI-Ala83-84 supported thrombin generation poorly (Figure 3D), consistent with the premise that thrombin activates fXI in this system.
Thrombin generation in XII-deficient plasma triggered by TF or thrombin
We verified the results of the previous experiments in a second system using fXII-deficient plasma, where endogenous fXI has not been exposed to fXIIa, effectively preventing contact activation-mediated generation of fXIa during plasma preparation. The PTT of this plasma was more than 200 seconds, and fXII cross-reactive material was not detected with a sensitive ELISA. Exogenous fXI is not used in this system, and the anti-fXI IgG O1A6 was used to generate the equivalent of a fXI-deficient state when required.27 Chimeras of fXI and the related protein PK were used to localize the O1A6 binding site to the fXI A3 domain (Figure 4A).29 Subsequent studies (not shown) indicated that O1A6 blocks access to residues required for fIX binding to fXIa.26 This system addresses the argument that supplementation of plasma with exogenous fXI may result in nonphysiologic interactions of fXI with some of its binding partners.9
Anti-fXI monoclonal antibodies. Western blots of fXI and PK using (A) anti–human fXI IgG O1A6 or (B) anti–murine fXI IgG 14E11 as primary antibody. H indicates human fXI; M, murine fXI; A1, A2, A3, and A4, human fXI with PK domains A1, A2, A3, or A4, respectively; and PK, human PK. Positions of molecular mass standards are shown on the left. The uppercase D and M to the right of each panel indicate positions of fXI dimer and monomer (no interchain disulfide bond), respectively. Note that fXI/PKA4 is half the molecular mass of other fXI species because the fXI A4 domain mediates dimer formation. (C) Activation of 25 nM fXI with 5 nM fXIIa (○, ●) or 15 nM α-thrombin (□, ▵) in the presence (●, ▵) or absence (○, □) of 100 nM IgG 14E11.
Anti-fXI monoclonal antibodies. Western blots of fXI and PK using (A) anti–human fXI IgG O1A6 or (B) anti–murine fXI IgG 14E11 as primary antibody. H indicates human fXI; M, murine fXI; A1, A2, A3, and A4, human fXI with PK domains A1, A2, A3, or A4, respectively; and PK, human PK. Positions of molecular mass standards are shown on the left. The uppercase D and M to the right of each panel indicate positions of fXI dimer and monomer (no interchain disulfide bond), respectively. Note that fXI/PKA4 is half the molecular mass of other fXI species because the fXI A4 domain mediates dimer formation. (C) Activation of 25 nM fXI with 5 nM fXIIa (○, ●) or 15 nM α-thrombin (□, ▵) in the presence (●, ▵) or absence (○, □) of 100 nM IgG 14E11.
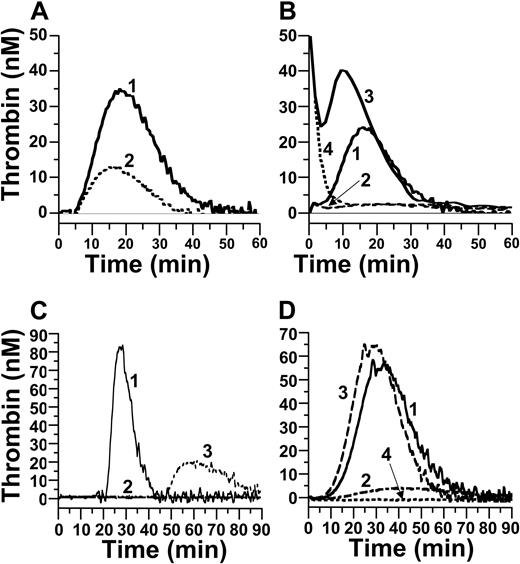
Thrombin generation was observed in fXII-deficient plasma when coagulation was initiated with 0.23 pM TF (Figure 5A) or 5 nM α-thrombin (Figure 5B), and was reduced when O1A6 was included in the reactions. Similar results were obtained by depleting fXII-deficient plasma of fXI by antibody-chromatography (data not shown).37 It is interesting to note that, when a larger amount of α-thrombin (50 nM) was used as an initiator, a fXI-dependent component of thrombin generation was still observed. In Figure 5B, the initial signal abutting the ordinate is the result of cleavage of fluorogenic substrate by the initiator. Note the subsequent peak of thrombin generation and its absence when O1A6 is included in the reaction. This contrasts with results when TF and fXa were used as initiators, where the fXI-dependence of thrombin generation disappeared at higher initiator concentrations (Figure 1C-D) and is consistent with thrombin being a fXI activator in this system, as suggested by an earlier study.37
Thrombin generation in fXII-deficient plasma. Thrombin generation was initiated by addition of Ca2+ and (A) 0.23 pM TF or (B) 5 nM (1 and 2) or 50 nM (3 and 4) α-thrombin. Reactions were run in the absence (1 and 3) or presence (2 and 4) of 50 nM O1A6. (C) Thrombin generation initiated with Ca2+ and 1 nM fXIIa in the presence of (1) vehicle, (2) 50 μg/mL CTI, or (3) 50 nM 14E11. (D) Thrombin generation initiated with Ca2+ and 5 nM α-thrombin (1-3) or no initiator (4). Reactions were run with (1) vehicle, (2) 50 nM O1A6, or (3) 50 nM 14E11.
Thrombin generation in fXII-deficient plasma. Thrombin generation was initiated by addition of Ca2+ and (A) 0.23 pM TF or (B) 5 nM (1 and 2) or 50 nM (3 and 4) α-thrombin. Reactions were run in the absence (1 and 3) or presence (2 and 4) of 50 nM O1A6. (C) Thrombin generation initiated with Ca2+ and 1 nM fXIIa in the presence of (1) vehicle, (2) 50 μg/mL CTI, or (3) 50 nM 14E11. (D) Thrombin generation initiated with Ca2+ and 5 nM α-thrombin (1-3) or no initiator (4). Reactions were run with (1) vehicle, (2) 50 nM O1A6, or (3) 50 nM 14E11.
Finally, we tested the sensitivity of the fXII-deficient system to fXIIa. Interestingly, whereas subpicomolar fXIa stimulated thrombin generation, 0.1 to 1 nM fXIIa was required to generate similar results (Figure 5C). As expected, addition of CTI blocked fXIIa-initiated thrombin generation. Antibody 14E11 binds to the fXI A2 domain (Figure 4B), prolongs the PTT of human plasma, and immunoprecipitates fXI from plasma (data not shown). When fXIa was incubated with 14E11 at room temperature for 30 minutes, its specific activity was not affected in a clotting assay, indicating that 14E11 interferes with fXI activation in plasma, but not subsequent fXIa activity. Consistent with this, in a purified system, 14E11 partially inhibited fXI activation by fXIIa but not activation by α-thrombin (Figure 4C). In the thrombin generation assay, 14E11 significantly reduced fXIIa-initiated thrombin generation (Figure 5C) but did not affect thrombin generation triggered by TF (not shown) or α-thrombin (Figure 5D). This indicates that 14E11 interferes with a specific pathway for fXI activation (through fXIIa) that is not required for fXI-dependent thrombin generation in assays triggered by TF or α-thrombin.
Discussion
Establishing the physiologic mechanism by which fXI is activated during hemostasis has been difficult and remains controversial. During contact activation-initiated clotting of plasma in vitro, such as in the PTT assay, fXII, fXI, and fIX are activated in sequence.4 This series of reactions may also occur in vivo. Studies with fXII-deficient mice indicate that a fXII-initiated process contributes to arterial thrombosis and central nervous system ischemia-reperfusion injury in a manner that is dependent on fXI.13,14 However, when the bleeding diatheses associated with deficiencies of fXII, fXI, and fIX are considered, it is clear that hemostasis in vivo must either be different or more complex. Factor VIIa/TF activates fIX,2,3,38,39 explaining the severe bleeding in fIX deficiency relative to the milder disorder in fXI deficiency.5 Similarly, fXI activation by a protease other than fXIIa would explain the different phenotypes of fXI and fXII deficiency.
A body of work supports a model in which fXI contributes to coagulation in the absence of fXII.18-23 von dem Borne et al showed that clot resistance to fibrinolysis depends on fXI in plasma treated with low concentrations of thrombin or TF.19 As with thrombin generation in the present study, clot degradation was prevented by picomolar fXIa. This study used fXII-deficient plasma depleted of fXI, and fXIa contamination was considered unlikely to account for the antifibrinolytic effect. This group also showed that a stable form of the thrombin precursor meizothrombin promoted fXI-dependent resistance to fibrinolysis, consistent with fXI activation by a product of prothrombin activation.18 Cawthern et al20 and Keularts et al21 showed that fXI enhanced thrombin generation in blood and plasma, respectively, when coagulation was initiated with less than or equal to 5 pM TF.
Recently, using a capture assay for fXIa in complex with the plasma serpin C1-inhibitor (C1-INH), Pedicord et al did not detect fXIa generation when thrombin or TF was added to plasma.9 In this study, plasma was prepared from the blood of healthy normal donors collected into CTI. fXIa was detected only if CTI was omitted from the collection process, and an anti-fXI antibody blocked a general marker of coagulation (chromogenic substrate cleavage) only in plasma prepared without CTI. It was concluded, justifiably, that fXI was activated by fXIIa during plasma preparation, and it was postulated that prior studies did not take adequate steps to account for fXIa contamination, leading to the erroneous conclusion that fXI activation had occurred independent of fXII. We addressed this issue using plasma systems designed to reduce the effects of fXIa contamination on thrombin generation by avoiding exposure of fXI to fXIIa. One system used fXI-deficient plasma containing CTI to which DFP-treated fXI was added, whereas the second used fXII-deficient plasma in which endogenous fXI has never been exposed to fXIIa. Thrombin generation did not occur in either system with recalcification in the absence of an initiator of thrombin generation (TF or fXa), or α-thrombin itself. Whereas this showed that contaminating fXIa, if present, was below a threshold for initiating thrombin generation, it did not completely rule out the possibility that traces of fXIa could synergize with an initiator to enhance thrombin generation.
To address this, we tested the sensitivity of the thrombin generation assay to fXIa, and it is important to emphasize these results. Thrombin generation was weak after addition of 3.0 pM fXIa to fXI-deficient plasma but was substantial with 0.3 pM fXIa in plasma containing fXI. If the latter result was due to contamination of fXI with sufficient fXIa to promote thrombin generation (> 3.0 pM), the 0.3-pM fXIa trigger should not have been required. This was not observed. That a fraction of the fXIa needed to trigger thrombin generation in the absence of fXI, induced thrombin generation in its presence, strongly indicates that fXIa is generated after addition of the fXIa initiator. That this process does not involve fXIIa is demonstrated by the results with fXII-deficient plasma, and by the observation that an antibody that interferes with fXIIa activation of fXI did not affect the contribution of fXI to thrombin generation initiated by TF or α-thrombin. We also tested the hypothesis that a thrombin-initiated feedback loop involving activation of fXI was required for activation of fIX. In thrombin- or TF-treated plasma, fXIa activation of fIX is required, as disrupting or blocking a fIX-binding site on fXIa prevented thrombin generation. Furthermore, fXI-Ala83-84, which is activated slowly by thrombin, supported thrombin generation poorly.
Any consideration of fXI activation in plasma must take into account the substantial literature on the effects of platelets on this reaction. Previous studies suggested that platelets enhance fXI activation by thrombin through a GP1b-dependent mechanism,40 although subsequent work could not confirm this.41 Oliver et al observed that fXI activation was enhanced by platelets,22 and Wielders et al showed that thrombin initiates and propagates thrombin generation in CTI-treated plasma only when fXI and platelets are present.23 These data are at odds with our results, possibly because we used phospholipid vesicles to supplement plasma. In our hands, phospholipids and platelets behave similarly, and platelet dependence in previous studies may have been related to various levels of phospholipids in different systems.
There are several possible explanations for the discrepancies between our results and those of Pedicord et al.9 The amount of fXIa needed to promote thrombin generation appears to be very small and may be below the detection limit of the fXIa-C1-INH capture assay (∼ 5 pM). In addition, fXIa forms complexes with several plasma inhibitors,42 and it is not clear that measuring a single fXIa-inhibitor complex provides a complete picture of fXI activation in all systems. Furthermore, it is not certain that all fXIa is completely inhibited during the course of the experiments, and some portion of the protease would, therefore, not be detectable by the serpin-capture technique. Whereas fXIa is a homodimer with 2 active sites,2-4 fXI activation in solution and in plasma proceeds through an intermediate with one active site (1/2-fXIa) that can activate fIX.43 Given the small amount of fXI that is probably activated in our assays, it is possible that 1/2-fXIa is a major species. The plasma half-life of 1/2-fXIa and its interaction with serpins have not been studied, and this protease may not be detected well by the C1-INH capture assay, which was calibrated with fully activated fXIa.
Finally, when considering fXI activation, it is illustrative to view the process in the context of vertebrate evolution. fXI is the most recent addition to vertebrate plasma coagulation, probably making its appearance in marsupial mammals. In comparison, the vitamin K-dependent coagulation proteases and fXII were present in early land vertebrates (amphibians).1 However, a gene for a protein of unknown function that is clearly ancestral to fXI and its homolog PK (another fXIIa substrate) also first appears in amphibians.1 It is probable that all terrestrial nonmammalian vertebrates have this fXI/PK predecessor. As the fXI/PK predecessor and fXII both made their debuts in early tetrapods,1 fXIIa may well be an activator of the fXI/PK predecessor, retaining the capacity to activate fXI and PK after the relatively recent duplication event responsible for generating the genes for these proteins from the predecessor gene. Given these observations, it is interesting to note that fXII expression has been lost in at least 2 vertebrate lineages. Cetaceans (whales, porpoises, and dolphins) share a common terrestrial ancestor and lack fXII as a result of a point mutation that inactivated the fXII gene.44,45 Despite this, there is no evidence of deterioration of the fXI gene,44 indicating that its product remains under selection pressure because it provides an adaptive function. Similarly, birds lack fXII46-48 but have an intact gene for the fXI/PK predecessor.1 Here, there is convincing evidence that the fXII gene was lost along the lineage leading from reptiles to birds.1 fXI and its predecessor, therefore, persist in the absence of fXII, supporting the conclusion that other proteases can activate these proteins to allow them to fulfill their functions.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Earl Davie (University of Washington, Seattle, WA) for his thoughtful comments on an early version of the manuscript.
This work was supported by National Heart, Lung, and Blood Institute (Bethesda, MD) grants HL58837, HL81326 (D.G.), and HL46213 (P.N.W.).
National Institutes of Health
Authorship
Contribution: D.V.K. and A.M. performed the thrombin generation experiments and analyzed all data; E.I.T. developed and characterized monoclonal antibodies; M.-f.S. generated and characterized the recombinant proteins; P.N.W. analyzed the thrombin activation mutant of fXI and contributed to manuscript writing; A.G. developed and characterized monoclonal antibodies, analyzed data, and assisted in manuscript writing; and D.G. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Gailani, Division of Hematology/Oncology, Vanderbilt University, 777 Preston Research Bldg, 2220 Pierce Ave, Nashville, TN 37232-6307; e-mail: dave.gailani@vanderbilt.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal