Abstract
Dendritic cells (DCs) are key coordinators of the immune response, governing the choice between tolerance and immunity. Despite their importance, the mechanisms controlling the size of the DC compartment are largely unknown. Using a mouse model allowing continuous DC depletion, we show that maintenance of DC numbers in spleen is an active process mediated by Flt3-L–dependent regulation of precursor differentiation into DCs, rather than by changes in proliferation of the differentiated DCs. In particular, the frequency and differentiation potential of intrasplenic DC precursors increased in response to reduced DC numbers. Levels of Flt3-L, a cytokine required for DC differentiation, increased in the blood after DC depletion and returned to normal levels once the DC compartment filled up again. Our data suggest a feedback regulation of DC homeostasis whereby reduction of the DC pool size promotes differentiation of their precursors, via increased Flt3-L availability. This mechanism is different to those known for other immune cell types, such as the B- and T-cell compartments, whereby lymphopenia induces proliferation of already differentiated lymphocytes.
Introduction
Dendritic cells (DCs) are rare immune cells scattered in lymphoid and nonlymphoid organs throughout the body serving as pivotal coordinators of the immune system.1,2 A key feature of DCs is that they set a fine balance between protective and pathologic immune responses. On the one hand, DCs are required to generate immunity against invading pathogens3-7 and T-cell tolerance to self,1,8-10 depending on their activation status. On the other hand, a relatively small increase in DC numbers results in T-cell hyperactivation and autoimmunity.11,12 Thus, it is crucial to maintain an optimal number of DCs whereby both T-cell immunity and tolerance are achieved. Despite the critical function of DCs in regulating innate and adaptive immune response,1-7 very little is known about the homeostatic control of DC numbers.
In addition, lineage commitment and differentiation of DC precursors are only beginning to be understood.13-16 As hematopoietic cells, DCs originate from myeloid precursors (MPs) in bone marrow (BM).17 A common macrophage and DC precursor (MDP) has been identified in BM.18 Specialized precursors with the capacity to differentiate exclusively into DCs have also been identified such as the common DC precursor (CDP)19 and pro-DC20 in BM, and the immediate pre-DC precursor in BM, spleen, and lymph nodes.20-22 Recently, a linear differentiation from MPs to MDPs, CDPs, pre-DCs, and DCs has been suggested.23 Pro-DCs and CDPs in the BM share phenotypical characteristics19,20 and both differentiate into pre-DCs,19,20,23 but whether they represent the same cell population remains to be formally elucidated. In recent years, it has become evident that BM DC precursors enter the circulation and extravasate into secondary lymphoid organs, skin, and lung where they finally differentiate into DCs.18,19,21,22,24-28 In the steady state, spleen and lymph node DCs do not seem to be derived from monocytes but from specialized precursors.21,29
Regulation of cellular homeostasis in other immune compartments is relatively well defined. For example, the size of the lymphocyte compartment is mainly controlled by the proliferation of mature T and B cells in the periphery, where competition for growth factors, such as IL-7, and MHC recognition by T cells dictates the extent of cell division.30-34 In contrast to lymphocytes, homeostasis of neutrophils is partly achieved by the regulated release of neutrophils from the BM via GM-CSF, CXCR4, and SDF-1.35,36 Regarding the DC compartment, Flt3 ligand (Flt3-L) is known to be required for its generation37 by promoting proliferation and differentiation of DC precursors.17,26 However, it is not known whether the maintenance of DC numbers in lymphoid organs during noninfectious states is the result of a passive or active process. The former implies that a steady rate of generation from precursors equals the rate of DC death, resulting in a constant size of the DC compartment (eg, 3 × 106 DCs per mouse spleen). In a regulated process, however, the number of DCs generated from a starting precursor population may vary depending on the physiologic need for generating more DCs or generating them faster.
Here, we demonstrate the existence of regulated homeostasis in the DC compartment and show that there are active mechanisms ensuring optimal numbers of conventional DCs (cDCs) in peripheral lymphoid tissues. Using a transgenic mouse line that allows continuous depletion of CD11c+ DCs by diphtheria toxin (DT), we observed that the development of cDCs from their precursors was increased after DC depletion, indicating a feedback regulation of DC generation by the size of the DC compartment. Our results suggest that this enhanced DC generation resulted from increased differentiation of pro-DCs into pre-cDCs and then cDCs. Flt3-L was required for the increased differentiation of DC precursors in DC-depleted mice. In agreement with this, serum levels of Flt3-L were increased after depletion of DCs, which express Flt3, the receptor for Flt3-L. Our findings are consistent with a mechanism by which Flt3+ DCs consume Flt3-L and thereby regulate its availability for their precursors. In contrast, the rate of cDC division was independent of the size of the DC compartment, indicating that DC proliferation is not modulated to maintain constant numbers. Thus, the homeostatic control of cDC numbers is distinct to those known for other immune cells such as lymphocytes and neutrophils.
Methods
Mice and DC depletion in vivo
C57BL/6N mice (B6; CD45.2) and congenic B6.SJL-Ptprca Pep3b/BoyJ (CD45.1) mice were purchased from Charles River Laboratories and bred in our animal facility. Flt3-L−/−37 mice were purchased from Taconic Farms. Heterozygous RA/EG mice with ubiquitous expression of eGFP have been described.38 Mice were maintained in the facilities of the German Cancer Research Center (DKFZ) in specific pathogen-free conditions. All mice experiments were conducted according to institutional guidelines and regulations (Zentrales Tierlabor, DKFZ) and were approved by the DKFZ.
To deplete DCs in vivo, we used BAC transgenic CD11c.DOG mice that express the human diphtheria toxin receptor (DTR) under the CD11c promoter.39 In this particular mouse strain, the eGFP component of the fusion protein fails to give a fluorescence signal. For systemic DC depletion, mice were injected intraperitoneally with 8 ng/g body weight DT (Sigma-Aldrich) in PBS. Sustained DC depletion was achieved by DT injection daily or every second day.
Generation of mixed BM chimera mice
Mixed BM chimera mice were made by transferring 2 × 106 Thy1.2+-depleted donor BM cells into 10 Gy-irradiated recipient B6 mice. Donor BM consisted of a mixture of cells from CD11c.DOG × CD45.1 F1 mice (DTR+) and eGFP+ mice (DTR−) at a ratio of 80:20. Because the CD11c.DOG mice do not show eGFP fluorescence, the CD45.1 marker together with eGFP enabled us to discriminate cells from CD11c.DOG, eGFP+ mice, and recipient mice by flow cytometry. Experiments were started 8 to 10 weeks after reconstitution.
Enumeration of DCs and their precursors
Heparinized blood was collected, and red blood cells were lysed with ammonium chloride. Spleens and BM were digested in PBS containing 1 mg/mL Collagenase IV (Sigma-Aldrich) and 50 U/mL DNase I (Roche) for 30 minutes at 25°C under constant shaking. Cells were then stained for flow cytometric analysis as detailed below. The percentage of cDCs was obtained by gating on live CD11chi MHC-II+ cells. pDCs were defined as CD11cint PDCA-1+ cells. The percentage of pre-cDCs was obtained by gating on either Lin (Ter119 CD19 CD3 NK1.1)− IL7Rα− CD45RA−/int MHC-II− CD11cint/hi CD172αint,20,21 or Lin (Ter119 CD19 CD3 NK1.1 B220)− MHC-II− CD11cint/hi CD172αint Flt3+.23 The number of DCs or precursors was calculated based on their percentage and the total viable count obtained by trypan blue exclusion.
Flow cytometric analysis and cell sorting
Biotinylated or fluorochrome-labeled antibodies directed against the following antigens were obtained from BD Biosciences, eBioscience, or Invitrogen: CD45.1 (A20), CD45.2 (104), CD11c (HL3), I-Ab (KH74), I-A/I-E (2G9 or M5/114.15.2), CD4 (GK1.5 or RM4-5), CD8α (53-6.7), PDCA-1 (JF05-1C2.4.1), CD19 (ID3), CD3ϵ (145-2C11), NK1.1 (PK136), CD11b (M1/70), IL-7Rα (A7R34), CD172a (Sirp1α; P84), CD45R (B220; RA3-6B2), CD45RA (14.8), CD135 (Flt3; A2F10.1), Gr-1 (RB6-8C5), F4/80 (Cl:A3-1), anti–natural killer (NK) cell (DX5), Sca-1 (Ly6A/E; D7), CD117 (c-kit, 2B8), CD115 (MCSFR; AFS98), and antierythrocyte (Ter119). Phycoerythrin- or APC/Cy7-labeled streptavidin was obtained from BD Biosciences. Propidium iodide was used as a viability dye. For cell-cycle analysis, cells were first fixed using the Foxp3 staining kit (eBiosciences) and then resuspended in PI/RNAse staining buffer (BD Bioscience) according to the manufacturer's protocol. Labeled cells were measured on a FACSCalibur, FACSCanto II, or FACSAria (BD Biosciences) and analyzed using FlowJo 6.4.7 (TreeStar Inc) or Diva 6.0 (BD Biociences) software.
Adoptive transfer of CD11c− spleen cells and quantification of donor-derived DCs
Spleens from CD45 congenic donor mice were digested with collagenase IVas described in “Enumeration of DCs and their precursors.” CD11c+ cells were depleted twice using anti-CD11c microbeads (Miltenyi Biotec) and the AutoMACS deplS program. The percentage of remaining CD11c+ spleen cells was consistently less than 0.03%. In cotransfer experiments, 5 × 106 cells of each donor were adoptively transferred intravenously into 6 Gy–irradiated recipient mice. In all other experiments, a total of 107 cells were transferred intravenously into nonirradiated recipient mice. Donor mice received 2 daily DT doses before adoptive transfer.
To calculate the number of donor-derived cDCs per donor spleen, the number of donor-derived cDCs in the recipient spleen per 107 transferred splenocytes was determined by flow cytometry (CD45.1+ CD11chi MHC-II+). This figure was then adjusted to the number of total splenocytes in the donor mice.
Flt3-L enzyme-linked immunoabsorbent assay
Serum Flt3-L titer was quantified with the use of the Quantikine mouse Flt3-L immunoassay (R&D Systems) following the manufacturer's instructions.
Real-time qPCR
RNA and cDNA from organ samples were prepared as described elsewhere40 after 2 daily doses of DT. Briefly, RNA from sample homogenates was prepared with the RNAeasy Minikit (QIAGEN). RNA was translated into single-stranded cDNA with the Superscript cDNASynthesis kit (Invitrogen) and random hexamers (Amersham Biosciences). Gene expression levels were determined using real-time quantitative polymerase chain reaction (qPCR) TaqMan technology and SYBR green incorporation (Applied Biosystems). The murine Flt3-L primers used were (5′ to 3′): GCGCTGGATAGAGCAACTGAA, CTCCAGAAGCGTTTGCATCTT. PCR was performed on 43 ng cDNA template per 25-μL reaction volume for all samples following instructions provided by the manufacturer. Results are displayed as Flt3-L Ct (cycle threshold) value, indicating the number of PCR cycles required to obtain the half-maximal amount of amplified Flt3-L DNA. High Ct values indicate a low expression level of Flt3-L mRNA, whereas low Ct values indicate high expression levels.
Statistical analysis
Comparisons between 2 samples were performed by the Student t test or by nonparametric signed rank test. Comparisons between multiple samples were carried out by 1-way analysis of variance (ANOVA) or by nonparametric Kruskal-Wallis 1-way ANOVA on ranks, and subsequent pairwise analysis was carried out by the Tukey multiple comparison tests. Statistical significance was set at P value less than .05. Data are expressed as mean values plus or minus SEM.
Results
DC numbers recover quickly after depletion
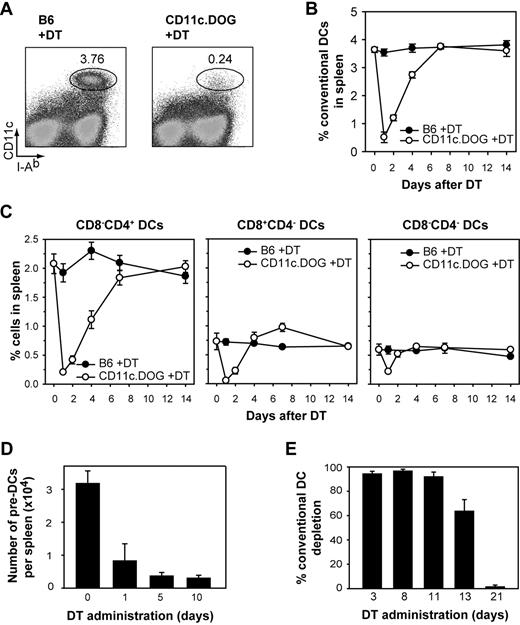
To study homeostatic control in the DC compartment, we made use of the BAC transgenic CD11c.DOG mice in which expression of the human DTR is under the control of the CD11c promoter.39 A single injection of 8 ng DT/g body weight into CD11c.DOG mice resulted in approximately 95% depletion of cDCs (CD11chigh MHC class II+) in the spleen (Figure 1A).39 cDC numbers quickly returned to normal levels approximately 4 to 6 days after DT application (Figure 1B), confirming previous reports of a fast DC turnover.5,41 Different subpopulations of cDCs (CD8−CD4+, CD8+CD4−, and CD8−CD4−) were depleted to a similar extent and returned to normal levels with comparable kinetics (Figure 1C). There exist various described DC precursor populations which are either CD11c− or CD11cint.19-22,26,42 Of these, the immediate DC precursors CD11cint pre-DCs in spleen and BM were partially depleted (∼ 75%), probably because of their lower CD11c expression (Figure 1D; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article; and data not shown).
DC depletion in vivo by DT administration in CD11c.DOG mice. (A) Flow cytometry of splenocytes 24 hours after a single diphtheria toxin (DT) injection. Numbers indicate percentage of CD11chi MHC-II+ conventional DCs (cDCs). (B) Kinetics of cDC depletion and recovery in the spleen after a single DT injection (n = 4 for each time point). (C) Depletion kinetics and recovery of the CD8−CD4+, CD8+CD4−, and CD8−CD4− subpopulations of cDCs in the spleen after a single DT injection (n = 4 for each time point). (D) Number of pre-DCs per spleen after DT treatment. Results are expressed as mean values ± SEM (n = 4). The gating strategy to identify pre-DCs is shown in supplemental Figure 1. (E) Continuous depletion of cDCs in the spleen after daily DT application. Results are expressed as mean value ± SEM (n = 4 for each time point). Shown is 1 representative of 3 for all experiments.
DC depletion in vivo by DT administration in CD11c.DOG mice. (A) Flow cytometry of splenocytes 24 hours after a single diphtheria toxin (DT) injection. Numbers indicate percentage of CD11chi MHC-II+ conventional DCs (cDCs). (B) Kinetics of cDC depletion and recovery in the spleen after a single DT injection (n = 4 for each time point). (C) Depletion kinetics and recovery of the CD8−CD4+, CD8+CD4−, and CD8−CD4− subpopulations of cDCs in the spleen after a single DT injection (n = 4 for each time point). (D) Number of pre-DCs per spleen after DT treatment. Results are expressed as mean values ± SEM (n = 4). The gating strategy to identify pre-DCs is shown in supplemental Figure 1. (E) Continuous depletion of cDCs in the spleen after daily DT application. Results are expressed as mean value ± SEM (n = 4 for each time point). Shown is 1 representative of 3 for all experiments.
DCs could be successfully depleted for a period of 11 to 12 days by daily application of DT in CD11c.DOG mice (Figure 1E) without any apparent sign of toxicity or weight loss.39 Because 11 days is sufficient for renewing the complete DC pool (Figure 1B), it is possible to investigate the homeostatic control of the DC compartment with this mouse strain by continuous administration of DT.
Regulation of cell numbers in the DC compartment is an actively regulated process
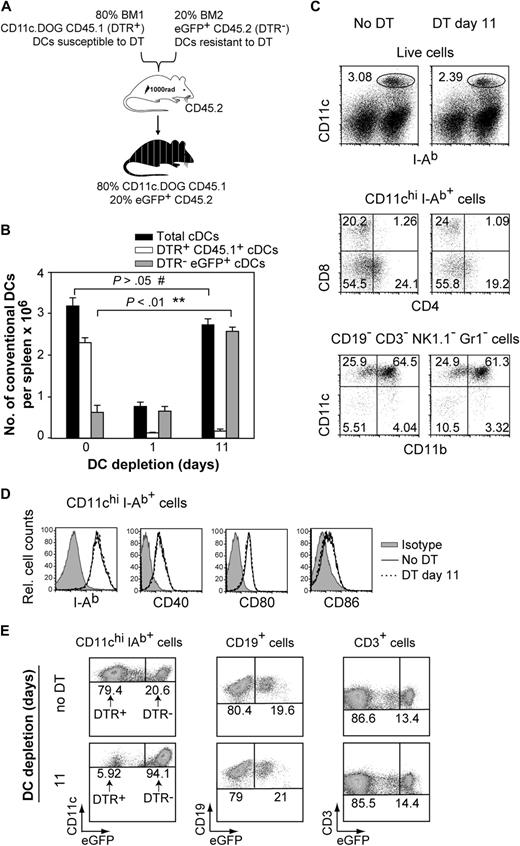
To investigate whether a starting number of precursors generates a constant or a variable number of DCs depending on the size of the CD11c+ cell compartment, we produced mixed BM chimera from DTR+ and DTR− mice that would enable us to deplete a large fraction of DCs and then monitor whether there are compensatory mechanisms leading to the expansion of the remaining DTR− DC pool over time. The mixed BM chimeras were generated using 80% BM from CD11c.DOG CD45.1+ mice (DTR+ CD45.1+) and 20% BM from eGFP+ CD45.2+ mice (DTR− eGFP+; Figure 2A). In these chimeras, most of the DCs could be eliminated in vivo by DT (derived from DTR+ CD45.1+ BM), whereas approximately 20% of DCs derived from DTR− eGFP+ BM were not susceptible to DT. One day after a single DT administration, most DTR+ CD45.1+ cDCs were depleted in the spleen, leading to an approximately 80% decrease in total cDC numbers (Figure 2B, 0 vs 1 day of DC depletion). To investigate whether there was a compensatory response aimed at restoring the size of the DC compartment, we quantified the numbers of DTR− eGFP+ cDCs (not susceptible to DT) after DT administration every second day. Interestingly, the total number of splenic DCs was increased significantly after 11 days of DT application compared with 1 day of depletion, resulting in full repopulation of the cDC compartment (Figure 2B, 0 vs 11 days of DC depletion). This increase in cDC numbers was exclusively due to DTR− eGFP+ DCs (Figure 2B, 0 vs 11 days of DC depletion; supplemental Figure 2), and it was observed for the 3 main cDC populations in the spleen (CD8−CD4+, CD8+CD4−, CD8−CD4−; Figure 2C; supplemental Figure 3). The resulting cDC population obtained after 11 days of DT administration was similar to steady-state cDCs in terms of CD11c, CD11b, CD4, and CD8 expression (Figure 2C), indicating that they do not represent inflammatory DCs (CD11cintCD11bint/hiCD8−)21,43 but steady-state cDCs. Furthermore, the expression level of MHC-II and costimulatory molecules CD40, CD80, and CD86 was not altered in the expanding DC pool (Figure 2D). The percentage or number of other immune cells such as eGFP+ B and eGFP+ T cells was not altered over the period of DT treatment (Figure 2E; supplemental Figure 4), indicating that the increase in DC numbers was specific to this compartment. Because specific expansion of the DTR− eGFP+ DC compartment was only found after DT treatment, we suggest that an active process of DC homeostasis is in place, whereby the output of a minor starting population of DC precursors can be altered and is sufficient to repopulate the DC compartment.
Active homeostatic regulation of DC numbers. (A) Schematic representation of the mixed bone marrow (BM) chimeras used in this study. (B) Numbers of CD11chi MHC-II+ cDC (gated as shown in Figure 2C top) per spleen in mixed BM chimera mice after continuous DT application. Results are expressed as mean value ± SEM; **P < .01; #not statistically significant, analysis of variance (ANOVA). (C) Phenotypic analysis of expanded cDCs in spleen after 11 days of DT application (DT day 11) compared with DCs from untreated mice (No DT). Shown are FACS dot plots showing the percentage of cDCs (C top), cDC subpopulations (C middle), and CD11b expression on DCs (C bottom) from 1 representative mouse spleen. Cells were gated as indicated on top of FACS plots, and numbers indicate percentages in gates shown. (D) Representative histogram overlays of I-Ab, CD40, CD80, CD86 expression by splenic CD11chi MHC-II+ cDCs after no DT treatment (full line) or 11 days of DT administration (open line). (E) Specific increase of DTR− eGFP+ cells in the DC compartment after prolonged depletion of DTR+ DCs. Shown are FACS dot plots from 1 representative mouse spleen. For each cell population, an equal number of total cellular events are shown for groups “no DT” and “11” days of DC depletion. Numbers in the dot plot indicate the percentage within the cDC (CD11chi MHC-II+), B-cell (CD19+) and T-cell (CD3+) populations. All experiments were repeated 3 times with similar results using 4 to 5 mice per group.
Active homeostatic regulation of DC numbers. (A) Schematic representation of the mixed bone marrow (BM) chimeras used in this study. (B) Numbers of CD11chi MHC-II+ cDC (gated as shown in Figure 2C top) per spleen in mixed BM chimera mice after continuous DT application. Results are expressed as mean value ± SEM; **P < .01; #not statistically significant, analysis of variance (ANOVA). (C) Phenotypic analysis of expanded cDCs in spleen after 11 days of DT application (DT day 11) compared with DCs from untreated mice (No DT). Shown are FACS dot plots showing the percentage of cDCs (C top), cDC subpopulations (C middle), and CD11b expression on DCs (C bottom) from 1 representative mouse spleen. Cells were gated as indicated on top of FACS plots, and numbers indicate percentages in gates shown. (D) Representative histogram overlays of I-Ab, CD40, CD80, CD86 expression by splenic CD11chi MHC-II+ cDCs after no DT treatment (full line) or 11 days of DT administration (open line). (E) Specific increase of DTR− eGFP+ cells in the DC compartment after prolonged depletion of DTR+ DCs. Shown are FACS dot plots from 1 representative mouse spleen. For each cell population, an equal number of total cellular events are shown for groups “no DT” and “11” days of DC depletion. Numbers in the dot plot indicate the percentage within the cDC (CD11chi MHC-II+), B-cell (CD19+) and T-cell (CD3+) populations. All experiments were repeated 3 times with similar results using 4 to 5 mice per group.
Proliferation rate of DCs is not related to DC number
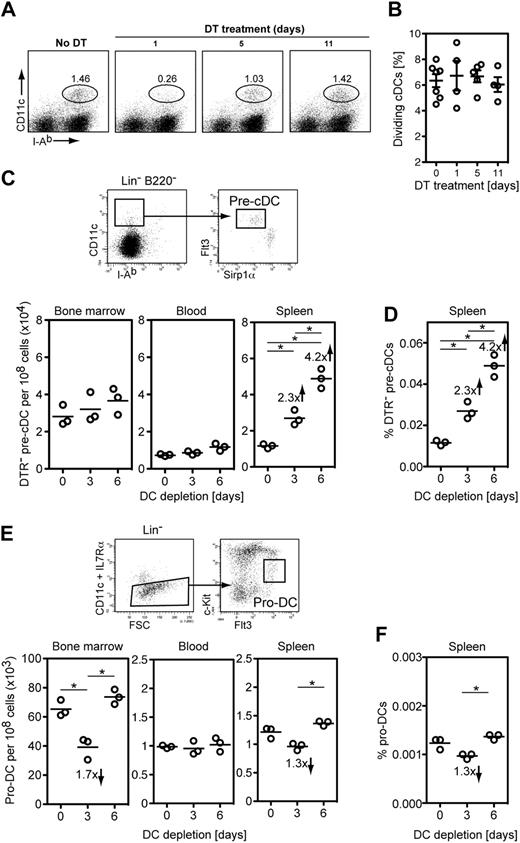
It has been reported that approximately 5% of cDCs are dividing in the steady state.22,25,44 Therefore, we monitored whether the percentage of dividing cDCs in the spleen can be modulated by the size of the DC pool, thus actively contributing to cDC homeostasis. To investigate this, we quantified dividing cDCs in mice with a normal DC pool and in mice in which the DC pool was expanding by assessing DNA content with propidium iodide (supplemental Figure 5). We used mixed BM chimeras as depicted in Figure 2A and compared DTR− eGFP+ cDCs before DT application and at different times after DT application, when the initially small pool of DTR− DCs expanded (Figure 2B and Figure 3A). In agreement with previous reports, there was a low 6.3% of cDCs undergoing proliferation in the steady state, but this figure did not significantly increase during the expansion phase (6.6%, 6.6%, and 5.9% at days 1, 5 and 11 of DT treatment, respectively; P = .48, ANOVA; Figure 3B). These results indicate that modulation of cDC proliferation is unlikely to participate in the expansion of the cDC compartment in situations of reduced cDC numbers. This is in striking contrast with other cells of the immune system, such as T and B cells, which are induced to proliferate in lymphopenic situations.
Immediate DC precursors are responsive to a decrease in number of differentiated DCs and DC precursors. Mixed BM chimera mice depicted in Figure 2A were treated with DT for the indicated period of time. (A) Splenic cDCs (CD11chiMHC-11+ cDCs) were analyzed for DNA content with propidium iodide at various time points after DT application, when the DC compartment was reduced (day 1 of DT treatment) and then expanding (days 5 and 11 of DT treatment). One representative mouse is shown for each time point. (B) Scatter plot summarizing the percentage of dividing (PI+) cDCs at different times points of DT administration from experiment shown in panel A (n = 7 for day 0, n = 4 for day 1, n = 5 for day 5, n = 4 for day 11 of DT treatment). Shown are individual mice, mean ± SEM. No statistically significant differences were observed (P = .406, ANOVA). (C top) Summarized gating strategy to identify pre-cDCs (full gating details on supplemental Figure 6); (bottom) scatter plot summarizing the number of DTR− pre-cDCs in BM, blood, and spleen after DC depletion in mixed BM chimeras as depicted in Figure 2A. (D) Scatter plot showing the frequency of DTR− pre-cDCs in the spleen after DT treatment. Note that DTR− pre-cDCs are only approximately 20% of the total pre-cDC compartment before DT treatment. (E top) Summarized gating strategy to identify pro-DCs (full gating details on supplemental Figure 9); (bottom) scatter plot summarizing the number of pro-DCs in BM, blood, and spleen in CD11c.DOG mice after DC depletion. (F) Scatter plot showing the frequency of pro-cDCs in the spleen of CD11c.DOG mice after DT treatment. (C-F) Representative of 3 experiments showing 3 individual mice (*P < .05, ANOVA).
Immediate DC precursors are responsive to a decrease in number of differentiated DCs and DC precursors. Mixed BM chimera mice depicted in Figure 2A were treated with DT for the indicated period of time. (A) Splenic cDCs (CD11chiMHC-11+ cDCs) were analyzed for DNA content with propidium iodide at various time points after DT application, when the DC compartment was reduced (day 1 of DT treatment) and then expanding (days 5 and 11 of DT treatment). One representative mouse is shown for each time point. (B) Scatter plot summarizing the percentage of dividing (PI+) cDCs at different times points of DT administration from experiment shown in panel A (n = 7 for day 0, n = 4 for day 1, n = 5 for day 5, n = 4 for day 11 of DT treatment). Shown are individual mice, mean ± SEM. No statistically significant differences were observed (P = .406, ANOVA). (C top) Summarized gating strategy to identify pre-cDCs (full gating details on supplemental Figure 6); (bottom) scatter plot summarizing the number of DTR− pre-cDCs in BM, blood, and spleen after DC depletion in mixed BM chimeras as depicted in Figure 2A. (D) Scatter plot showing the frequency of DTR− pre-cDCs in the spleen after DT treatment. Note that DTR− pre-cDCs are only approximately 20% of the total pre-cDC compartment before DT treatment. (E top) Summarized gating strategy to identify pro-DCs (full gating details on supplemental Figure 9); (bottom) scatter plot summarizing the number of pro-DCs in BM, blood, and spleen in CD11c.DOG mice after DC depletion. (F) Scatter plot showing the frequency of pro-cDCs in the spleen of CD11c.DOG mice after DT treatment. (C-F) Representative of 3 experiments showing 3 individual mice (*P < .05, ANOVA).
DC precursor frequency in DC-depleted mice
We next investigated whether the frequency of various DC precursors is modulated in response to DC depletion. It has become evident that DC development is a continuous process involving initial precursors in the BM, migration to secondary lymphoid organs, and terminal differentiation into DCs.19,21,22,25,26,45 First, we monitored the immediate CD11c+ pre-cDC precursors.21 As shown in Figure 1, the large majority of CD11chi DCs as well as a fraction of these CD11cint pre-cDCs are depleted in CD11c.DOG mice after DT treatment. Thus, we made use of mixed BM chimeras (Figure 2A) to investigate whether DC depletion has an effect on the frequency of DTR− pre-cDC precursors. Pre-cDCs were identified in BM, blood, and spleen at similar frequencies as previously reported (Figure 3C; supplemental Figure 6).20,21,23 The number and frequency of DTR− pre-cDCs in spleen continuously increased during the 6 days of DC depletion (Figure 3C-D), when the cDC pool was undergoing full expansion (Figure 3A), whereas BM and blood DTR− pre-cDC numbers were not altered during the same period of time (Figure 3C). Because we observed a specific increase of DTR− pre-cDCs in spleen, these results suggest an increased in situ generation of splenic pre-DCs in response to DC depletion.
We next monitored the frequencies of earlier CD11c− DC precursors after DC depletion. We focused on known DC precursors such as myeloid precursors (MP),17 common macrophage and DC precursors (MDP),18 and the DC-restricted pro-DCs20 and common DC precursors (CDP).19 As recently published,23 we found significant numbers of MPs, MDPs, and CDPs only in BM (supplemental Figure 7), but their numbers were not altered after DC depletion (supplemental Figure 8). Pro-DCs were identified in BM as reported earlier20 and also in blood and spleen (Figure 3E; supplemental Figure 9) albeit at 40 to 60 times lower frequency than in the BM, suggesting that pro-DCs are migratory DC precursors. Further experiments are required to investigate the differentiation potential of these splenic and blood pro-DCs. The pool sizes of pro-DCs in BM and spleen were transiently reduced after 3 days of DC depletion and returned to normal values after 3 more days of DC depletion (Figure 3E-F; supplemental Figure 9). Because pro-DCs differentiate to cDCs via the pre-cDC stage,20 these findings suggest a fast differentiation of BM and spleen pro-DCs leading to increased numbers of splenic pre-DCs.
Besides the analysis of known DC precursors, we also quantified the frequency of Flt3-L–responsive hematopoietic progenitors in spleen of DC-depleted mice using a colony-forming unit (CFU) assay. Spleens of DT-treated CD11c.DOG mice contained approximately 10 times more CFUs than those of DT-treated B6 mice with a normal DC compartment (supplemental Figure 10), indicating that DC depletion induced increased numbers of Flt3-L–responsive progenitor cells. Presently, we do not know the nature of these Flt3-L–responsive CFUs in spleen, but they are not likely to be pre-cDCs because their proliferative capacity is limited and they are not able to form colonies in semisolid media.21
Differentiation of cDCs from splenic precursors is increased after DC depletion
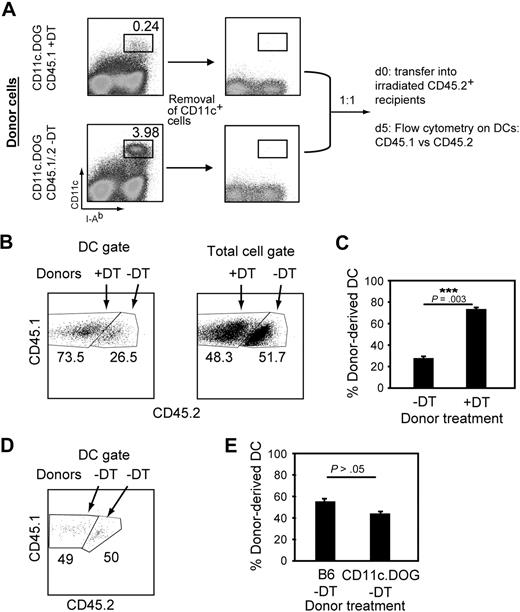
We next investigated whether DT treatment leads to enhanced generation of cDCs from splenic precursors. To address this question, we compared the generation of cDCs from spleens of mice with or without DCs. For this, we performed competitive cotransfers of equal numbers of splenocytes from DT-treated or untreated CD11c.DOG mice and then quantified the number of cDCs derived from each transferred population. Besides the immediate pre-DC precursor, DC lineage commitment in spleen is still ill defined.13-15 Therefore, as a source of DC precursors we used spleens from which only CD11c+ cells had been removed by magnetic sorting (Figure 4A), taking into account that we also removed approximately 50% of the immediate splenic CD11cint pre-DC precursors (supplemental Figure 11A). However, it was necessary to remove CD11c+ cells from the spleen preparation used as DC precursor population to avoid contamination with donor cDCs (supplemental Figure 11B). CD11c+-depleted splenocytes from mice with a normal DC compartment (untreated CD45.1/.2 CD11c.DOG mice) were cotransferred with an equal number of those from DT-treated mice (DT-treated CD45.1 CD11c.DOG mice) into sublethally irradiated CD45.2 mice (Figure 4A). Irradiation of recipient mice is frequently used to study differentiation of hematopoietic precursors to obtain clearly detectable numbers of donor-derived cells, because it is thought to increase the space/resources for transferred precursors. Five days after transfer, precursor cells from donor mice (CD45.1) treated with DT 2 days previously gave rise to almost 3 times more cDCs compared with those derived from mice with normal numbers of DCs (CD45.1/2, 73.5% and 26.5%, respectively; Figure 4B left and C). The increase in cDC cellularity was specific for the DC pool, because the percentage of total cells from each donor was approximately 50% in the recipient mice (Figure 4B right).
DT treatment results in increased differentiation of DC precursors. (A) Competitive cotransfer approach to investigate splenic DC precursor activity in vivo. The DC precursor population was obtained from spleens of mice treated or not with DT for 2 days and MACS-depleted of CD11c+ cells. Five million cells from each donor were then cotransferred into irradiated recipient. Five days later, donor-derived DCs were quantified by flow cytometry based on CD45 congenic markers. (B) DCs predominantly derive from splenic precursors obtained from DC-depleted mice in cotransfer experiments. Representative flow cytometry data showing the relative percentages of cells from DT-treated (CD45.1) or untreated (CD45.1/.2) CD11c.DOG donor mice. (C) Bar diagram summarizing data shown in panel B from 4 individual mice. Results are expressed as mean percentage ± SEM. The experiment was repeated 3 times with comparable results. ***P < .005, Student t test. (D) CD45.1 and CD45.2 splenocytes have an equal capacity to generate DCs. The experiment was performed as in panels A to C except that DT treatment was omitted. (E) Bar diagram summarizing data shown in panel D from 3 individual mice. The experiment was repeated 2 times with comparable results. No statistically significant differences were observed (P = .109, Student t test).
DT treatment results in increased differentiation of DC precursors. (A) Competitive cotransfer approach to investigate splenic DC precursor activity in vivo. The DC precursor population was obtained from spleens of mice treated or not with DT for 2 days and MACS-depleted of CD11c+ cells. Five million cells from each donor were then cotransferred into irradiated recipient. Five days later, donor-derived DCs were quantified by flow cytometry based on CD45 congenic markers. (B) DCs predominantly derive from splenic precursors obtained from DC-depleted mice in cotransfer experiments. Representative flow cytometry data showing the relative percentages of cells from DT-treated (CD45.1) or untreated (CD45.1/.2) CD11c.DOG donor mice. (C) Bar diagram summarizing data shown in panel B from 4 individual mice. Results are expressed as mean percentage ± SEM. The experiment was repeated 3 times with comparable results. ***P < .005, Student t test. (D) CD45.1 and CD45.2 splenocytes have an equal capacity to generate DCs. The experiment was performed as in panels A to C except that DT treatment was omitted. (E) Bar diagram summarizing data shown in panel D from 3 individual mice. The experiment was repeated 2 times with comparable results. No statistically significant differences were observed (P = .109, Student t test).
Differences in the potential of DC generation between congenic strains that would explain the obtained results were excluded because the output of donor-derived cDCs was similar by precursors from congenic mice not treated with DT (Figure 4D-E). In addition, similar results were observed when the opposite combination of CD45 congenic strains was used (supplemental Figure 12A-B). Thus, the number of DCs generated from splenic precursors is not constant, and depletion of CD11c+ cells induced an increased generation of DCs in the spleen. These results indicate that DC precursor differentiation in spleen actively contributes to the regulation of DC homeostasis.
Preexisting precursors in spleen generate higher numbers of DCs in a Flt3-L–dependent manner after transfer into DC-depleted mice
The aforementioned increase in DC numbers may be explained by increased DC generation by the preexisting splenic DC precursor population, and/or enhanced recruitment of DC precursors from the BM into the spleen in response to DT treatment. We thus investigated whether in situ splenic DC precursors increase their activity after transfer into mice with an empty DC compartment. To this end, we transferred splenic DC precursors (within CD11c+ magnetic-activated cell sorting (MACS)–depleted splenocytes) from the spleens of untreated CD45.1+ congenic mice into nonirradiated CD11c.DOG mice treated with DT or B6 mice treated with DT (Figure 5A). Quantification of the number of donor-derived cDCs (CD45.1+ CD11chi MHC II+) at different times after transfer allowed us to investigate whether the output of the preexisting DC precursor population in the spleen can be modulated. When DC precursor cells were transferred into mice containing a full DC compartment, we detected generation of low numbers of donor-derived cDCs in the spleen (Figure 5B; supplemental Figure 13) as previously described.21,22 However, after transfer of the same number of splenic donor cells into DT-treated mice, we observed larger numbers of donor-derived cDCs which, by day 10 after transfer, reached an approximately 10-fold increase compared with those obtained in mice with a full DC compartment (Figure 5B; supplemental Figure 13). This enhanced DC generation is probably due to increased DC differentiation rather than increased DC division because depletion of CD11c+ cells in the mixed BM experiments described earlier resulted in 4 times more splenic DTR− pre-DCs (Figure 3C-D) but not in a higher DC proliferation rate (Figure 3B). In average, the in situ precursors located in one spleen generated approximately 6 × 105 cDCs in 10 days after transfer into DC-deficient mice (Figure 5B), which represents approximately 25% of DCs present in the spleen during steady state. This capacity is remarkable, taking into account that precursor transfer experiments into nonirradiated hosts may be very inefficient because of homing to the spleen and competition for resources with the endogenous precursors. In control studies, specific depletion of another cell population with similar presence in the spleen such as NK cells did not increase the activity of DC precursors in the spleen (supplemental Figure 14), showing that cellular death per se is not responsible.
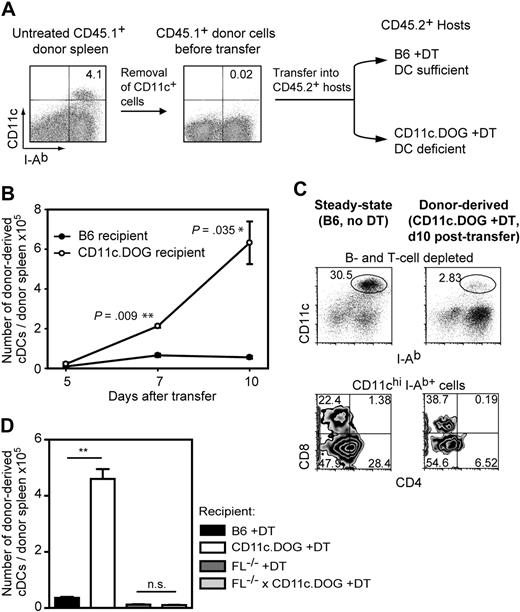
Increased differentiation of preexisting DC precursors in spleen after transfer into DC-depleted mice. (A) Experimental layout. Ten million of CD45.1+ CD11c− splenocytes (CD11c+ MACS-depleted splenocytes) obtained from untreated mice were transferred into CD45.2+ recipient mice with either normal DC numbers (B6 +DT) or reduced (CD11c.DOG +DT). In recipient mice, DT was applied daily from 1 day before precursor cell transfer until the end of the experiment. (B) Intrasplenic DC precursors differentiate into higher numbers of splenic DCs after transfer into DC-deficient environment. The number of donor-derived cDCs per donor spleen was calculated at 5, 7, and 10 days after adoptive transfer by flow cytometry using the CD45 congenic marker. cDC gate was set as in Figure 1A. Results are expressed as mean value ± SEM; *P < .05; ***P < .005, ANOVA. (C) Splenic DC precursors develop into all 3 major cDC populations in the spleen after transfer into DC-deficient environment. Shown are representative FACS diagrams of splenocytes derived from untreated B6 mice (B6, no DT) and donor-derived cells in DC-depleted mice (CD11c.DOG, +DT, day 10 after transfer). B and T cells were depleted before analysis. The experiment was repeated 4 times with comparable results. (D) The enhanced differentiation of splenic DC precursors in mice with reduced DC numbers is Flt3-L dependent. Similar experiment to that in panel B using different sets of recipient mice as indicated and injected with DT for 10 consecutive days. Results are expressed as mean value ± SEM; ***P < .005; #not statistically significant, ANOVA.
Increased differentiation of preexisting DC precursors in spleen after transfer into DC-depleted mice. (A) Experimental layout. Ten million of CD45.1+ CD11c− splenocytes (CD11c+ MACS-depleted splenocytes) obtained from untreated mice were transferred into CD45.2+ recipient mice with either normal DC numbers (B6 +DT) or reduced (CD11c.DOG +DT). In recipient mice, DT was applied daily from 1 day before precursor cell transfer until the end of the experiment. (B) Intrasplenic DC precursors differentiate into higher numbers of splenic DCs after transfer into DC-deficient environment. The number of donor-derived cDCs per donor spleen was calculated at 5, 7, and 10 days after adoptive transfer by flow cytometry using the CD45 congenic marker. cDC gate was set as in Figure 1A. Results are expressed as mean value ± SEM; *P < .05; ***P < .005, ANOVA. (C) Splenic DC precursors develop into all 3 major cDC populations in the spleen after transfer into DC-deficient environment. Shown are representative FACS diagrams of splenocytes derived from untreated B6 mice (B6, no DT) and donor-derived cells in DC-depleted mice (CD11c.DOG, +DT, day 10 after transfer). B and T cells were depleted before analysis. The experiment was repeated 4 times with comparable results. (D) The enhanced differentiation of splenic DC precursors in mice with reduced DC numbers is Flt3-L dependent. Similar experiment to that in panel B using different sets of recipient mice as indicated and injected with DT for 10 consecutive days. Results are expressed as mean value ± SEM; ***P < .005; #not statistically significant, ANOVA.
The expanding cDC pool expressed a similar level of surface CD11c and MHC class II as cDCs in steady-state mice (Figure 5C top), strongly suggesting that the former were not inflammatory CD11cint DCs. Donor-derived cDCs in the spleen consisted of all 3 major subpopulations, with CD8+CD4− and CD8−CD4− DCs being preferentially generated over CD8−CD4+ DCs (Figure 5C bottom). Therefore, our results suggest that the differentiation of the splenic DC precursor population is regulated by the number of existing DCs, as shown by enhanced differentiation of steady-state spleen precursors when transferred into DC-depleted mice compared with normal mice (Figure 5).
We next investigated whether the enhanced differentiation of splenic DC precursors in DC-depleted mice (Figure 5B) depended on Flt3-L. For this, we transferred wild-type (wt) CD11c-depleted spleen cells as DC precursor population into Flt3-L−/−, Flt3-L−/−CD11c.DOG, BL6, or CD11c.DOG mice treated with DT for 10 days. As expected, we observed a 10-fold increase in donor-derived cDCs in DT-treated CD11c.DOG versus B6 recipient mice (Figure 5D; supplemental Figure 15). However, wt DC precursors were able to generate only residual amounts of DCs in Flt3-L−/− and Flt3-L−/−CD11c.DOG mice treated with DT (Figure 5D; supplemental Figure 15), showing that wt DC precursors require Flt3-L to increase their differentiation in response to reduced DC numbers.
Flt3-L serum concentration is elevated after DC depletion
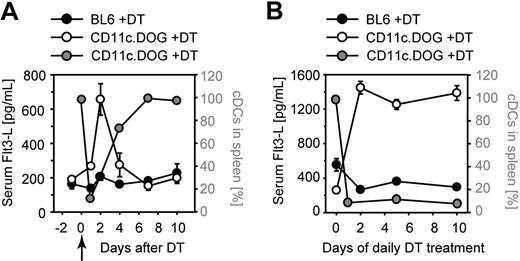
We hypothesized that DC depletion may result in increased Flt3-L levels and, thereby, enhanced generation of cDCs. To investigate this, we monitored serum levels of Flt3-L. After a single depletion, the concentration of serum Flt3-L increased approximately 3-fold, after a kinetics that inversely correlated with cDC depletion and recovery (Figure 6A). The high serum Flt3-L level was maintained but not further increased during continuous DC depletion (Figure 6B). These results suggest that the increased availability of Flt3-L may enhance DC precursor differentiation and thus the number of cDCs generated.
Serum Flt3-L levels are elevated after DC depletion. (A-B) Serum Flt3-L concentration in mice with a normal or depleted DC compartment. Sera of mice was collected at the indicated time points of DT treatment and analyzed for Flt3-L by enzyme-linked immunoabsorbent assay. (A) Single DT injection at day 0 (); (B) daily injections of DT for a period of 10 days. The y-scale on the left indicates the concentration of Flt3-L in serum (black and white circles); the y-scale on the right indicates the percentage of DC in CD11c.DOG mice (grey circles). The number of DCs without DT treatment was set at 100%. Shown is a representative of 3 experiments (n = 4 mice per group).
Serum Flt3-L levels are elevated after DC depletion. (A-B) Serum Flt3-L concentration in mice with a normal or depleted DC compartment. Sera of mice was collected at the indicated time points of DT treatment and analyzed for Flt3-L by enzyme-linked immunoabsorbent assay. (A) Single DT injection at day 0 (); (B) daily injections of DT for a period of 10 days. The y-scale on the left indicates the concentration of Flt3-L in serum (black and white circles); the y-scale on the right indicates the percentage of DC in CD11c.DOG mice (grey circles). The number of DCs without DT treatment was set at 100%. Shown is a representative of 3 experiments (n = 4 mice per group).
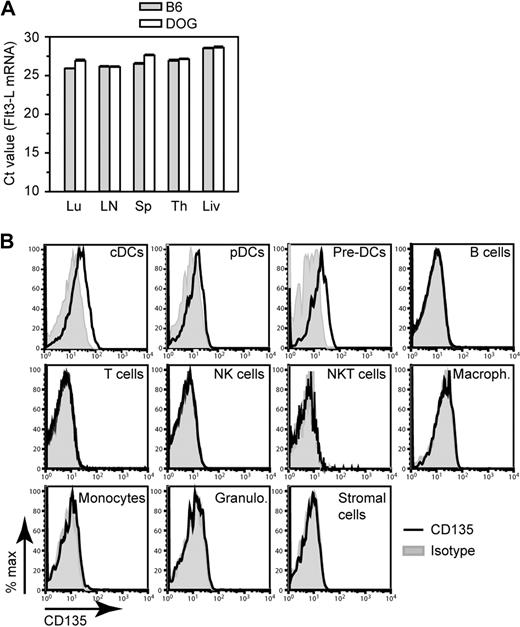
To investigate whether the increased Flt3-L levels were due to transcriptional up-regulation of the Flt3-L gene, we compared the Flt3-L mRNA amount present in different organs of B6 and CD11c.DOG mice after DT treatment by real-time qPCR. Flt3-L mRNA was detected in lung, lymph nodes, spleen, thymus, and liver, but the expression level did not increase after DC depletion as indicated by the number of PCR cycles required to obtain the half-maximal amount of amplified Flt3-L DNA (ie, Ct value; Figure 7A). The high Ct values (≥ 30) obtained with cDNA samples from BM and intestine indicate that Flt3-L mRNA expression is very low in these organs (data not shown). Within splenic cell populations, T cells, B cells, and NK cells expressed the highest Flt3-L mRNA, but again no increase was observed after DC depletion (data not shown). In contrast to other reports of studies that used stromal cell lines or BM stroma,46,47 we failed to observe significant Flt3-L transcription in primary splenic CD45− Ter119− stromal cells (data not shown). These expression studies suggest that the increase in serum Flt3-L levels may not be the result of enhanced Flt3-L transcription.
Flt3-L and Flt3-L receptor expression. (A) Flt3-L mRNA expression by different organs from B6 and CD11c.DOG after 2 daily injections of DT. Results are expressed as mean Ct (cycle threshold) value, that is, the number of PCR cycles required to obtain the half-maximal amount of amplified Flt3-L DNA starting from a fixed amount of total cDNA. High Ct values indicate low expression level of Flt-L mRNA, whereas low Ct values indicate high expression level. Error bars indicate SEMs (n = 6-10 mice). Lu indicates lung; LN, skin-draining lymph nodes; Sp, spleen; Th, thymus; Li, liver. (B) FACS histogram overlays showing expression of CD135 (Flt3, the Flt3-L receptor) by different spleen cell populations. Cell gates were set for cDCs (CD11chiMHC-II+), pDCs (CD11cintPDCA-1+); pre-DCs (CD19−CD3−NK1.1−CD11b−MHC-II−CD45RA−/intCD172aintCD11cint), B cells (CD19+), T cells (CD3+ NK1.1−), NK cells (CD3−NK1.1+), NKT cells(CD3+NK1.1+), macrophages (CD11b+MHC-II−/loF4/80+), monocytes (SSCloCD11b+Gr-1int), granulocytes (SSChiCD11b+Gr-1hi), and stromal cells (CD19−CD3−NK1.1−CD11c−CD11b−MHC-II−Ter119−CD45−). P < .01, ANOVA.
Flt3-L and Flt3-L receptor expression. (A) Flt3-L mRNA expression by different organs from B6 and CD11c.DOG after 2 daily injections of DT. Results are expressed as mean Ct (cycle threshold) value, that is, the number of PCR cycles required to obtain the half-maximal amount of amplified Flt3-L DNA starting from a fixed amount of total cDNA. High Ct values indicate low expression level of Flt-L mRNA, whereas low Ct values indicate high expression level. Error bars indicate SEMs (n = 6-10 mice). Lu indicates lung; LN, skin-draining lymph nodes; Sp, spleen; Th, thymus; Li, liver. (B) FACS histogram overlays showing expression of CD135 (Flt3, the Flt3-L receptor) by different spleen cell populations. Cell gates were set for cDCs (CD11chiMHC-II+), pDCs (CD11cintPDCA-1+); pre-DCs (CD19−CD3−NK1.1−CD11b−MHC-II−CD45RA−/intCD172aintCD11cint), B cells (CD19+), T cells (CD3+ NK1.1−), NK cells (CD3−NK1.1+), NKT cells(CD3+NK1.1+), macrophages (CD11b+MHC-II−/loF4/80+), monocytes (SSCloCD11b+Gr-1int), granulocytes (SSChiCD11b+Gr-1hi), and stromal cells (CD19−CD3−NK1.1−CD11c−CD11b−MHC-II−Ter119−CD45−). P < .01, ANOVA.
A possible explanation for the increased serum Flt3-L titer observed after DT treatment is a reduced consumption because of removal of cells expressing Flt3, the receptor for Flt3-L. Flt3 is expressed by differentiated DCs,17 their immediate precursors,20,26 and more primitive hematopoietic precursors such as common lymphoid and common myeloid precursors.17,48 Consumption of Flt3-L is most likely restricted to these cell populations. In agreement with these reports, our fluorescence-activated cell sorting (FACS) analysis of surface Flt3 expression in the spleen showed that only cDCs, pDCs, and pre-DCs expressed detectable Flt3 (Figure 7B). These results were confirmed by more sensitive reverse transcription qPCR (data not shown). Thus cDCs, which represent 3% of cells in the spleen, are likely to be the most avid consumers of Flt3-L compared with pre-DCs, which express comparable levels of Flt3 (Figure 7B) but represent only approximately 0.03% of splenocytes. Thus, altogether these experiments suggest that the increased Flt3-L concentration observed in the serum after DC depletion may be due to a decrease in Flt3-L–consuming DCs.
Discussion
The results presented here show the existence of active homeostatic mechanisms in the DC compartment which control the normal size of the cDC pool in vivo. Using CD11c.DOG mice in which DT administration results in depletion of cDCs (CD11chi MHC-II+), we report that the rate of cDCs generated from a given precursor population is not a constant event, but it can be modulated by the size of the cDC compartment to ensure normal numbers of cDCs. Thus, control of precursor differentiation via a feedback loop emerges as a key event in the homeostatic control of the cDC compartment.
We cannot exclude that the ability of a residual approximately 5% of cDCs to undergo cell division25,44 plays some role in maintenance of cDC numbers. However, in contrast to the lymphocyte compartment, our results show that cDCs do not homeostatically divide themselves to replenish their numbers after depletion (Figure 3A-B). It is therefore unlikely that proliferation of cDCs plays a crucial role in the control of cDC homeostasis. Instead, we show that the total cDC number is controlled by regulated differentiation of their precursors. This is shown by 2 independent sets of experiments. First, the spleen of mice treated with DT generated more cDCs than that of control mice as shown by competitive cotransfers of DC precursor–containing splenocytes from DT-treated or untreated mice (Figure 4). Second, splenic DC precursors were able to yield variable numbers of cDCs as shown by transfers of unmanipulated precursors into mice containing a full or empty DC compartment (Figure 5).
The frequency of known DC precursors was monitored in BM, blood, and spleen at different times of DC depletion. The size of the MP and CDP compartments was stable after DC ablation, indicating that there was not a net increase in their numbers (supplemental Figure 8). In contrast, the number of splenic pro-DCs and their progeny (pre-cDCs) changed during DC depletion. The number of splenic pro-DCs decreased in the first 3 days of DC depletion, whereas the DTR− pre-cDCs number in spleen increased continuously (Figure 3C-F), suggesting that DC depletion promotes splenic pro-DC differentiation into pre-cDCs and finally DCs. In addition, a fast mobilization of BM pro-DCs may also contribute to the increased frequency of splenic DTR− pre-cDCs in DC-depleted mice as suggested by an initial reduction of pro-DC numbers also in BM (Figure 3E). The extremely short transit time of DC precursors in blood (few minutes)25 may explain why we did not observe a net increased frequency of pro- and pre-cDCs in blood (Figure 3C).
The mechanism regulating the size of the cDC compartment remains to be fully elucidated. Our results point toward an important role for Flt3-L in this process because transfer of wt DC precursors into DC-depleted mice results in a high yield of donor-derived DCs only in the presence of endogenous Flt3-L (Figure 5B,D; supplemental Figure 15). Flt3-L is known to be crucial for DC development37 from DC precursors in BM17 and spleen.26 For example, Flt3-L−/− mice contain strongly reduced DC numbers, whereas administration of supraphysiologic amounts of Flt3-L into wt mice leads to increased DC numbers.49 Interestingly, we observed that the concentration of Flt3-L in the serum was increased after DC ablation (Figure 6). There are 2 potential reasons for the increased serum levels, namely, enhanced production rate and removal of cells that are able to consume Flt3-L. Because no up-regulation in Flt3-L transcription was observed after DC depletion (Figure 7A), we favor the second possibility, namely consumption of Flt3-L. Other mechanisms such as posttranscriptional regulation of Flt3-L production may also play a role and need to be investigated. Differentiated cDCs and DC precursors were found to express Flt3, the receptor for Flt3-L (Figure 7B), which is in agreement with previous data.17,20,26,50 Published reports suggest that Flt3-L binding by DCs and pre-DCs promotes DC survival51 and differentiation of pre-DCs,26 respectively. Because the production rate of Flt3-L mRNA appears not to increase, it is possible that removal of the CD11c+ Flt3+ cells results in increased Flt3-L availability, thereby stimulating a higher rate of cDC generation from precursors. Once sufficient numbers of cDCs are generated, they will consume the Flt3-L, thereby limiting its availability for the precursors. This is supported by the finding that Flt3-L in the serum returned to normal levels concomitantly with the restoration of the DC compartment. Because not only DCs but also pre-DCs express Flt3 and are partially depleted, it cannot be formally excluded that pre-DCs also consume significant amounts of Flt3-L. Because the ratio of cDC to pre-DC in the spleen is approximately 100:1, cDCs are probably the main consumers of Flt3-L. Likewise, because the number of Flt3+ common lymphoid and myeloid precursors is low in comparison to DCs, they probably do not contribute much to Flt3-L consumption.
This potential mechanism is partially reminiscent of the control of T-cell homeostasis, whereby T cells require the growth factor IL-7 for survival and proliferation.52 It is generally accepted that the amount of available IL-7 is regulated via consumption by T cells rather than by regulation of production.52-55 According to this, the size of the T-cell compartment is in equilibrium with the amount of IL-7. Once the number of T cells is increased, they compete for available IL-7 and, thereby, down-regulate their further homeostatic expansion. However, the major and important difference to the mechanism for DC homeostasis proposed here is that DCs consume Flt3-L which is required for their survival but also for their development from precursors.
Maintaining DC homeostasis is of importance during infection. Regulating DC numbers in noninfectious states is also critical. A moderate increase in DC number results in T-cell hyperactivation and autoimmunity.11,12 Interestingly, T cells react to a decrease in DC numbers by becoming unresponsive to subsequent antigenic challenge in the presence of antigen-presenting cells (data not shown), highlighting the physiologic relevance of DC homeostasis in the steady state. In conclusion, our studies identify an active homeostatic control of DC numbers and suggest that DCs control the activity of their precursors through consumption of Flt3-L.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Christine Schmidtt-Mbamunyo, Martin Wühl, and Carmen Heinrich-Kellner for expert assistance in experimental procedures and Dr Steffen Schmitt for cell sorting. We thank Bernd Arnold and Thilo Ölert (DKFZ, Heidelberg) and Thomas Schüler (Charité, Berlin) for helpful discussions and critical reading of the manuscript. We thank Ralf Gilsbach (University of Freiburg) for advice on RT-qPCR.
This work was supported partially by European Union projects NoE-MUGEN (LSHG-CT-2005-005203) and CancerImmunotherapy (LSH-2004-2.2.0-5), the German Ministry of Education and Research (NGFN-2 01GS0452), and DFG SFB 405.
Authorship
Contribution: K.H. and T.M. planned experiments and wrote the manuscript; J.S. planned experiments; S.N. contributed to planning and analysis of experiments in Figure 3; G.J.H. planned experiments and wrote the manuscript; and N.G. planned experiments, directed the study, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Natalio Garbi, Division of Molecular Immunology, German Cancer Research Center (DKFZ), Im Neuenheimer Feld 280, Heidelberg 69120, Germany; e-mail: n.garcia@dkfz.de.
References
Author notes
G.J.H. and N.G. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal