To the editor:
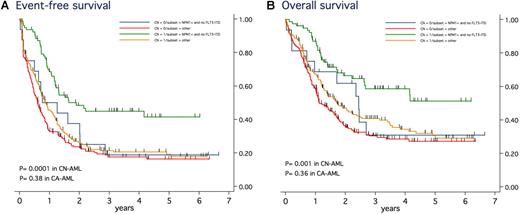
We read with interest the article by Haferlach et al1 that concluded that cytogenetic status has no significant impact on the prognosis of patients with nucleophosmin (NPM1)–mutated acute myeloid leukemia (AML). Authors suggested that the favorable genotype defined by the presence of NPM1 mutation without fms-like tyrosine kinase 3 (FLT3) internal tandem duplication (ITD), previously reported to be associated with favorable outcome in patients with cytogenetically normal (CN) AML,2 should be considered whatever the karyotype, that is, even in those with cytogenetically abnormal (CA) AML. We thus analyzed the role of these factors in newly diagnosed AML patients homogeneously treated in the Acute Leukemia French Association (ALFA) trials between 1990 and 2006 (ALFA 9000, 9801, 9802).3-5 Among 1529 patients (15-70 years of age), 554 patients were screened for NPM1 mutation, FLT3-ITD and have informative karyotype. Mutations screening were centrally performed according to previously described procedures.6,7 Karyotype was considered as normal if at least 20 normal marrow metaphases were present without any abnormal marrow metaphases. Favorable core binding factor (CBF) AML patients (n = 74) were excluded from this analysis. Among the 480 remaining patients, 137 (29%; median age 48 years; range, 17-68 years) were NPM1-mutated. As Haferlach et al, we found no difference between NPM1 mutated CN-AML patients (n = 115) and NPM1 mutated non-CBF CA-AML patients (n = 22) in term of complete remission rate (88% vs 83%; P = .49), overall survival (OS; 39% vs 38% at 5 years, P = .85), event-free survival (EFS; 33% vs 25% at 5 years, P = .39). However, in the context of the favorable genotype and in contrast with the results of Haferlach et al, EFS was significantly longer in the 79 CN- than in the 16 CA-AML patients (41% vs 19% at 5 years; P = .04) with a trend to better OS (51% vs 31% at 5 years; P = .12; Figure 1). No significant differences were observed in patients with NPM1-mutated AML but FLT3-ITD (5-year EFS, 12% vs 40%; 5-year OS, 20% vs 60%; P = .47 and .27, respectively), even if the numbers of patients were here very low (35 CN- vs 5 CA-AML). More importantly, the favorable genotype was predictive of a longer EFS and OS in the 267 patients with CN-AML (5-year EFS, 41% vs 19%; 5-year OS, 51% vs 30%; P < .001 and P = .001, respectively), whereas no differences were observed in the 213 patients with non-CBF CA-AML (5-year EFS, 19% vs 16%; 5-year OS, 31% vs 27%; P = .38 and .36, respectively; Figure 1). As shown, patients with the favorable genotype but CA-AML had an outcome relatively similar to those with a nonfavorable genotype and either CA- or CN-AML. As we recently reported for CCAAT/enhancer binding protein α (CEBPA) mutations,8 the favorable outcome associated with NPM1 mutation in the absence of FLT3-ITD might thus depend on the presence of a normal karyotype. We thus think that larger studies or overviews are needed to answer this question definitely.
EFS and OS in patients with NPM1-mutated and no FLT3-ITD AML (favorable genotype) versus other patients, according to the presence of a normal karyotype or not. In the presence of NPM1 mutation and no FLT3-ITD (favorable genotype), estimated 5-year EFS was 41% (95% CI, 29%-54%) in the 79 patients with CN-AML (green curves) and 19% (95% CI, 5%-40%) in the 16 patients with non-CBF abnormal karyotype (blue curves; P = .04). In these patients, estimated 5-year OS was 51% (95% CI, 36%-64%) in CN-AML patients and 31% (95% CI, 10%-55%) in the non-CBF CA-AML patients (P = .12). In the absence of this favorable genotype, estimated 5-year EFS was 19% (95% CI, 13%-26%) in the 188 patients with CN-AML (yellow curves) and 16% (95% CI, 11%-23%) in the 197 patients with non-CBF abnormal karyotype (red curves; P = .13). In these patients, estimated 5-year OS was 30% (95% CI, 21%-36%) in CN-AML patients and 27% (95% CI, 20%-35%) in the non-CBF CA-AML patients (P = .10). As shown, a favorable genotype was thus predictive of a longer EFS and OS in patients with CN-AML (P < .001 and P = .001, respectively), but not in those with non-CBF CA-AML (P = .38 and P = .36, respectively).
EFS and OS in patients with NPM1-mutated and no FLT3-ITD AML (favorable genotype) versus other patients, according to the presence of a normal karyotype or not. In the presence of NPM1 mutation and no FLT3-ITD (favorable genotype), estimated 5-year EFS was 41% (95% CI, 29%-54%) in the 79 patients with CN-AML (green curves) and 19% (95% CI, 5%-40%) in the 16 patients with non-CBF abnormal karyotype (blue curves; P = .04). In these patients, estimated 5-year OS was 51% (95% CI, 36%-64%) in CN-AML patients and 31% (95% CI, 10%-55%) in the non-CBF CA-AML patients (P = .12). In the absence of this favorable genotype, estimated 5-year EFS was 19% (95% CI, 13%-26%) in the 188 patients with CN-AML (yellow curves) and 16% (95% CI, 11%-23%) in the 197 patients with non-CBF abnormal karyotype (red curves; P = .13). In these patients, estimated 5-year OS was 30% (95% CI, 21%-36%) in CN-AML patients and 27% (95% CI, 20%-35%) in the non-CBF CA-AML patients (P = .10). As shown, a favorable genotype was thus predictive of a longer EFS and OS in patients with CN-AML (P < .001 and P = .001, respectively), but not in those with non-CBF CA-AML (P = .38 and P = .36, respectively).
Authorship
Acknowledgments: The authors are grateful to all ALFA investigators who participated in the ALFA-9000, ALFA-9801, and ALFA-9802 trials.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hervé Dombret, Department of Hematology, Hôpital Saint-Louis, 1 ave Claude Vellefaux, 75010 Paris, France; e-mail: herve.dombret@sls.aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal