Abstract
It was recently shown that bacterial thymus-independent (TI) antigens confer long-lasting immunity and generate memory B lymphocytes. However, reactivation of TI memory B cells is repressed in immunocompetent mice, thus raising the issue of the mechanism whereby TI vaccines confer immune protection. Here, we propose an explanation to this apparent paradox by showing that a Streptococcus pneumoniae capsular polysaccharide (PS) generates long-lived bone marrow (BM) plasma cells which frequency can be increased by CpG oligodeoxynucleotides (ODNs). The adjuvant effect of CpG ODNs on the PS3 Ab response is directly targeted to B cells and does not involve B-1a cells. We also demonstrated that BM plasma cells generated in response to the thymus-dependent (TD) form of the PS vaccine have a higher secretion capacity than those produced after immunization with the CpG-adjuvanted PS vaccine. Finally, we show that the PS-specific BM plasma cell compartment is sufficient to confer full protection of vaccinated mice against S pneumoniae infection. Altogether, our results show that TI antigens like their TD counterparts can generate both the lymphoid and the plasma cell component of B-cell memory. They also provide a framework for the improvement and widespread usage of TI vaccines.
Introduction
Despite advances in antimicrobial therapy, infections with extracellular polysaccharide (PS)–encapsulated bacteria remain an important clinical problem and a primary source of death worldwide. Pathogenic bacteria including Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae express capsular PSs that behave as prototypic thymus-independent (TI) antigens (Ags). These Ags have initially been defined on the basis of their ability to elicit an antibody (Ab) response in T cell–deficient or athymic mice by a mechanism that requires expression of a functional Bruton tyrosine kinase.1 They are mostly nonpeptidic molecules, which in their great majority, are unable to follow the conventional Ag-processing pathway leading to presentation by MHC class I and II molecules.2 To overcome systemic infections by encapsulated extracellular bacteria such as S pneumoniae, B cells rapidly generate a protective Ab response against capsular PS. B-1 and marginal zone B cells have been envisioned as key players in the humoral response against TI Ags.3 More recently, 2 studies, using Borrelia hermsii or capsular PSs from S pneumoniae as immunogens, have shown that B-1b cells are critically involved in bacterial clearance in vaccinated mice.4,5
It has long been thought that the generation of memory B cells is restricted to thymus-dependent (TD) responses. However, the recent observation that adoptive transfer of B-1b cells from donor mice immunized with whole bacteria can confer long-lasting TI immunity to immunodeficient mice shows that TI Ags generate B-cell memory and that this function can be assigned to the B-1b–cell subset.5 Nonetheless, the concept of TI B-cell memory is at odds with the fact that, unlike TD Ags, TI Ags fail to induce a bona fide amplified recall response on secondary immunization.6-9 This paradox was resolved by the demonstration that Ag-specific IgG generated during the primary response exerts a negative regulatory feedback control on TI memory B cells.10 Therefore, although B-1b memory cells isolated from immunized donors confer protection to immunodeficient recipients, the mechanism whereby they protect immunocompetent mice from bacterial infections is still obscure. It is now established that, in addition to memory B lymphocytes, TD Ags generate a second memory compartment constituted of long-lived BM plasma cells.11,12 It is also currently admitted that accumulation of plasma cells in the BM is strictly a T cell–dependent process that requires germinal center formation.13 In the present study, we demonstrate that TI Ags generate a BM compartment of long-lived plasma cells that is sufficient to confer full protection against infection with live bacteria.
Methods
Mice
C57BL/6J Igha and Ighb mice were purchased from The Jackson Laboratory and from Charles River Laboratories, respectively and maintained in pathogen-free conditions at the Plateau de Biologie Expérimentale de la Souris (Ecole Normale Supérieure de Lyon, France). μMT mice were obtained from the Transgénése et Archivage d'Animaux Models. CD3ϵ−/− mice were obtained from Dr M. Malissen (Centre d'Immunologie Marseille-Luminy). Prof Shizuo Akira (Research Institute for Microbial Diseases, Osaka University, Osaka, Japan) provided TLR9−/− mice. All these mice were on the C57BL/6J background. All studies and procedures were approved by the Comité Régional d'Ethique pour l'Expérimentation Animale.
Immunizations
Mice were immunized subcutaneously with 0.5 μg of the serotype 3 capsular polysaccharide (PS3) from S pneumoniae (Sanofi Pasteur) and with CpG1668 oligodeoxynucleotides (ODNs; 5′-TCCATGACGTTCCTGATGCT-3′) or control CpG1720 (5′-TCCATGAGCTTCCTGATGCT-3′; Eurofins MWG Operon) administrated subcutaneously and used at 80 μg/mouse. For immunization with the tetanus toxin–conjugated form of PS3 from S pneumoniae (PS3TT; Sanofi Pasteur), mice were injected subcutaneously with 0.5 μg of the conjugate vaccine emulsified in alum (Pierce Chemical) followed by a boost immunization in the same conditions 28 days later.
S pneumoniae infections
The encapsulated serotype 3 WU2 strain of S pneumoniae was grown in Todd-Hewitt broth supplemented with 0.5% yeast extract to mid-log phase, then enumerated by plating the suspension on blood agar plates, and finally frozen as glycerol stocks at −80°C. At the time of infection, different doses of bacteria were diluted in 200 μL sterile PBS for intraperitoneal injection. S pneumoniae challenges were performed in a biosafety level 2 animal facility.
ELISAs
Serum samples were incubated with cell wall PS (Statens Serum Institute) to block anti–phosphorylcholine antibodies. They were then incubated in 96-well MaxiSorb plates (Nunc) coated with PS3TT at a concentration of 1 μg/mL or the diphtheria toxin–conjugated form of PS3 from S pneumoniae (Sanofi Pasteur). Alkaline phosphatase–conjugated anti–mouse IgM, (Southern Biotechnology Associates), anti–mouse IgG antibodies (Invitrogen), or biotin-conjugated anti–mouse IgMa or IgMb (BD Biosciences) were then used. Enzyme-linked immunoabsorbent assays (ELISAs) were developed by adding alkaline phosphatase substrate (Sigma-Aldrich). ODs were measured using a microplate reader (Molecular Devices) at 450/490 nm. Endpoint titers were determined using 3-fold serial dilutions and were measured as the reciprocal dilution yielding an OD405/490nm value that was 3-fold higher than that of the negative control OD sera.
ELISPOTs
Splenocytes, BM cells, or peritoneal wash cells (reactivated or not with 1 ng PS3/mL) were distributed at various concentrations into Multiscreen HTS plates (Millipore) precoated with PS3TT or diphtheria toxin–conjugated form of PS3 from S pneumoniae at a concentration of 1 μg/mL in PBS overnight at 4°C. Enzyme-linked immunospots (ELISPOTs) were developed as previously described.14 Ab-secreting cells (ASCs) were enumerated with the KS ELISPOT (Zeiss).
Enrichment or depletion of BM plasma cells
BM cells from immunized mice were stained with a biotin-conjugated anti-CD138 mAb (BD Biosciences). After washings, cells were incubated with anti–biotin MicroBeads (Miltenyi Biotec), and CD138+ cells were positively or negatively selected by loading the suspension on LS or LD columns, respectively.
Generation of chimeras
BM chimeras were constructed, as previously described,15 using lethally irradiated (10 Gy [1000 rad], γ-ray source) C57BL/6J mice as recipients. Recipient mice received 2 different mixtures of 107 BM cells, each containing either 20% TLR9−/− and 80% μMT total BM cells or 20% WT C57BL/6 and 80% μMT total BM cells in sterile PBS by intravenous injections. Allotypic chimeras were constructed, as previously described,16 by reconstituting irradiated C57BL/6 mice with 107 BM cells from WT or TLR9−/− (Ighb) mice injected intravenously. In addition, cell sorting–purified B-1a or B-1b cells (2.105-5.105/mouse) from C57BL/6 (Igha) mice were injected intraperitoneally.
Depletion of naive and memory B lymphocytes
Immunized mice received total body irradiation 45 days after immunization, (7.5 Gy [750 rad,] γ-ray source). Mice were reconstituted with 107 μMT BM cells by intravenous injections. Evaluation of the B-1– and B-2 –cell depletion both in the peritoneal cavity and in the spleen was performed by flow cytometry as previously described.14
Statistical analysis
Data are expressed as mean plus or minus SEM values for individual mice. Statistical significance of differences was determined by the paired 2-tailed Student t test with the GraphPad (GraphPad Software Inc) software Version 4.0. Differences were considered statistically significant for P values less than or equal to .05 (*) or less than or equal to .01 (**).
Results
CpG1668 enhances the Ab response to PS3 in a T cell–independent manner
The longevity of humoral immunity is greatly influenced by the context in which Ag has been presented to the immune system. Plain PS vaccines, such as Pneumovax, can induce relatively short-lived immunity that is limited to 5 to 10 years in adults.17,18 We thus explored whether the use of a TLR9 agonist (CpG ODNs) expected to mimic some of the cosignals provided by whole bacteria could modulate the amplitude and duration of the Ab response to PS3. In a coadministration protocol, CpG ODNs have been reported to amplify the Ab response to PS–protein conjugates19,20 or to NP–Ficoll but not to a plain PS vaccine.21 To reconsider the effect of TLR agonists on TI B-cell responses, we conducted experiments in which CpG1668 was administrated subcutaneously before immunization, at the time of immunization, or after immunization with PS3. The anti-PS3 IgM response was not affected when CpG1668 was injected 1 day before PS3 immunization but was consistently inhibited when PS3 and CpG1668 were coinjected (Figure 1A). In contrast, CpG1668 administration 1, 2, or 3 days after PS3 immunization strongly increased anti-PS3 IgM titers (Figure 1A). Similar results were obtained after intraperitoneal injection of PS3 and CpG1668 (data not shown). The amplitude of the adjuvant effect of CpG1668 on serum PS3 Ab titers steadily increased from day 1 to day 3, but we reproducibly found that its administration at day 2 after immunization gave the best increase in splenic ASCs numbers (Figure 1B-C). Similar results were obtained with several TLR agonists that target different TLRs (data not shown).
T-independent boost of the primary Ab response to the PS3 vaccine by CpG1668. (A) Kinetics of CpG1668 administration. C57BL/6 mice (5 per group) were immunized with PBS or PS3 and injected with CpG1668 at the time of immunization (d0); 1 day before immunization (d−1); 1, 2, or 3 days after immunization (d1, d2, and d3). The anti-PS3 IgM titers were determined by ELISA from sera collected at day 7 after immunization. (B) Effect of delayed administration of CpG1668 on the PS3-specific ASC frequency in the spleen. Mice were injected subcutaneously with PBS or PS3 and injected 2 days later with either CpG1668 or CpG1720, as a negative control. Splenocytes were collected at day 5 after immunization, and PS3-specific ASCs were determined by ELISPOT. (C) Visualization of PS3-specific ASCs in ELISPOT wells from splenocytes of PS3-immunized C57BL/6 mice injected CpG1668 either at day 0 (top) or 2 days later (bottom). (D-E) WT and CD3 KO mice (5 per group) were immunized with PS3 and injected or not with CpG1668: at the time of immunization (d0) or 2 days (d2) after immunization. Spleens from each group were collected 5 days after immunization, and ASC numbers were determined by ELISPOT (D). Sera from each group were collected 5 days after immunization, and titers were determined by ELISA (E). Results are expressed as means ± SEM of the values collected in 5 individual mice.
T-independent boost of the primary Ab response to the PS3 vaccine by CpG1668. (A) Kinetics of CpG1668 administration. C57BL/6 mice (5 per group) were immunized with PBS or PS3 and injected with CpG1668 at the time of immunization (d0); 1 day before immunization (d−1); 1, 2, or 3 days after immunization (d1, d2, and d3). The anti-PS3 IgM titers were determined by ELISA from sera collected at day 7 after immunization. (B) Effect of delayed administration of CpG1668 on the PS3-specific ASC frequency in the spleen. Mice were injected subcutaneously with PBS or PS3 and injected 2 days later with either CpG1668 or CpG1720, as a negative control. Splenocytes were collected at day 5 after immunization, and PS3-specific ASCs were determined by ELISPOT. (C) Visualization of PS3-specific ASCs in ELISPOT wells from splenocytes of PS3-immunized C57BL/6 mice injected CpG1668 either at day 0 (top) or 2 days later (bottom). (D-E) WT and CD3 KO mice (5 per group) were immunized with PS3 and injected or not with CpG1668: at the time of immunization (d0) or 2 days (d2) after immunization. Spleens from each group were collected 5 days after immunization, and ASC numbers were determined by ELISPOT (D). Sera from each group were collected 5 days after immunization, and titers were determined by ELISA (E). Results are expressed as means ± SEM of the values collected in 5 individual mice.
We next explored whether the adjuvant effect of CpG1668 on the PS3 Ab response was T-cell independent using CD3ϵ-deficient mice. The PS3 Ab response was only slightly decreased in CD3ϵ-deficient mice (Figure 1D-E), thus showing that PS3 is indeed a bona fide TI Ag. Furthermore, CpG ODNs promoted a comparable enhancement of the splenic PS3 ASC numbers in WT mice (18-fold) and in T cell–deficient mice (14.5-fold; Figure 1D). Accordingly, the CpG-mediated enhancement of the serum anti-PS3 IgM titers was 8-fold in WT mice versus 7.5-fold in T cell–deficient mice (Figure 1E). Thus, CpG1668 exerted a similar adjuvant effect in both T cell–competent and T cell–deficient mice, suggesting that a switch of the PS3 Ab response from a TI to a TD type cannot explain the adjuvant effect of CpG.
Adjuvant effect of CpG1668 on the PS3 Ab response is targeted to B cells
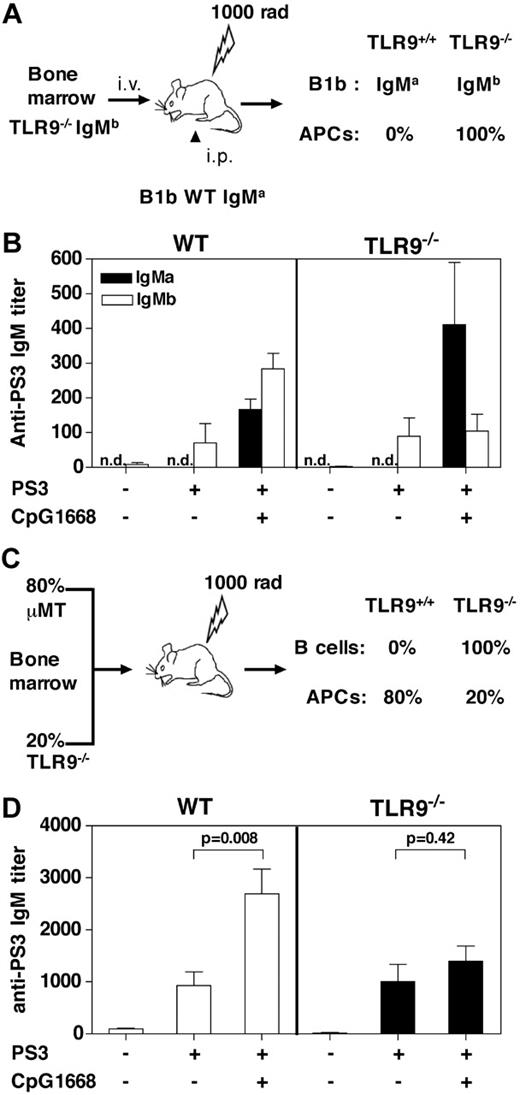
The second signal required for B-cell differentiation in response to TI Ags is provided by antigen-presenting cell (APC)–derived factors, such as BAFF.22 Therefore, B cells and APCs represent the 2 most likely targets of the adjuvant effect of TLR agonists on the PS3 Ab response. First, allotype chimeras were generated to study the role of APCs in the adjuvant effect of CpG1668. To this end, irradiated C57BL/6 recipients were reconstituted with BM from WT or TLR9−/− (Ighb) mice and by B-1b cells from WT (Igha) mice (Figure 2A). Reconstitution of the B-1 compartment in the different groups of mice was similar with approximately 5% of B-1b cells expressing the IgMa allotype (supplemental Figure 1A-B, available on the Blood website; see the Supplemental Materials link at the top of the online article). Splenocytes from TLR9−/− chimeras did not produce IL-12, a cytokine produced by APCs, showing that in these mice, APCs were unable to respond to CpG stimulation (supplemental Figure 1C). The PS3 Ab response generated by WT transferred B-1b cells was monitored by quantification of the IgMa PS3-specific titers. The endogenous PS3 Ab response originating from either WT or TLR9−/− B-1b cells in both chimeras was estimated by quantification of the IgMb PS3-specific titers. Analysis of the PS3-specific IgMb response showed that the PS3 Ab response was not modified in chimeras in which B cells and APCs are TLR9−/−, whereas there was a complete inhibition of the CpG-adjuvant effect (Figure 2B). These results confirmed previous data obtained in TLR9−/− mice (data not shown). However, analysis of the PS3-specific IgMa response clearly showed that, in the presence of TLR9-deficient APCs, CpG1668 was still able to adjuvant the PS3 Ab response, even if in the absence of adjuvant the response was too low to be detected. This was probably the consequence of the low number of B-1b IgMa transferred into recipient mice.
B cells but not accessory cells are the cellular targets of the adjuvant effect of CpG1668. (A) Allotype-chimeric mice with TLR9 gene deficiency restricted to all cells (in particular APCs) were generated by reconstituting irradiated C57BL/6 mice with BM from TLR9−/− (Ighb) mice (B right panel, TLR9−/−) and by intraperitoneal injection of B-1b cells from C57BL/6 (Igha) mice. The control group was reconstituted with WT BM from C57BL/6 (Ighb) mice (B left panel, WT) and by intraperitoneal injection of B-1b cells from C57BL/6 (Igha) mice. (B) Seven weeks after reconstitution, chimeric mice (4 per group) were immunized intraperitoneally with PBS, PS3 alone, or PS3 followed by injection 2 days later with 80 μg CpG1668. The PS3-specific IgM titers of both allotype (IgMa and IgMb) were determined by ELISA from blood samples collected 7 days after immunization. n.d. indicates not detected. (C) BM-chimeric mice with TLR9 gene deficiency restricted to B cells were generated by reconstituting irradiated C57BL/6 mice with 80% μMT BM and 20% TLR9−/− BM (C right panels, TLR9−/−). The control group received 80% μMT BM and 20% wild-type (WT) C57BL/6 BM (C left panel, WT) to generate a TLR9 proficient B-cell compartment. (D) Eight weeks after reconstitution, chimeric mice (6 per group) were immunized subcutaneously with PBS, PS3 alone, or PS3 followed by injection 2 days later with 80 μg CpG1668. The IgM titers were determined by ELISA from blood samples collected 7 days after immunization. Results are expressed as means ± SEM of the values collected in 4 to 6 individual mice.
B cells but not accessory cells are the cellular targets of the adjuvant effect of CpG1668. (A) Allotype-chimeric mice with TLR9 gene deficiency restricted to all cells (in particular APCs) were generated by reconstituting irradiated C57BL/6 mice with BM from TLR9−/− (Ighb) mice (B right panel, TLR9−/−) and by intraperitoneal injection of B-1b cells from C57BL/6 (Igha) mice. The control group was reconstituted with WT BM from C57BL/6 (Ighb) mice (B left panel, WT) and by intraperitoneal injection of B-1b cells from C57BL/6 (Igha) mice. (B) Seven weeks after reconstitution, chimeric mice (4 per group) were immunized intraperitoneally with PBS, PS3 alone, or PS3 followed by injection 2 days later with 80 μg CpG1668. The PS3-specific IgM titers of both allotype (IgMa and IgMb) were determined by ELISA from blood samples collected 7 days after immunization. n.d. indicates not detected. (C) BM-chimeric mice with TLR9 gene deficiency restricted to B cells were generated by reconstituting irradiated C57BL/6 mice with 80% μMT BM and 20% TLR9−/− BM (C right panels, TLR9−/−). The control group received 80% μMT BM and 20% wild-type (WT) C57BL/6 BM (C left panel, WT) to generate a TLR9 proficient B-cell compartment. (D) Eight weeks after reconstitution, chimeric mice (6 per group) were immunized subcutaneously with PBS, PS3 alone, or PS3 followed by injection 2 days later with 80 μg CpG1668. The IgM titers were determined by ELISA from blood samples collected 7 days after immunization. Results are expressed as means ± SEM of the values collected in 4 to 6 individual mice.
Second, BM-chimeric mice were generated in which TLR9 was selectively invalidated in B cells (Figure 2C). Both groups of chimeras responded equally well to PS3 immunization, whereas a control group of mice, reconstituted with BM from μMT mice only, did not respond (Figure 2D; data not shown). However, the adjuvant effect of CpG1668 on the PS3 Ab response was almost completely abolished for chimeric mice in which the B-cell compartment was TLR9−/−. Altogether, these findings indicate that the integrity of the TLR9 pathway in B cells but not in APCs is required for the adjuvant effect of CpG ODNs on the PS3 Ab response.
B-1a cells are not recruited during the Ab response to the CpG-adjuvanted PS3 vaccine
Recent evidence has suggested that only B-1b cells adoptively transferred into immunodeficient recipient mice can generate an adaptive PS3-specific Ab response.4 However, one possible explanation for the adjuvant effect of CpG1668 was the recruitment of another B-cell population, in particular B-1a cells. To answer this question, allotype chimeras were generated by reconstituting irradiated mice with BM from C57BL/6 Ighb mice and by intraperitoneal injection of B-1a cells from C57BL/6 Igha mice. Five weeks after transfer, the B-1 compartment was mainly reconstituted with B-1b cells expressing the IgMb allotype, whereas the B-1a compartment was still poor and heterogeneous with approximately 50% of B-1a cells of both allotypes (Figure 3A). IgM-secreting transferred B-1a cells (Igha) were found in the serum of each group of recipient mice at similar levels (Figure 3B), showing that these transferred B-1a cells not only accumulated in the peritoneal cavity but were also functional. However, PS3-specific IgMa titers were very low after immunization with PS3 alone and did not increase after injection of CpG1668. In contrast, in the same mice, CpG1668 boosted the PS3-specific IgMb response (Figure 3C), suggesting that B-1a cells are not involved in the PS3 Ab response or in the adjuvant effect of CpG.
B-1a cells are not involved in the PS3 Ab response in the presence or absence of CpG1668. For production of allotype chimeras, irradiated C57BL/6 mice were reconstituted with BM from C57BL/6 mice Ighb and with B-1a cells from C57BL/6 mice Igha. Five weeks after reconstitution, chimeric mice (3 per group) were immunized intraperitoneally with PBS, PS3 alone, or PS3 followed by injection 2 days later with 80 μg CpG1668. Seven days after immunization, blood samples were collected, and peritoneal washings were harvested and analyzed by fluorescence-activated cell sorting staining. (A) Percentage of B-1a and B-1b cells expressing IgMa or IgMb in a mice immunized with PBS alone. R1 corresponds to the total B-1 population in the peritoneal washings. Percentages of each quadrant are indicated. (B) The total IgM titers of both allotype (IgMa and IgMb) were determined by ELISA. (C) The PS3-specific IgM titers of both allotype (IgMa and IgMb) were determined by ELISA. Results are expressed as means ± SEM of the values collected in 3 individual mice.
B-1a cells are not involved in the PS3 Ab response in the presence or absence of CpG1668. For production of allotype chimeras, irradiated C57BL/6 mice were reconstituted with BM from C57BL/6 mice Ighb and with B-1a cells from C57BL/6 mice Igha. Five weeks after reconstitution, chimeric mice (3 per group) were immunized intraperitoneally with PBS, PS3 alone, or PS3 followed by injection 2 days later with 80 μg CpG1668. Seven days after immunization, blood samples were collected, and peritoneal washings were harvested and analyzed by fluorescence-activated cell sorting staining. (A) Percentage of B-1a and B-1b cells expressing IgMa or IgMb in a mice immunized with PBS alone. R1 corresponds to the total B-1 population in the peritoneal washings. Percentages of each quadrant are indicated. (B) The total IgM titers of both allotype (IgMa and IgMb) were determined by ELISA. (C) The PS3-specific IgM titers of both allotype (IgMa and IgMb) were determined by ELISA. Results are expressed as means ± SEM of the values collected in 3 individual mice.
Plain and adjuvanted PS3 vaccines generate a persistent pool of PS3-specific Abs and BM plasma cells
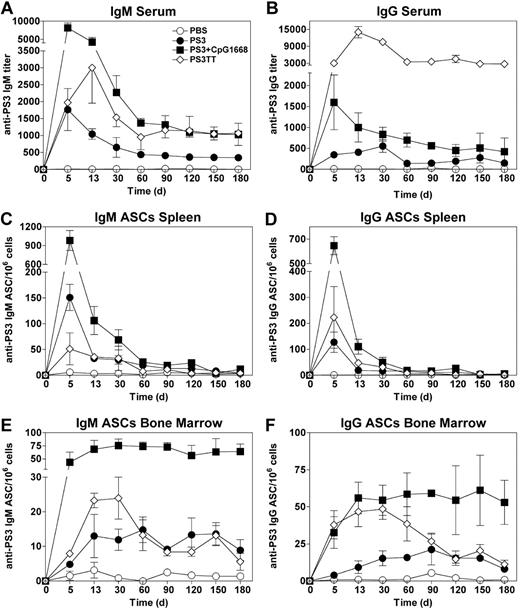
We next studied the effect of CpG1668 on the maintenance of high PS3 Ab titers in the serum. For this purpose, mice either received a single injection of PS3 followed or not by CpG1668 injection 2 days later or were immunized with the conjugate vaccine PS3TT. This vaccine was chosen as control because it induces long-term protection not only in adults but also in children and in the elderly for whom the plain PS vaccine is inefficient.23 Mice immunized with the PS3 vaccine elicited an IgM production that peaked at day 5, then decreased until day 60, and finally stabilized at an IgM titer slightly greater than 500 from day 60 onward (Figure 4A). The time peak of IgM production and the kinetics of serum IgM decay were comparable in mice immunized with the CpG-adjuvanted PS3 vaccine except that the IgM titers at the plateau of the response were approximately 3- to 4-fold higher than in mice immunized with the PS3 vaccine. Serum anti-PS3 IgG followed the same decrease kinetics as did IgM except for mice immunized with PS3TT in which serum IgG titers were maintained greater than 2700, up until 180 days after immunization, a titer approximately 7 times higher than that obtained after immunization with the CpG1668-adjuvanted PS3 vaccine (Figure 4B).
The plain and CpG-adjuvanted PS3 vaccines generate a persistent pool of BM plasma cells. Ten-week-old mice received a single injection of PS3 followed or not by CpG1668 injection 2 days later or were immunized twice with PS3TT with a 28-day interval. (A-B) Sera from 5 mice in each group were collected at different time points after primary immunization (PS3 and CpG-adjuvanted PS3) or secondary immunization (PS3TT), and IgM (A) and IgG (B) titers were determined by ELISA. (C-D) Numbers of PS3-specific IgM (C) and IgG (D) ASCs in the spleen. Spleens from 5 mice in each group were collected at the indicated time points after primary immunization (PS3 and CpG-adjuvanted PS3) or secondary immunization (PS3TT), and ASC numbers were determined by ELISPOT. (E-F) Numbers of PS3-specific IgM (E) and IgG (F) ASCs in the BM. ASC numbers were determined as in panels C and D. Results are expressed as means ± SEM of the values collected in 5 individual mice.
The plain and CpG-adjuvanted PS3 vaccines generate a persistent pool of BM plasma cells. Ten-week-old mice received a single injection of PS3 followed or not by CpG1668 injection 2 days later or were immunized twice with PS3TT with a 28-day interval. (A-B) Sera from 5 mice in each group were collected at different time points after primary immunization (PS3 and CpG-adjuvanted PS3) or secondary immunization (PS3TT), and IgM (A) and IgG (B) titers were determined by ELISA. (C-D) Numbers of PS3-specific IgM (C) and IgG (D) ASCs in the spleen. Spleens from 5 mice in each group were collected at the indicated time points after primary immunization (PS3 and CpG-adjuvanted PS3) or secondary immunization (PS3TT), and ASC numbers were determined by ELISPOT. (E-F) Numbers of PS3-specific IgM (E) and IgG (F) ASCs in the BM. ASC numbers were determined as in panels C and D. Results are expressed as means ± SEM of the values collected in 5 individual mice.
Because the PS3 vaccine adjuvanted with CpG1668 allowed the maintenance of enhanced levels of anti-PS3 IgM and IgG, we next investigated whether this sustained Ab production was associated with the persistence of plasma cells in the spleen or in the BM. The highest frequency of splenic PS3-specific ASCs was found 5 days after immunization and from day 60 onward, the IgM and IgG ASC numbers were reduced to 90% to 97% of those generated at the peak of the response, whatever the condition of immunization (Figure 4C-D). This shows that the sustained serum PS3 Ab titers obtained with the plain and adjuvanted PS3 vaccines are not correlated with a persistent pool of splenic plasma cells. Surprisingly, in the BM, PS3-specific plasma cells start to be detected as soon as day 5, and their numbers increase until day 30 (Figure 4E-F). We estimated that approximately 5% to 10% of the total plasma cells generated in the spleen at day 5 have relocated in the BM 30 days later. Furthermore, CpG1668 enhanced approximately 5-fold the numbers of IgG and IgM PS3-specific BM plasma cells generated in response to the PS3 vaccine (Figure 4E-F).
At early time points, the numbers of BM PS3-specific ASCs induced by PS3TT were lower than (IgM ASCs) or comparable to (IgG ASCs) those generated in response to the CpG-adjuvanted PS3 vaccine. Unexpectedly, from day 30 to day 60, these numbers slowly declined to reach a plateau that persisted up to day 180 after immunization. By contrast, BM plasma cells generated in response to the TI form of the PS3 vaccine (adjuvanted or not by CpG1668) only slightly decreased until day 180 after immunization (Figure 4E-F). Again, PS3-specific BM plasma cells generated after immunization with the plain or the CpG-adjuvanted vaccines are produced by and persist in a TI fashion (supplemental Figure 2).
BM plasma cells generated in response to PS3TT have a higher secretion capacity than those produced after immunization with the CpG-adjuvanted PS3 vaccine
The number of PS3-specific ASCs detected in the BM after immunization with CpG-adjuvanted PS3 vaccine exceeded that observed after PS3TT immunization, whereas, in the same time, the serum titer was similar for IgM and 10 to 15 times higher for IgG in response to PS3TT. To understand this contradiction, the secretion capacity of BM plasma cells generated by this TD or TI immunization was studied. Because the frequency of PS3-specific BM plasma cells was very low, CD138+-enriched BM cells were seeded both in ELISPOT plates (to assess the number of PS3-specific IgM and IgG ASCs) and in cell culture plates (to assess the level of PS3-specific IgM and IgG secretion). The secretion capacity of BM plasma cells produced in response to PS3TT was 2-fold higher for IgM and 38-fold higher for IgG than that generated in response to the CpG-adjuvanted PS3 vaccine (Figure 5A). This is also shown by the bigger size of the IgM and IgG spots obtained after immunization with PS3TT compared with PS3 and CpG (Figure 5B). These results suggest that TD memory plasma cells are functionally different from their TI counterparts.
Secretion capacity of BM plasma cells generated in response to the CpG-adjuvanted PS3- and PS3TT-conjugated vaccine. Seven- to 10-week-old mice (10 per group) were immunized with PS3 and injected 2 days later with CpG1668 or twice with PS3TT at a 21-day interval. Two weeks after immunization, CD138+ BM cells were enriched by positive selection and seeded both in ELISPOT plates for 24 hours (to assess the number of PS3-specific IgM and IgG ASCs) and in cell culture plates for 5 days (to assess the level of PS3-specific IgM and IgG secretions). (A) Secretion capacity of BM plasma cells expressed as the mean of the titer/ASCs of triplicate determinations for both type of immunizations. One of 2 representative experiments is shown. (B) Photos of ELISPOT wells showing the difference of spot size generated after immunization with CpG-adjuvanted PS3 or PS3TT.
Secretion capacity of BM plasma cells generated in response to the CpG-adjuvanted PS3- and PS3TT-conjugated vaccine. Seven- to 10-week-old mice (10 per group) were immunized with PS3 and injected 2 days later with CpG1668 or twice with PS3TT at a 21-day interval. Two weeks after immunization, CD138+ BM cells were enriched by positive selection and seeded both in ELISPOT plates for 24 hours (to assess the number of PS3-specific IgM and IgG ASCs) and in cell culture plates for 5 days (to assess the level of PS3-specific IgM and IgG secretions). (A) Secretion capacity of BM plasma cells expressed as the mean of the titer/ASCs of triplicate determinations for both type of immunizations. One of 2 representative experiments is shown. (B) Photos of ELISPOT wells showing the difference of spot size generated after immunization with CpG-adjuvanted PS3 or PS3TT.
BM plasma cells generated by the CpG-adjuvanted PS3 vaccine are long lived
The existence of a persistent pool of BM plasma cells provides a plausible explanation for the maintenance of high titers of serum PS3 Abs up to 6 months after PS3 vaccination. But how are BM plasma cells numbers maintained? Two scenarios can be envisaged: BM plasma cells generated in response to PS3 are long lived or their pool is replenished by stimulation of PS3-specific B lymphocytes at the periphery.24 To address this question, mice immunized with the CpG-adjuvanted PS3 vaccine were exposed to mild whole-body irradiation, known to deplete naive and memory B lymphocytes12,25,26 while sparing plasma cells (PCs) in the BM.27 Irradiation depleted more than 93% and 97% of peritoneal B-1a and B-1b cells, respectively, and more than 98% B-1 and B-2 cells in the spleen whatever the time point considered (Figure 6A-B). The effect of irradiation on PS3-specific B cells was also studied by quantifying the number of plasma cells generated by in vitro stimulation of peritoneal cells with PS3. This frequency reached 2.63 × 10−4 in control nonirradiated mice, whereas it dropped down to 3 × 10−6 for irradiated mice 180 days after irradiation (Figure 6C). On average, irradiation caused a 100-fold reduction of the numbers of PS3-specific B cells in the peritoneal cavity. Having shown that mild irradiation profoundly depletes PS3-specific B cells, we analyzed how ASCs generated by immunization with the CpG1668-adjuvanted PS3 vaccine persist in the absence of PS3-specific B cells. Except the difference of frequency observed for IgM-producing BM plasma cells at day 180 and for IgG-producing BM plasma cells at day 90 between control and irradiated mice, the numbers of ASCs at the other time points were not significantly different (Figure 6D-E). This suggests that most of the BM plasma cells generated by the CpG-adjuvanted PS3 vaccine are long-lived and that their renewal rate by PS3-specific B lymphocytes is low.
The CpG-adjuvanted PS3 vaccine generates long-lived BM plasma cells. (A-E) Ten-week-old mice were immunized with PS3 and injected 2 days later with CpG1668 before being irradiated at 7.5 Gy (750 rad) to deplete naive and memory B lymphocytes 45 days later. (A) Numbers of B-1a and B-1b cells in peritoneal washings determined by numeration and flow cytometry at the indicated time points, in control and irradiated mice. (B) Numbers of B-1 and B-2 cells in the spleen remaining 60 days after irradiation, determined as in panel A. (C) Numbers of B cells responsive to in vitro PS3 stimulation in peritoneal washings of mice immunized with the CpG-adjuvanted PS3 vaccine 180 days after irradiation. (D-E) The numbers of PS3-specific IgM (D) and IgG (E) ASCs in the BM of control and irradiated mice were determined by ELISPOT assay at the indicated time points. (F) Adoptive transfer of PS3-specific plasma cells results in prolonged PS3-specific Ab production in B cell–deficient mice. Donor C57BL/6 mice received a single injection of the CpG-adjuvanted PS3 vaccine or 2 sequential injections of PS3TT with a 28-days interval. Forty days after the last injection, total femoral BM cells or CD138-depleted BM cells were adoptively transferred into naive B cell–deficient (μMT) mice. Each recipient mice received 3.5 × 104 PS3-specific ASCs (from donors immunized with the CpG-adjuvanted PS3 vaccine) or 2.25 × 104 PS3-specific ASCs (from donors immunized with the PS3-TT vaccine). Sera were collected at the indicated time points after transfer, and PS3-specific IgM titers were determined by ELISA. Results are expressed as means ± SEM of the values collected in 5 individual mice.
The CpG-adjuvanted PS3 vaccine generates long-lived BM plasma cells. (A-E) Ten-week-old mice were immunized with PS3 and injected 2 days later with CpG1668 before being irradiated at 7.5 Gy (750 rad) to deplete naive and memory B lymphocytes 45 days later. (A) Numbers of B-1a and B-1b cells in peritoneal washings determined by numeration and flow cytometry at the indicated time points, in control and irradiated mice. (B) Numbers of B-1 and B-2 cells in the spleen remaining 60 days after irradiation, determined as in panel A. (C) Numbers of B cells responsive to in vitro PS3 stimulation in peritoneal washings of mice immunized with the CpG-adjuvanted PS3 vaccine 180 days after irradiation. (D-E) The numbers of PS3-specific IgM (D) and IgG (E) ASCs in the BM of control and irradiated mice were determined by ELISPOT assay at the indicated time points. (F) Adoptive transfer of PS3-specific plasma cells results in prolonged PS3-specific Ab production in B cell–deficient mice. Donor C57BL/6 mice received a single injection of the CpG-adjuvanted PS3 vaccine or 2 sequential injections of PS3TT with a 28-days interval. Forty days after the last injection, total femoral BM cells or CD138-depleted BM cells were adoptively transferred into naive B cell–deficient (μMT) mice. Each recipient mice received 3.5 × 104 PS3-specific ASCs (from donors immunized with the CpG-adjuvanted PS3 vaccine) or 2.25 × 104 PS3-specific ASCs (from donors immunized with the PS3-TT vaccine). Sera were collected at the indicated time points after transfer, and PS3-specific IgM titers were determined by ELISA. Results are expressed as means ± SEM of the values collected in 5 individual mice.
To confirm the longevity of the BM plasma cell pool generated in a TI fashion, the persistence of PS3-specific IgM was followed in naive B cell–deficient (μMT) recipient mice reconstituted with BM cells isolated from PS3-immunized mice. To address the possible replenishment of the transferred plasma cell pool by naive or memory B cells that may be present in the BM inoculum, control groups of mice were injected with BM cells from immunized mice that had been depleted of CD138-expressing cells. An ELISPOT performed on CD138− BM cells showed a 90% to 95% reduction in the numbers of PS3-specific ASCs, thus confirming the efficacy of our depletion procedure (supplemental Figure 3). The PS3-specific IgM titers underwent a steep decline during the first 30 days after transfer, most probably because a fraction of plasma cells transferred failed to relocalize to the BM and rapidly died (Figure 6F). After this initial phase of rapid decay, the PS3-specific IgM levels gradually and slowly declined to half 4 months after transfer. This means that the apparent half-life of PS3-specific serum IgM in those experimental groups is largely superior to the half-life of 2 days that has been described for passively transferred mouse IgM.28 By contrast, the IgM titers in mice reconstituted with CD138-depleted BM became almost undetectable as soon as 15 days after transfer. These results suggest that the maintenance of serum PS3-specific IgM Abs in the recipients is neither due to passively transferred Abs nor driven by restimulation of donor B cells, but rather by long-lived plasma cells.
BM plasma cells generated in a TI fashion confer immune protection against live S pneumoniae
It has been described that B-1b cells provide long-lasting T cell–independent IgM memory and confer protection against Borrelia hermsii infections.5 We thus sought to explore the contribution of long-lived PS3-specific BM plasma cells to the protection afforded by the plain and adjuvanted PS3 vaccines. All vaccinated mice were protected against a 106 cfu inoculum (Figure 7A), whereas 100% of naive and CpG-injected mice succumbed after injection of a 103 cfu inoculum (data not shown). Only 35% of the mice vaccinated with the plain PS vaccine survived the injection of 107 live bacteria. By contrast, all mice immunized with the conjugated or CpG-adjuvanted PS3 vaccine survived this inoculum of live bacteria (Figure 7B; data not shown), showing that delayed administration of TLR agonists enhances both the primary PS antibody response and the efficiency of the immune protection conferred by a PS vaccine. Furthermore, there was no significant difference in the survival rate of irradiated and nonirradiated mice after challenge with 107 cfu of live S pneumoniae (Figure 7B), suggesting that PS3-specific long-lived BM plasma cells are sufficient to protect vaccinated mice against acute S pneumoniae infections.
PS3-specific BM plasma cells are sufficient to promote full protection against Streptococcus pneumoniae infections. Ten-week-old mice were immunized with PS3 or PS3 followed 2 days later by injection of CpG1668 and irradiated or not 45 days later. Fifteen days after irradiation, 2 mice per group were checked for depletion of total B-1, B-1a, B-1b, and B-2 cells in peritoneal washings as well for the frequency of the PS3-specific lymphocytes as described in Figure 6A and B. In all irradiated groups, B-cell depletion was superior to 98% irrespective of the B-cell subset considered, and the frequencies of PS3-specific lymphocytes in peritoneal washings were reduced at least 100-fold compared with control mice. Fifteen days after irradiation, the survival of nonirradiated and irradiated immune mice (8 per group) was then monitored after challenge with 106 (A) or 107 cfu (B) of live S pneumoniae.
PS3-specific BM plasma cells are sufficient to promote full protection against Streptococcus pneumoniae infections. Ten-week-old mice were immunized with PS3 or PS3 followed 2 days later by injection of CpG1668 and irradiated or not 45 days later. Fifteen days after irradiation, 2 mice per group were checked for depletion of total B-1, B-1a, B-1b, and B-2 cells in peritoneal washings as well for the frequency of the PS3-specific lymphocytes as described in Figure 6A and B. In all irradiated groups, B-cell depletion was superior to 98% irrespective of the B-cell subset considered, and the frequencies of PS3-specific lymphocytes in peritoneal washings were reduced at least 100-fold compared with control mice. Fifteen days after irradiation, the survival of nonirradiated and irradiated immune mice (8 per group) was then monitored after challenge with 106 (A) or 107 cfu (B) of live S pneumoniae.
Discussion
The goal of an antibacterial vaccine is to generate memory B cells able to rapidly elicit massive secretion of neutralizing Abs. Plain PS vaccines, such as Pneumovax, can induce relatively short-lived immunity in adults17,18 limited to 5 to 10 years, compared with several decades of anamnestic responses that can be induced by protein Ags and by whole virus.29 Our results open new scopes for the use of plain PS vaccines for the following reasons. First, we clearly showed that the administration of a TLR agonist to mice vaccinated with a plain PS vaccine enhances up to 10 times the primary antibody response. Second, the efficiency of the protection against S pneumoniae infections conferred by the adjuvanted PS3 vaccine with CpG ODNs was highly increased and comparable to that obtained with the conjugate vaccine that is considered as the gold standard for vaccination of young children (data not shown). Whereas the adjuvant effect of GpG ODNs for TD Ags and for the TD form of PS in particular (PS–protein conjugates) is uncontested,19,20 contradictory results have been published about the potential adjuvant effect of TLR agonists on TI Ab responses. Kovarick et al21 demonstrated that, although CpG ODNs significantly enhance Ag-specific IgM and IgG responses to TNP-Ficoll, they fail to increase Ig titers in response to any of the 18 PS serotypes from S pneumoniae tested. Note that in their report, the researcher only tested coadministration of the CpG ODNs with the PS vaccine.21 By contrast coadministration of Pneumovax with the TLR4 agonist, monophosphoryl lipid A, has been shown to enhance the antibody response in adult and aged mice.30
From a mechanistic point of view, the reason why TLR agonists require to be administrated at least 1 day after immunization to exert their adjuvant effects remains an open question. Because we bring evidence that CpG1668 directly targets B cells, the existence of a negative cross talk between the TLR and the BCR signaling pathways appears as a plausible scenario. Interestingly, Btk, which is a critical component of the BCR signaling machinery for B-1 cells, is also involved in TLR signaling in B cells.31 On concomitant engagement of TLRs and the BCR, the available pool of cytosolic Btk might split between the 2 receptors, thus preventing optimal activation of both signaling pathways. This hypothesis is compatible with the partial inhibition of the PS3 Ab response obtained when TLR agonists and PS3 were concomitantly injected.
Our present finding showing that TLR signaling in B cells is instrumental for the adjuvant effect of TLR agonists on TI Ab responses is in agreement with the current concept that amplification of the B-cell responses to TD Ags by TLR agonists is targeted to B cells and not to APCs.32,33 This observation is nonetheless in contradiction with a report from Pasare et al32 which shows that the TI B-cell response and in particular the IgG3 Ab response against flagellin is deficient in MyD88−/− mice but not in μMT mice that had received MyD88−/− B cells, suggesting that TLR signaling in dendritic cells or macrophages but not in B cells were required for optimal response to TI Ags.
Our data clearly establish that the delayed administration of a TLR agonist enhances the protection conferred by the PS3 vaccine; however, the nature of the cells responsible for this protection was unexpected. Indeed, the pioneering study of Alugupalli et al5 has described that immune protection conferred by TI vaccination can be transmitted to immunodeficient mice by the transfer of lymphoid (B-1b) cells. It is now established that, in addition to memory B lymphocytes, TD Ags generate a second memory compartment constituted of long-lived BM plasma cells.11,12 However, it was admitted that accumulation of plasma cells in the BM is strictly a T cell–dependent process.34 Despite this, expression of CXCR4, which is known to be involved in the homing of plasmablasts to the BM, is up-regulated during the plasma cell differentiation process of human B cells in response to TI stimuli.35 Furthermore, B-1 cell–derived plasma cells express high levels of Blimp-1,14,36 which has been shown to play a central role in BM plasma cell survival.37 Our present data showing that PS3 by itself elicits few but persistent plasma cells in the BM indicate that plasmablasts generated in response to TI Ags can migrate to the BM and possibly become long lived. Moreover, the apparent differential lifespan of BM plasma cells generated in response to TI Ags and those generated in response to TD Ags, evokes the possibility that the 2 types of PCs have either different intrinsic survival capacities or occupy different niches in the BM. At face value, the latter observation is in contradiction with the fact that the titers of serum PS3 Abs at the plateau phase remain either comparable (IgM) or higher (IgG) in mice immunized with PS3TT than in mice that were vaccinated with PS3 and CpG. However, our results point out that the Ig secretion capacity of ASCs generated by PS3TT is higher than that of ASCs produced by the PS vaccine, suggesting that plasma cells generated by TD and TI Ags are functionally different. Altogether, these results suggest that the memory plasma cell compartment is heterogeneous.
Finally, TI immunologic memory appears to be more complex than expected because it involves both a lymphocyte (B-1b) and a plasma cell compartment. It is unclear at present in which situation the B-1b cell memory compartment comes into play. The respective contribution of TI memory B cells and TI memory plasma cells could vary depending on the bacterial type, the site, and the chronicity of infection. In the case of chronic infections the first episodes of bacteremia will generate B-1b memory cells as well as Ag-specific IgGs that, as previously reported by Obukhanych and Nussenzweig,10 repress their reactivation. It is possible that the recurrent bacterial infection could exhaust the inhibitory IgG Abs through formation of immune complexes, thereby allowing activation of memory B-1b cells.
In conclusion, our present work brings 4 major outcomes. First, we showed that a prototypic TI Ag generates a compartment of long-lived BM plasma cells. Some elements in the literature suggest that this conclusion may also apply to TI Ab response in humans. It was indeed recently described that patients treated for several months with rituximab, which depletes all CD19-expressing blood lymphocytes, maintained their levels of serum anti-pneumococcal capsular PS IgM and IgG.38 Second, this compartment of long-lived BM plasma cells can be enlarged by the administration of TLR agonists. Third, the memory plasma cell compartment generated in response to a TI antigen differs from that produced in response to its TD counterpart. Fourth, BM plasma cells generated by a PS vaccine are sufficient to confer full protection of vaccinated animals toward challenge with live S pneumoniae bacteria. Altogether, our results shed some light on the mechanisms of action of PS vaccines and provide a framework for their improvement and widespread usage.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Nadine Aguilera for help at the animal care facility, Chantal Bella for help with flow cytometry, and André Fleer (University Medical Center Utrecht, The Netherlands) for the gift of the WU2 S pneumoniae bacteria.
This work was supported by Sanofi-Pasteur and Inserm.
Authorship
Contribution: M.T. conceived, designed, and performed experiments, analyzed data, and wrote the paper; G.H., P.M., M.-J.A., and H.G. performed experiments and analyzed data; N.B. contributed to the materials and scientific discussion; T.D. conceived and designed the experiments and wrote the paper; and L.G. conceived, designed, and performed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laurent Genestier, Inserm, U851, 21 Ave Tony Garnier, Lyon, F-69007, France; e-mail: laurent.genestier@inserm.fr.
References
Author notes
T.D. and L.G. contributed equally to this study.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal